Abstract
Much debate still exists regarding the appropriate extent of lymphadenectomy for gastric adenocarcinoma. In high incidence countries in Eastern Asia, more extensive (e.g. D2) lymphadenectomies are standard, and these surgeries are generally done by experienced surgeons with low morbidity (<20 %) and mortality (<1 %). In United States and Western Europe, where the incidence of gastric adenocarcinoma is much lower, the majority of patients are treated at non-referral centers with less extensive (e.g. D1 or D0) lymphadenectomy. This symposium article first reviews early studies that led to recommendations for less extensive lymphadenectomy. Two large prospective, randomized trials performed in the United Kingdom and the Netherlands in the 1990s failed to demonstrate a survival benefit of D2 over D1 lymphadenectomy, but these trials have been criticized for inadequate surgical training and high surgical morbidity (43–46 %) and high mortality rates (10–13 %) in the D2 group. We then discuss more contemporary studies that support more extensive lymphadenectomy with a minimum of 16 lymph nodes for adequate staging. The 15-year follow-up of the Netherlands trial now demonstrates an improved disease-specific survival and locoregional recurrence in the D2 group. A prospective, randomized trial from Taiwan found a survival benefit of more extensive lymphadenectomies, and another randomized trial from Japan found adding dissection of para-aortic nodes to a D2 lymphadenectomy did not improve survival. Western surgeons have increasingly accepted the importance of performing more than a D1 node dissection, and Eastern surgeons are accepting that more than a D2 node dissection does not improve survival and increases morbidity. Thus both Eastern and Western approaches are favoring D2 lymphadenectomy as a standard, and on this topic we appear to be harmonizing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is estimated that there are over one million cases of gastric cancer worldwide per year making it the fourth most common cancer [1]. Nearly three-quarters of cases occur in developing countries, and nearly half of cases occur in Eastern Asia (mainly in China). Gastric cancer is the second leading worldwide cause of cancer death for both men and women, with a total of over 700,000 deaths each year. The incidence of gastric cancer in the United States and Western Europe is only about one-fifth to one-seventh that of the highest incidence countries in Eastern Asia [2].
In addition to the global differences in gastric cancer epidemiology, there are also significant differences in Eastern and Western philosophies regarding the role and extent of lymph node dissection for gastric cancer. The Eastern view generally contends that extensive lymphadenectomy provides better cancer cell clearance and therefore improves survival, whereas the Western view generally holds that lymphadenectomy provides prognostic information and when done adequately, improves staging and guides adjuvant treatment decisions. From either perspective, there is strong agreement that lymphadenectomy is an integral part of high-quality gastric cancer operation—but is more extensive lymphadenectomy always better? This article examines the issue of lymphadenectomy for gastric adenocarcinoma from the perspective of historical randomized, prospective trials up to more current studies in order to define a reasonable perspective and guideline regarding the appropriate balance to achieve the safest and most oncologically sound operation for our patients with gastric cancer.
Definitions
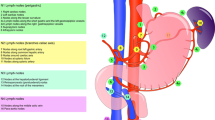
Prior to discussion of differences in lymphadenectomy for gastric adenocarcinoma, one should define the terms to be used. The lymph node stations surrounding the stomach were precisely defined by the JGCA (formerly known as the Japanese Research Society for Gastric Cancer) in 1973 [3] (Fig. 1; Table 1). The terminology for the extent of lymph node dissection has changed over the years, leading to some confusion. The JGCA defined four levels of lymph node stations identified as N1 through N4. The designation of N1–N4 nodes varied according to the site of the primary tumor (i.e. upper, middle, or lower third of stomach). The D level of lymphadenectomy was based on the JGCA definitions of lymph node station levels that were dissected [5]. A D1 lymphadenectomy was defined as removal of all N1 level nodes, and a D2 dissection was defined as removal of all N1 and N2 level nodes. In the most recent “Japanese gastric cancer treatment guidelines 2010,” the JGCA changed the definitions such that the D level of lymphadenectomy is now defined according to the type of gastrectomy performed rather than the location of the tumor (Table 2) [6]. To broadly summarize, a D1 lymphadenectomy removes the first tier of perigastric lymph nodes and a D2 lymphadenectomy removes the second tier of lymph nodes, which generally fall along branches of the celiac axis (i.e. left gastric artery, splenic artery, common hepatic artery, proper hepatic artery). A D1 + lymphadenectomy is intermediate between D1 and D2. The “Japanese gastric cancer treatment guidelines 2010” recommends a D2 lymphadenectomy for all gastric carcinomas beyond a clinical T1 tumor (a.k.a. tumor invades lamina propria, muscularis mucosae, or submucosa). They define a D2 + lymphadenectomy as an “extended lymphadenectomy beyond D2.”
Older randomized controlled trials from Western countries
During the 1990s, two landmark randomized, prospective studies were performed that subsequently dominated the Western approach to lymphadenectomy for gastric cancer. These studies provided the foundation for a generation of surgeons in the West to advocate for D1 lymphadenectomy. However, many investigators now consider these studies to be outdated. The first study, by Bonenkamp et al. [7], was a randomized study from the Netherlands in 1995 and has commonly been referred to as the Dutch study. This study randomized 711 patients to D1 versus D2 lymphadenectomy. D2 lymphadenectomy included a splenectomy and distal pancreatectomy for proximal tumors. This study found that patients in the D2 group had greater perioperative morbidity (43 vs 25 %) and mortality (10 vs 4 %) but no difference in 5-year overall survival (47 vs 45 %). The authors concluded that D2 lymphadenectomy does not provide superior results compared to D1 lymphadenectomy but was associated with increased perioperative morbidity and mortality and thus should not be recommended.
The second study was a randomized study from England that was published in the Lancet in 1999 and randomized 400 patients to D1 versus D2 lymphadenectomy [8]. The D2 group was found to have higher perioperative morbidity (46 vs 28 %) and mortality (13 vs 6.5 %), and there was no difference in 5-year overall survival (33 vs 35 %). Again, the D2 lymphadenectomy included a splenectomy and distal pancreatectomy for proximal tumors. The authors concluded that the added morbidity and mortality from the more extensive operation “nullify any survival benefit from D2 procedures”.
Current randomized controlled trials
In response to the findings of these earlier trials, many surgeons, predominantly in the East, questioned their validity on the basis of inadequate surgical training (D2 lymphadenectomy is performed at high-volume Eastern centers with <20 % morbidity and <1 % mortality) and lack of sufficient lymphadenectomy (many patients included in these studies had fewer than 16 lymph nodes retrieved). Furthermore, several studies have now demonstrated that D2 lymphadenectomies can be performed without the need for distal pancreatectomy [9] or splenectomy [10, 11]. More recent studies support the notion that a D2 lymphadenectomy may be optimal.
In 2006, Wu et al. [12] from Taiwan published their randomized controlled trial of D1 versus “D3” dissection in Lancet Oncology. The D3 lymphadenectomy described in this study essentially includes a JGCA D2 lymphadenectomy and nodes around the retropancreatic region and superior mesenteric vein. That study randomized 221 patients to lymphadenectomy, there was excellent quality control, and the primary endpoints were 5-year overall- and disease-free survival. At a median follow-up of 95 months, there was a 5-year overall survival of 63 % for patients who underwent D3 surgery and 58 % for patients undergoing D1 surgery (p = 0.006). The recurrence rate at 5 years was 51 % after D1 surgery and 40 % after D3 surgery. The authors concluded that D3 nodal dissection compared with D1 offers a survival benefit for patients with gastric cancer.
In 2010, the Dutch trial was updated with a 15-year follow-up [13]. Gastric cancer-related death was 48 % for the D1 group and 37 % for the D2 group (p = 0.01), whereas death from other causes was the same in both groups. The authors acknowledged that the original D2 procedure was associated with significantly higher perioperative morbidity and mortality, but also stated that the D2 lymphadenectomy has become a safer option since pancreas and spleen-preserving resections are now more routinely done. Therefore, they recommended D2 lymphadenectomy as the standard approach.
Last comes the randomized, prospective study from Sasako et al. [14] from Japan, published in the New England Journal of Medicine in 2008. That study questioned whether D2 lymphadenectomy with para-aortic nodal dissection was better than standard D2 lymphadenectomy. In it, 523 patients with curable stage gastric cancer were randomly assigned to D2 lymphadenectomy (n = 264) or D2 plus para-aortic node dissection (n = 260). The results included a longer median operative time and more blood loss in the extensive lymphadenectomy group but no significant differences in morbidity. The 5-year overall survival rate was 69 % for the D2 lymphadenectomy group and 70 % for the D2 plus para aortic nodal dissection group. These researchers concluded that treatment with more extensive lymphadenectomy does not improve the survival rate for gastric cancer.
Current standards for lymphadenectomy in the Western world
The studies summarized in the preceding section highlight the progression of our understanding of lymphadenectomy and appropriate levels of dissection. Earlier studies were conducted at a time when more extensive en bloc resections were performed and morbidity and mortality rates were higher. In addition, the supplemental survival benefits of improved chemotherapy options were not as developed as they are today. Although the Japanese study did confirm that more extensive resections such as para-aortic nodal dissection do not further improve survival, contemporaneous studies from the East and West confirm that D2 lymphadenectomy is a standard for a sound oncologic resection.
Can Western surgeons perform more extensive lymphadenectomies safely? The Italian Gastric Cancer Study Group approached the issue of Western surgeons performing more extensive lymphadenectomies in Western patients in a series of two prospective clinical trials [15, 16]. After extensive training of 16 surgeons in the performance of D2 lymphadenectomy, a phase II trial of D2 lymphadenectomy was instituted in which all surgeries were performed by the two attending surgeons. Of the 191 patients enrolled in the study, 106 patients (55 %) were ultimately found to be ineligible, usually because of the presence of more extensive disease. The mean number of lymph nodes removed was 39 (range: 22–93). Overall postoperative morbidity and mortality were impressively low at 20.9 and 3.1 %, respectively. Subsequent to that study, the surgeons from the five highest volume centers performed a randomized trial of D1 versus D2 lymphadenectomy [17]. Among 267 randomized patients, total morbidity and mortality were 12.0 and 3.0 % in the D1 group and 17.9 and 2.2 % in the D2 group. Survival results are pending. The experience of the Italian Gastric Cancer Study Group clearly demonstrates that after a period of fairly rigorous training Western surgeons can perform D2 lymphadenectomies on Western patients with morbidity and mortality results similar to those reported from high-volume centers in Korea and Japan.
Several tertiary referral centers in Western countries routinely perform D2 lymphadenectomies for gastric cancer with low morbidity and mortality [18, 19], but lymphadenectomies for gastric cancer in Western countries are limited and often do not reach the D1 threshold. There are several reasons why more extensive lymphadenectomies are not more commonly performed. One significant obstacle is the relative paucity of gastric adenocarcinomas seen in any Western institution. For more extensive lymphadenectomy to benefit gastric cancer patients, the procedure must be performed without excessive morbidity and mortality, and this can only be achieved with adequate surgical training and adequate case volume. Contributing to the lack of high-volume centers for the treatment of gastric cancer surgery is a significant reluctance of general surgeons to refer gastric cancer patients to tertiary referral centers given that gastric surgery has historically been the realm of the general surgeon [20]. Finally, geographical and language barriers make international dissemination of information and techniques on the surgical treatment of gastric cancer difficult.
Recommendations
Overall, it appears that despite major early differences in the approach to lymphadenectomy by surgeons in the East and West, as surgeons develop similar understanding and values, and as they adopt similar approaches to lymph node dissection in patients with gastric cancer, the differences are fading. Although the more extensive D2 lymphadenectomy with paraaortic node dissection originally espoused in the East appears to be excessively invasive with increased morbidity, the less extensive D1 lymphadenectomy typically carried out in the West is not sufficient to adequately stage and guide treatment for patients. There now appears to be increasing understanding that the approach to lymph node removal may be better guided by the stage of disease. Thus, select groups are evaluating the application of less aggressive lymphadenectomy for the earliest stage gastric cancers, via endoscopic mucosal resection without lymph node dissection or wedge resection with limited regional lymphadenectomy for early lesions—although results and long-term follow-up are not yet complete for such applications. Nevertheless, the general understanding that treatment should be individualized based on the stage of disease and perhaps concomitant co-morbidities, is allowing a more thoughtful approach to caring for patient with gastric cancer. At the very least, it appears that the major differences once perceived from the East and West may not actually be as extensive as once thought.
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62:10–29
Japanese Research Society for Gastric Cancer (1973) The general rules for the gastric cancer study in surgery. Jpn J Surg 3:61
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14:101–112
Nishi M, Omori Y, Miwa K (1995) Japanese classification of gastric carcinoma. Kanehara & Co., Ltd, Tokyo
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer 14:113–123
Bonenkamp JJ, Songun I, Hermans J et al (1995) Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 345(8952):745–748
Cuschieri A, Weeden S, Fielding J et al (1999) Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer 79:1522–1530
Maruyama K, Sasako M, Kinoshita T et al (1995) Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 19:532–536. doi:10.1007/BF00294714
Uyama I, Ogiwara H, Takahara T et al (1996) Spleen- and pancreas-preserving total gastrectomy with superextended lymphadenectomy including dissection of the para-aortic lymph nodes for gastric cancer. J Surg Oncol 63:268–270
Biffi R, Chiappa A, Luca F et al (2006) Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: low morbidity and mortality rates in a single center series of 250 patients. J Surg Oncol 93:394–400
Wu CW, Hsiung CA, Lo SS et al (2006) Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol 7:309–315
Songun I, Putter H, Kranenbarg EM et al (2010) Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 11:439–449
Sasako M, Sano T, Yamamoto S et al (2008) D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 359:453–462
Degiuli M, Sasako M, Ponti A et al (1998) Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol 16:1490–1493
Degiuli M, Sasako M, Calgaro M et al (2004) Morbidity and mortality after D1 and D2 gastrectomy for cancer: interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol 30:303–308
Degiuli M, Sasako M, Ponti A (2010) Morbidity and mortality in the Italian Gastric Cancer Study Group randomized clinical trial of D1 versus D2 resection for gastric cancer. Br J Surg 97:643–649
D’Angelica M, Gonen M, Brennan MF et al (2004) Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 240:808–816
Schmidt B, Chang KK, Maduekwe UN et al. (2013) D2 lymphadenectomy with surgical ex vivo dissection into node stations for gastric adenocarcinoma can be performed safely in Western patients and ensures optimal staging. Ann Surg Oncol (in press)
Karpeh MS Jr (2005) Should gastric cancer surgery be performed in community hospitals? Semin Oncol 32(6 Suppl 9):S94–S96
Conflict of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strong, V.E., Yoon, S.S. Extended Lymphadenectomy in Gastric Cancer Is Debatable. World J Surg 37, 1773–1777 (2013). https://doi.org/10.1007/s00268-013-2070-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-013-2070-1