Abstract
Background
Appendiceal neuroendocrine tumors (aNET) are a common entity in routine medical care, with a rate per appendectomy as high as 0.3–0.9 %. Considering the relatively young age at diagnosis for these patients, exact information about the long-term prognosis of aNET is required. Survival rates vary substantially between 71 and 100 % and are mostly limited to 5 years. This investigation assessed the long-term mortality rates of patients who underwent aNET resections at fifteen hospitals.
Methods
Between 1990 and 2003, the 10-year survival rates of 79 patients were analyzed using risk-adjusted Cox proportional hazard regression models adjusted for population-based baseline mortality. Additionally, prognostic factors for the oncologic outcomes were assessed.
Results
The median follow-up of all patients was 12.1 and 13.7 years for those alive. All patients underwent curative R0 resections. No distant metastases were diagnosed. A total of 31 (39.2 %), 29 (36.7 %), 18 (22.8 %), and 1 (1.3 %) patients had stage I, IIA, IIB, and IIIB aNET, respectively, according to the latest classification by the European Neuroendocrine Tumor Society. The 10-year overall and relative survival rates were 83.6 % (95 % CI 75.5–92.6 %) and 96.7 % (95 % CI 87.5–107 %), respectively. The 10-year relative survival rate after resection of aNET did not differ from the survival of the average national population with the same age and gender (p = 0.947). Second primary malignancies (hazard ratio of death 7.0, 95 % CI 1.6–30.6) were identified as a significant prognosticator for long-term survival.
Conclusions
Long-term survival is not significantly depreciated after the curative resection of aNET.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of neuroendocrine tumors (NET) varies between 3.24 and 6.50/100,000 [1]. Appendiceal NET (aNET) comprise one of the largest subgroups, although the percentage ranges from 3 to 44 % of all NET sites [1–3], which corresponds to an incidence of 0.15–0.21/100,000 [2]. Although these numbers are small, aNET represent a common problem in routine medical care because appendectomy is one of the most common surgical procedures, with a lifetime cumulative incidence rate of up to 12 % for males and 23.1 % for females [4, 5] and a rate of aNET per appendectomy of 0.3–0.9 % [6, 7].
With regard to those numbers and considering the relatively young mean age at diagnosis, exact information about the long-term prognosis of aNET is required. 5-year relative survival rates vary substantially between 71 and 100 %, and the surveillance rates beyond 5 years are rarely described [8]. The vast majority of available relative survival data are based on cancer registries, such as the surveillance, epidemiology, and end results (SEER) program, and not on clinical trials with higher data resolutions. Cancer registries typically house the data from patients treated with and without surgery, and a relevant percentage of data on cancer staging might be incorrect or missing, leading to potentially biased conclusions [9].
The aim of the present study was to assess long-term survival in a sample of 79 patients with aNET, including data from both, two secondary centers and small affiliated hospitals. Uni- and multivariate survival analyses were performed after adjusting for the Swiss population-based baseline mortality, applying the relative survival approach to provide internationally comparable results. Prognostic factors for the oncologic outcome were assessed [10].
Patients and methods
Data collection and definitions
For the present retrospective study, patients diagnosed with aNET were identified from the registries of the pathological institutes St. Gallen and Chur, Switzerland. Between January 1990 and December 2003, 81 patients undergoing resection for histologically proven aNET at fifteen hospitals affiliated with the institutes of pathology in St. Gallen and Chur were identified. Consistent with the histological definition of aNET in the literature, goblet cell carcinomas were excluded from our trial. In-hospital mortality occurred in one of the 81 patients (1.2 %; 95 % CI 0.1–6.7 %). Additionally, one patient was lost to follow up. Hence, a total of 79 patients were available for analysis. Data regarding the patients’ demographics and postoperative mortality were ascertained from their medical charts. Histological results were obtained from the registries of the two institutes of pathology. No information was available about any form of adjuvant therapy.
Follow-up
To gather up-to-date survival information, the patients and their family doctors were contacted. If no information was available, the appropriate resident registration offices were contacted to obtain the most complete follow-up information. Follow-up was completed in July 2012.
Pathology
All aNET were reviewed by a pathologist trained in the field of NET, and all specimens were stained for synaptophysin and chromogranin A to immunohistologically verify the neuroendocrine tumor entity. Additionally, the tumor specimens were stained for Ki-67, and the mitotic count per 10 HPF was measured. All original pathological data were re-classified to meet the 2012 ENETS classification criteria [10].
Statistical analyses
Statistical analyses were performed using the R statistical software (www.r-project.org). A two-sided p value <0.05 was considered to be statistically significant. Continuous data are expressed as the mean ± SD. The main outcome measured was the relative survival, which is the ratio of the observed overall survival proportion and the expected population-based survival proportion (“background mortality”). Overall survival was also assessed to provide comparability with previous research and to serve as a sensitivity analysis.
The population tables for background mortality in the relative survival analyses were obtained from the Federal Statistical Office [11]. The relative survival analyses were performed using the R package relsurv with the Ederer estimator [12, 13]. Population mortality rates were included as time-dependent covariates (multiplicative Cox regression model) [14].
The risk set for multivariable survival analyses included age (continuous), gender, body mass index (continuous), second primary malignancies (SPM, yes vs. no), extent of the primary and completion operation (appendectomy only vs. additional lymphadenectomy, hemicolectomy, etc.), infiltration of the mesoappendix (yes vs. no), tumor size (<1 vs. ≥1 cm), and Ki-67 index (<2 vs. 3–20 vs. >20), which was determined using monoclonal antibodies against one epitope of a proliferation-related antigen. To cope with the small number of events, the categories were summarized in the risk set. Age and gender were included in the risk set for relative survival to allow for informative censoring, although the baseline mortality was already estimated based on age and gender [15]. Missing data were imputed using the random survival forest method [16]. The risk set was assessed as putative prognostic factors for relative and overall survival in unadjusted and risk-adjusted Cox regressions, with a backward variable selection procedure from the full Cox regression model based on the Akaike’s information criterion. The proportional hazard assumption was tested by scaled Schoenfeld residuals and inspection of the hazard ratio plots. No violations of the assumption of proportional hazards were observed.
Results
Patient and treatment characteristics
A total of 79 patients with histologically proven aNET were included in the present analysis (Table 1). The median follow-up for all patients was 12.1 years (range: 1.6–22.4 years) and 13.7 years (range: 2.3–22.3 years) for all living patients. A total of 16 patients died during the follow-up. Second primary malignancies occurred in 12 patients (15 %). The following preceding or synchronal malignancies were recorded: colon cancer, N = 3; ovarian cancer, N = 2; rectal cancer, N = 1; prostatic cancer, N = 1; and cervix uteri cancer, N = 1. The following metachronous malignancies were recorded: colon cancer, N = 1; rectal cancer, N = 1; cervix uteri cancer, N = 1; and ovarian cancer N = 1. The initial operation for most of the patients was either a laparoscopic (N = 19) or open appendectomy (N = 56), which were complemented by a second operation in seven patients. In ten patients, the extent of the initial or the complementary operation met the criteria for an oncologic resection.
Histopathology
Table 2 summarizes the pathological staging information. No patient was diagnosed with distant metastasis. One patient had a regional lymph node metastasis. Stage I aNETs were found in 31 patients (39.2 %), stage IIa in 29 (36.7 %) patients, stage IIb in 18 (22.8 %) patients, and stage IIIb in one patient (1.3 %). When cross tabulating the criteria for an oncologic resection and the stage of the aNET, a relevant proportion of the patients were under- or overtreated, showing no significant relationship between the oncologic resection and aNET stage (p = 0.105) (Table 2).
Survival pattern
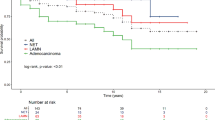
The 10-year overall survival rate was 83.6 % (95 % CI 75.5–92.6 %), (Fig. 1). According to gender, age, and calendar year at the time of operation, the expected 10-year survival rate was 87.8 % (using the relative survival approach). Hence, 4.2 % of the patients died because of the aNET (“excess mortality”), and 12.2 % died because of other causes (“baseline-mortality”) during the 10-year horizon. The 10-year relative survival rate was 96.7 % (95 % CI 87.5–107 %) and did not differ from that of the average Swiss population, according to gender, age, and calendar year at the time of operation (p = 0.947).
Prognostic factors for survival
The 10-year overall survival rates for patients with stage I, IIa, and IIb/IIIb aNET were 83 % (95 % CI 70–98 %), 78 % (95 % CI 64–95 %), and 86 % (95 % CI 70–100 %), respectively. The 10-year relative survival rates for patients with stage I, IIa, and IIb/IIIb aNET were 99 % (95 % CI 84–117 %), 85 % (95 % CI 70–103 %), and 104 % (95 % CI 85–126 %), respectively. Table 3 summarizes the multivariable Cox regression analysis for relative and overall survival. Because of the low number of events, a limited risk set with summarized categorical prognosticators was applied. Second primary malignancies were uniformly identified as a significant adverse prognostic factor. Regarding the extent of the oncologic operation, a tendency towards a protective influence was observed.
Discussion
To the best of our knowledge, to date, this study is one of the largest investigations of a homogeneous sample of clinically followed aNET patients to provide relative survival results by adjusting for population-based baseline mortality. The key result of the present study is a relative survival rate of 96 % in the 10-year perspective. The patient survival rate in our series did not differ from that of the general Swiss population (matched by age and gender). Hence, after a complete (R0) resection of aNET, patients can be considered to be cured. After multivariable adjustment, second primary malignancies had a significant prognostic impact on the oncologic outcome.
The observed long-term relative survival of 96 % confirms previous clinical studies that reported an excellent prognosis after aNET. Concordant with our findings, two other clinical studies demonstrated overall survival rates of 100 % [6, 7]. However, both investigations were based on small sample sizes and suffered from a short follow-up period. At this point, the present clinical study, which has a fairly complete 10-year follow-up, provides additional certainty regarding excellent patient prognosis on a 10-year horizon; this result is of utmost importance considering the young ages of patients diagnosed with aNET.
The excellent prognosis of aNET might reflect its less aggressive histology compared to other gastrointestinal NET entities, for example, colonic NET [1]. Another reason for the good prognosis might be caused by early symptoms and diagnosis through appendectomy.
The coincidence of second primary malignancies with aNET was significantly associated with impaired survival. To the best of our knowledge, the present study is the first clinical investigation to address this association. The observed rate of 15 % of patients with second primary malignancies aligned with that in the literature [17–20]. The significant influence of second primary malignancies on survival underlines the excellent prognosis of aNET itself and implies a risk-adapted follow-up.
Currently, there are two discrete classification systems for aNET in use: the 2010 WHO-classification system (according to the UICC) and the 2012 ENETS classification system. The main difference between these two systems is the distinction between T2 and T3 tumors. This differentiation is based on the size of the tumor and the extension of its infiltration into the mesoappendix. Regardless of its size, a tumor with an infiltration of the mesoappendix exceeding 3 mm is classified as T3, according to ENETS. However, there is insufficient data regarding the prognostic impact of the staging systems and their risk factors. Previous studies have proven tumor size to be a negative predictor of survival [21]. In addition to tumor size, the Ki-67 index is suggested to aid in the decision-making process concerning the extent of the operation in those intermediate tumors >1 cm but <2 cm and is included in the recent ENETS guidelines. Nevertheless, studies proving a significant prognostic influence of the Ki-67 index on survival are lacking [10, 22, 23]. The present study also failed to demonstrate worse outcomes for the histopathological criteria. This fact might be well explained by the small sample size. Deducing a lack of influence from a missing significant association between potential risk factors and survival could be misleading. When considering the small sample size, the small number of events and the retrospective study design, any absence of evidence must not be interpreted as evidence of absence; instead, it indicates the need for larger studies with more complete data [24].
When comparing appendectomy alone versus extended operations, a tendency for better survival after extended operations was observed in the present study, even with its small sample size. Hence, no conclusive statement can be derived addressing the important question as to whether an extended operation leads to a better prognosis.
We acknowledge the limitations of the present study. First, it is a cohort study and not a prospective trial. However, based on the research question at hand and the low incidence of aNET, a cohort study is most likely the most appropriate study design. Second, although a study using cancer registries can include larger patient numbers, the present study has the high resolution of a clinical investigation that is unlikely to be matched by cancer registry studies. Another drawback of the present study is the insufficient information concerning recurrence due to several reasons, i.e., a low autopsy rate and non-validated follow-up guidelines. However, this issue was the reason to apply the relative survival approach as an alternative statistic method. Thus, the mortality caused by a certain cancer entity can be estimated even with lacking information about the cause of death or relapse by comparison with the general population [12].
We conclude that patients with aNET face an excellent prognosis with a long-term relative survival rate of 96 % when the tumor is completely resected, regardless of whether the operation conformed to the guidelines. We recommend the strict treatment of patients following actual guidelines until further clinical trials with a large number of patients or highly developed prospective registries can be realized to validate these therapeutic guidelines. Second primary malignancies are an independent prognostic factor and should be explicitly considered in the further development of therapeutic guidelines, particularly for follow-up.
References
Hauso O, Gustafsson BI, Kidd M et al (2008) Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer 113:2655–2664
Maggard MA, O’Connell JB, Ko CY (2004) Updated population-based review of carcinoid tumors. Ann Surg 240:117–122
Modlin IM, Lye KD, Kidd M (2003) A 5-decade analysis of 13,715 carcinoid tumors. Cancer 97:934–959
Addiss DG, Shaffer N, Fowler BS et al (1990) The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol 132:910–925
Anderson JE, Bickler SW, Chang DC et al (2012) Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995–2009. World J Surg 36:2787–2794. doi:10.1007/s00268-012-1749-z
Shapiro R, Eldar S, Sadot E et al (2011) Appendiceal carcinoid at a large tertiary center: pathologic findings and long-term follow-up evaluation. Am J Surg 201:805–808
In’t Hof KH, van der Wal HC, Kazemier G et al (2008) Carcinoid tumour of the appendix: an analysis of 1485 consecutive emergency appendectomies. J Gastrointest Surg 12:1436–1438
Rorstad O (2005) Prognostic indicators for carcinoid neuroendocrine tumors of the gastrointestinal tract. J Surg Oncol 89:151–160
Robinson D, Sankila R, Hakulinen T et al (2007) Interpreting international comparisons of cancer survival: the effects of incomplete registration and the presence of death certificate only cases on survival estimates. Eur J Cancer 43:909–913
Pape UF, Perren A, Niederle B et al (2012) ENETS consensus guidelines for the management of patients with neuroendocrine neoplasms from the jejuno-ileum and the appendix including goblet cell carcinomas. Neuroendocrinology 95:135–156
Bevölkerungsbewegung–Analysen–Jährliche Sterbetafeln, Bundesamt für Statistik, 2013. http://www.bfs.admin.ch/bfs/portal/de/index/themen/01/06/blank/dos/la_mortalite_en_suisse/tabl02.html. Accessed 18 Nov 2014
Pohar M, Stare J (2007) Making relative survival analysis relatively easy. Comput Biol Med 37:1741–1749
Ederer F, AXTELL LM, CUTLER SJ (1961) The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr 6:101–121
Andersen PK, Borch-Johnsen K, Deckert T et al (1985) A Cox regression model for the relative mortality and its application to diabetes mellitus survival data. Biometrics 41:921–932
Danieli C, Remontet L, Bossard N et al (2012) Estimating net survival: the importance of allowing for informative censoring. Stat Med 31:775–786
Ishwaran H, Kogalur UB (2010) Consistency of random survival forests. Stat Probab Lett 80:1056–1064
Habal N, Sims C, Bilchik AJ (2000) Gastrointestinal carcinoid tumors and second primary malignancies. J Surg Oncol 75:310–316
Godwin JD (1975) Carcinoid tumors. An analysis of 2837 cases. Cancer 36:560–569
Moertel CG, Dockerty MB, Judd ES (1968) Carcinoid tumors of the vermiform appendix. Cancer 21:270–278
Sandor A, Modlin IM (1998) A retrospective analysis of 1570 appendiceal carcinoids. Am J Gastroenterol 93:422–428
McGory ML, Maggard MA, Kang H et al (2005) Malignancies of the appendix: beyond case series reports. Dis Colon Rectum 48:2264–2271
Grozinsky-Glasberg S, Alexandraki KI, Barak D et al (2013) Current size criteria for the management of neuroendocrine tumors of the appendix: are they valid? Clinical experience and review of the literature. Neuroendocrinology 98:31–37
Plockinger U, Couvelard A, Falconi M et al (2008) Consensus guidelines for the management of patients with digestive neuroendocrine tumours: well-differentiated tumour/carcinoma of the appendix and goblet cell carcinoma. Neuroendocrinology 87:20–30
Altman DG, Bland JM (1995) Absence of evidence is not evidence of absence. BMJ 311:485
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Thomas Steffen and Sabrina M. Ebinger have contributed equally.
Rights and permissions
About this article
Cite this article
Steffen, T., Ebinger, S.M., Warschkow, R. et al. Long-Term Survival is not Impaired After the Complete Resection of Neuroendocrine Tumors of the Appendix. World J Surg 39, 2670–2676 (2015). https://doi.org/10.1007/s00268-015-3164-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-015-3164-8