Abstract
Background
Although thrombocytosis has been reported in patients with various types of cancer, the association between thrombocytosis and the clinicopathological features of patients with colorectal cancer (CRC) has not been fully investigated. We evaluated the clinical features associated with thrombocytosis in CRC.
Materials and methods
The medical records of 636 consecutive CRC patients undergoing surgery in our department between January 2002 and July 2008 were retrospectively reviewed. The correlation between the clinicopathological variables and the preoperative platelet count was analyzed by univariate and multivariate analyses. The impact of thrombocytosis on the prognosis of these patients was assessed, in comparison with the other clinicopathological variables.
Results
Platelet count showed significant correlation with gender, age, venous involvement, tumor size, depth of invasion, regional lymph node metastasis, distant metastasis in univariate analysis, and tumor size and depth of invasion were independent factors in multivariate analysis. The cancer-specific survival (CSS) of CRC patients with thrombocytosis was significantly shorter than that for those without thrombocytosis (P < 0.001), specifically in patients with stage III CRC (P < 0.001). Multivariate analysis indicated that thrombocytosis was an independent prognostic factor of CSS (hazard ratio = 2.96, 95% confidence interval [CI] = 1.72–5.00). Moreover, within stage II CRC, the univariate analysis revealed that disease-free survival (DFS) was associated with preoperative thrombocytosis, but not the other clinicopathological variables.
Conclusions
Preoperative thrombocytosis is not only an independent indicator of poor CSS in CRC patients but also an independent predictor of poor DFS in patients with stage II CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related death in developed countries [1]. Although surgical resection remains the only established curative treatment for colon cancer, at least one-third of patients receiving potentially curative resection die within 5 years of diagnosis [2]. Therefore, it is pivotal to define reliable prognostic indicators to identify CRC patients at high risk of recurrence who might benefit from adjuvant treatment, such as chemotherapy. Although TNM staging strongly correlates with postoperative prognosis [3], prognosis varies even among the cases in the same stage. Therefore, many reports have been directed at evaluating the prognostic value of numerous molecular based factors [4]. There remains a continuing need to find clinically relevant indicators that would improve the prediction of survival in patients receiving potentially curative resection for CRC [5].
For more than a century, many abnormalities of the hemostatic system have been reported to be associated with malignancy. Elevated plasma fibrinogen has been demonstrated to be associated with tumor progression in many kinds of malignancies [6–8]. Levitan et al. showed that the frequency of deep vein thrombosis or pulmonary embolus was significantly higher in patients with malignancy, as compared with those without malignancy [9]. Among these coagulation abnormalities, thrombocytosis has been suggested to be strongly associated with various types of malignancies [10–14]. Thrombocytosis has been reported to have prognostic significance in patients with lung cancer, endometrial cancer, esophageal cancer, and breast cancer.
There are some reports on the association of thrombocytosis and clinicopathological features or prognosis of CRC, but the results are conflicting, and consensus has not been achieved [13, 15–17]. Thus, the clinical association between thrombocytosis and prognostic relevance remain to be investigated. In the present study, we attempted to assess the relationship between the preoperative platelet count and tumor progression, metastasis, recurrence, and outcome in CRC patients.
Patients and methods
The medical records of 814 consecutive patients who underwent surgical resection for CRC in our department between January 2002 and July 2008 were retrospectively reviewed. Among them, 42 patients were excluded on the basis of the following criteria (Table 1): (1) synchronous extracolonic malignancy, or association with apparent acute inflammatory disease, such as colorectal perforation, ulcerative colitis, Crohn’s disease, cholecystitis, or pneumonia, in which the platelet count can be positively affected, or (2) association with severe liver cirrhosis, which may negatively affect the platelet count [18, 19]. Ninety-nine patients were excluded because of preoperative treatment, such as radiation or chemoradiation. Thirty-seven patients with noncurative resection were also excluded, leaving 636 patients enrolled in the study.
Preoperative blood samples were obtained from patients with CRC within 2 weeks prior to operation. Platelet count was measured, and platelet count ≥ 370,000/μl was defined as thrombocytosis, according to the normal range of platelet count of our institution. In our institution, the normal platelet count ranges from 140,000/μl to 370,000/μl of blood. These limits are the 2.5th lower and upper percentiles of the values obtained by examination of the blood from 100 healthy volunteers in our institution in 2004. After resection of CRC, all specimens were histopathologically analyzed by the pathologists, and the pathological TNM classification and staging were given, according to the classification of the American Joint Committee on Cancer [20]. Of the 636 patients enrolled in the study, 30 had two or more primary CRCs. In these cases with multicentric cancer, the histopathological variables were determined by assessing the dominant lesion, that is, the largest/deepest one with respect to tumor size or invasion.
Univariate analyses of the correlation between platelet counts and clinicopathological variables were carried out according to nonparametric methods. The individual statistical methods used were as follows: the variables categorized into two groups, i.e., gender, age (<65 years, >64 years), tumor location, tumor size (<50 mm, >49 mm), histological type, lymphatic involvement, venous involvement, tumor depth (T1-2, T3-4), lymph node involvement, and presence of metastasis, were analyzed using the Mann–Whitney U-test. The ordinal variable categorized into four groups, i.e., depth of invasion (T-factor), was analyzed with the Kruskal–Wallis test. The continuous variable, i.e., tumor size, were analyzed with the Spearman rank correlation coefficient. The association between thrombocytosis and clinicopathological variables was examined by the Chi-square test. Significant risk factors affecting platelet counts were assessed by multivariate analysis with a logistic regression model. The Kaplan–Meier estimator, log-rank test, and Cox proportional hazard model were used for survival analysis. For patients who remained alive, data were censored at the date of the last contact. Cancer-specific survival (CSS) is calculated for all patients from the date of surgery until death from colorectal cancer, and disease-free survival (DFS) is calculated from the date of surgery until the time of relapse. Two-sided P values < 0.05 were considered to be statistically significant.
Results
Platelet count and the clinicopathological variables
The general characteristics of the patients are shown in Table 2. Of the 636 CRC patients analyzed, 398 (62.6%) were male and 238 (37.4%) female. The age of the patients ranged from 26 to 94 years (mean, 65.9 ± 10.8 years), and the mean platelet count in the study population was 27.0 ± 8.6 × 10,000/μl. Thrombocytosis, defined as platelet count > 370,000/μl, was observed in 77 patients (12.1%). Table 3 represents the results of univariate analysis of the association between preoperative platelet count and clinicopathological variables. The variables associated with higher platelet counts were: female gender (P < 0.001), age under 65 years old (P < 0.001), large tumor size (P < 0.001), venous involvement (P = 0.045), deep tumor invasion (P < 0.001), regional lymph node metastasis (P = 0.006), and distant metastasis (P = 0.003). Table 4 presents the results of univariate analysis of the association between preoperative thrombocytosis and clinicopathological variables. The variables associated with thrombocytosis were: age under 65 years old (P = 0.015), large tumor size (P < 0.001), venous involvement (P = 0.035), deep tumor invasion (P < 0.001).
The positive correlation between the platelet count and maximum tumor diameter (Spearman correlation = 0.307; P < 0.001) was observed, as shown in the scatter diagram in Fig. 1. Also, the positive correlation between the platelet count and the progression of T-factor (P < 0.001) was found, as shown in the boxplot in Fig. 2. The results of the multinomial logistic regression analysis to identify independent variables that correlate with the platelet count are shown in Table 5. Tumor size (Odds ratio 3.79; P = 0.006) and depth of tumor invasion (Odds ratio 2.58; P = 0.019) were independently correlated with thrombocytosis, and regional lymph node involvement or distant metastasis were not.
Relation between platelet count and the depth of tumor invasion. The boxplot shows that platelet count also positively correlated with the depth of tumor (P < 0.001). Horizontal lines within boxes represent median values, and boxes denote values between the 25th and 75th percentiles. Horizontal lines without boxes indicate the 0th and 100th percentile. The P values were determined using Kurskal-Wallis test
Prognostic significance of platelet count
We then assessed the prognostic impact of the high preoperative platelet count. The median follow-up period of the study population was 49.1 months. Seventy-one patients (11.2%) died of cancer, and a further 18 patients (2.8%) died of an intercurrent disease.
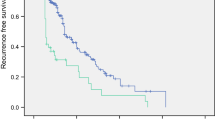
Figure 3 shows the Kaplan–Meier survival curves of patients with or without thrombocytosis. Both the CSS and the DFS of those patients with thrombocytosis were markedly shorter compared to those without thrombocytosis (P < 0.001 and P < 0.001, respectively). In those patients with thrombocytosis, the 5-year CSS rate was 62.9% (95% confidence interval [CI], 50.1–74.2), whereas patients without thrombocytosis had a longer 5-year CSS rate, 89.9% (95% CI 86.5–92.5). In those patients with thrombocytosis, the 5-year DFS rate was 41.7% (95% CI 30.8–53.6), whereas patients without thrombocytosis had a longer 5-year DFS rate, 79.6% (95% CI 75.8–82.9).
Kaplan-Meier survival curves of 636 patients, according to the preoperative platelet counts. Patients were allocated into the high or normal platelet groups according to the preoperative platelet counts. Platelet count between 140,000–370,000/μl was considered normal and that >370,000/μl, high platelet. a Cancer-specific survival (P < 0.001) and b disease-free survival (P < 0.001). The P values were determined using the log-rank test
Next, we evaluated whether the preoperative thrombocytosis could be a predictive factor of the prognosis of patients with each tumor stage (Fig. 4). In stage III, the group of patients with thrombocytosis, 34/212 patients (16.0%), have significantly shorter CSS (P < 0.001) and DFS (P < 0.001) than the group of patients without thrombocytosis. In stage I and IV, CSS and DFS of those patients with thrombocytosis were not significantly different in comparison to those patients without thrombocytosis. In stage II, notably, the group of patients with thrombocytosis, 27/194 patients (13.9%), had significantly shorter DFS than the group of patients without thrombocytosis (P = 0.001), but significance could not be achieved for CSS. In the univariate DFS analysis of these patients with stage II CRC, only thrombocytosis (P = 0.001) was the independent risk factor among the clinicopathological variables evaluated (Table 6).
Kaplan-Meier survival curves of each stage colorectal cancer patients, according to the platelet counts. Patients were allocated into the high or normal platelet groups according to the preoperative platelet counts. Platelet count between 140,000–370,000/μl was considered normal and that >370,000/μl, high platelet. Cancer specific survival and disease-free survival of each stage were shown. The P values were determined using the log-rank test
To investigate the independent prognostic factors in those patients with CRC, Cox’s proportional hazard model was applied. In the multivariate Cox hazard model of cancer-specific survival, depth of tumor invasion (Hazard ratio 3.81, 95% CI 1.73–10.1; P < 0.001), regional lymph node metastasis (Hazard ratio 1.85, 95% CI 1.13–3.08; P = 0.014), and platelet count (Hazard ratio 3.04, 95% CI 1.82–4.96; P < 0.001) were the independent risk factors of poor survival (Table 7). In the multivariate Cox hazard model of DFS, depth of tumor invasion (Hazard ratio 3.22, 95% CI 1.90–5.85; P < 0.001), regional lymph node metastasis (Hazard ratio 1.86, 95% CI 1.31–2.66; P < 0.001), distant metastasis (Hazard ratio 4.20, 95% CI 2.83–6.15; P < 0.001), and platelet count (Hazard ratio 2.54, 95% CI 1.75–3.60; P < 0.001) were independent risk factors for DFS (Table 7).
As the criteria of thrombocytosis vary among the institutions, CSS was analyzed with three different criteria: (1) >370,000 (the criteria in our institution; Fig. 3), (2) >400,000, and (3) >450,000 (Fig. 5). The number of cases with thrombocytosis decreased, according to the criteria applied, from 77 in (1), to 47 in (2), and 19 in (3). In determining the CSS of those patients with thrombocytosis using other criteria, both (2) and (3) were markedly shorter than in those patients without thrombocytosis (P < 0.001). In addition, to evaluate the applicability of the criteria used in our institution, the 5-year CSS was analyzed in the following groups of patients: (1) platelet count < 370,000 (n = 559), (2) 370,000 ≤ platelet count < 400,000 (n = 30), (3) 400,000 ≤ platelet count < 450,000 (n = 28), and (4) platelet count ≥ 450,000 (n = 19). As shown in Table 8, the significant difference in the CSS was found only between group (1) and group (2) (P < 0.001), but not between groups (2) and (3) (P = 0.836) or groups (3) and (4) (P = 0.418).
Kaplan-Meier survival curves of 636 patients, according to the other criteria of preoperative thrombocytosis. a Platelet count between 140,000–400,000/μl was considered normal and that >400,000/μl, high platelet. Cancer-specific survival was shown (P < 0.001). b Platelet count between 140,000–450,000/μl was considered normal and that >450,000/μl, high platelet. Cancer-specific survival was shown (P < 0.001). The P values were determined using the log-rank test
Discussion
We examined the preoperative platelet count as a possible prognostic factor in 636 patients with CRC. In the univariate analysis, platelet count was associated with disease progression, higher values being observed in cases with deeper tumor invasion, presence of regional lymph node metastasis, and presence of distant metastasis. In the multivariate analysis, high platelet count correlated with tumor size and depth of invasion. In addition, the progression of T-factor, especially T3-T4, was strongly correlated with the platelet count (Fig. 2). From these results, we can speculate that platelet count increases along with local tumor progression, and especially that serosal invasion of cancer, is markedly correlated with increased circulating platelets.
The cut-off level of preoperative platelet count of patients with colorectal cancer was defined from 310,000/μl to 450,000/μl in previous reports [13, 15–17]. Although some reports defined thrombocytosis as a platelet count higher than 400,000/μl or 450,000/μl [15–17], we defined it as a platelet count higher than 370,000/μl according to the normal range of platelet count of our institution. From our results using three different criteria, the number of cases with thrombocytosis decreased according to the criterion applied, but independent of the criterion applied, thrombocytosis was found to be a significant predictor of poor prognosis in patients with CRC after surgery. In addition, the significant difference in cancer-specific survival was found only between group (1) (platelet count < 370,000) and group (2) (370,000 ≤ platelet count < 400,000), but not between groups (2) and (3) (400,000 ≤ platelet count < 450,000) or groups (3) and (4) (platelet count ≥ 450,000). Therefore, we concluded that the criterion applied in our institution is appropriate for the determination of thrombocytosis, and for the detection of patients at higher risk of poor prognosis of CRC.
The presence of thrombocytosis in some solid tumors has been associated with poor prognosis [10–12, 21, 22]. Also in CRC, preoperative thrombocytosis has been shown to correlate with poor prognosis [13, 16, 17]. In addition, Qiu et al. demonstrated the cumulative effect of anemia and thrombocytosis as a prognostic marker in patients with CRC. In cancer environment, anemia and thrombocytosis might be closely associated [16]. On the other hand, Nyasavajjala et al. [15] have denied the value of preoperative thrombocytosis as a prognostic indicator of survival in CRC, regardless of pathological stage, and the positive association between thrombocytosis and poor survival found in previous studies was attributed to the small number of cases compared to their large-scale series, in which 627 patients were investigated. Therefore, the association between preoperative thrombocytosis and poor prognosis in patients with CRC remained debatable.
In our series, we enrolled 636 patients, a number close to the earlier study by Nyasavajjala et al. [15], but contradictory to their finding, we clearly demonstrated that preoperative thrombocytosis is a potential predictive factor of poor survival and high recurrence rate in patients with CRC who undergo curative resection. The basic differences between the previous report [15] and ours are the definition of thrombocytosis and the length of the follow-up period. We defined thrombocytosis as platelet count >370,000/μl, according to the normal range of our institution, whereas they defined it as platelet count >450,000/μl. As a result, the incidence of thrombocytosis was higher in our series than in theirs (12.1% [77/636] versus 8.1% [51/627], respectively). In addition, the median period of follow-up was longer in our series than in theirs (49.1 versus 22 months, respectively). In our series, 35 patients (49.3%, 35/71) died of cancer during the postoperative period, between the 22nd and the 49th months. Therefore, we speculated that both the follow-up period and the definition of thrombocytosis, but in particular, the follow-up period, influenced the results obtained, and different results in prognosis were obtained. Therefore, here we identified preoperative thrombocytosis as a potential and useful prognostic marker of CRC. Because platelet count is a routinely available test, without the need of an additional cost to the routine preoperative testing, it would be easily applied in clinical setting.
In our study, the most significant result was that preoperative thrombocytosis was an independent prognostic factor for both CSS and DFS in the multivariate analysis. Thrombocytosis was found to be a predictor of poor prognosis, as well as a marker to predict patients at high risk of recurrence. The detailed analysis of the prognostic usefulness of thrombocytosis was evaluated in each tumor stage. Thrombocytosis was a prognostic factor of CSS in patients at stage III, but it lost significance in patients at stage I, II, and IV. Also, thrombocytosis was found to be a prognostic factor for DFS in patients at stages II and III, but significance was lost in patients at stage I and IV. Although a tendency toward lower survival in the group of patients with thrombocytosis was also observed in stage I and IV cases, statistical significance could not be achieved, and this was attributed to the small number of samples. Also, significance was not achieved for CSS in stage II patients, which may have been affected by the period of postoperative follow-up (median: 49 months). A longer follow-up period may be necessary to confirm the usefulness of thrombocytosis as a prognostic factor of CSS in this group of patients. The most evident prognostic significance of thrombocytosis for both CSS and DFS could be observed in stage III cases.
Based on the predictive factors of prognosis, it is possible to improve survival by the addition of an adjuvant treatment, such as chemotherapy. Adjuvant chemotherapy is the standard of care for stage III colon cancer, but its role in stage II disease remains controversial [23]. At present the NCCN guideline does not recommend routine administration of adjuvant chemotherapy for all stage II CRC patients receiving complete resection [24]. However, its administration should be considered for patients with high-risk factors, including T4 tumor, lymphovascular invasion, and poorly differentiated histology [25]. In our results, the cumulative 5-year DFS of stage II patients with preoperative thrombocytosis was 55%, similar to that of stage III patients in previous reports [3], but shorter than that of stage III patients in our series (64.3%). In contrast, stage II patients without thrombocytosis had a 5-year DFS rate of 83.9%. Accordingly, from our present results, we believe that preoperative thrombocytosis should be considered a useful indicator to predict the postoperative prognosis and should be taken into consideration in determining those cases that will benefit from adjuvant chemotherapy.
Although the pathophysiological mechanisms of cancer-thrombocytosis association have not been fully clarified, several hypotheses have been suggested. Cancer cells, by secreting thrombopoietic factors such as interleukin-6, can stimulate the bone marrow to produce megakaryocytes, leading to thrombocytosis [26, 27]. Similarly, cancer cells secrete vascular endothelial growth factor (VEGF), which activates the coagulation cascade, leading platelets to be activated, increasing platelet adhesion to endothelial cells of tumor vessels [28]. The peripheral blood platelets can also be activated when they circulate through the tumor vasculature. Activated platelets produce various stimulators of angiogenesis, such as platelet-derived growth factor or VEGF, and the resulting angiogenesis accelerates tumor growth [28, 29]. Activated platelets also release thrombopoietin, which stimulates bone marrow to generate new platelets [30]. Thus, platelets and cancer cells cooperate, forming a positive feedback cascade in which each stimulates the other, potentiating the effect.
Moreover, cancer cells express various membrane receptors that can bind directly to platelets and mediate cancer cell-platelet binding and activate platelets [31]. When activated, platelets release microparticles, which increase the invasive potential of cancer cells by increasing the abilities of adhesion, proliferation, and chemotaxis [32]. These observations suggested that platelet-tumor cell emboli contribute to the formation of distant metastatic foci by tumor cells. Additionally, it has been proposed that platelets form a sheath around the tumor cells, interfering with the recognition of tumor cells by natural killer cells [33]. These observations corroborate our findings on the pivotal role of platelets in the prognosis of colorectal cancer, which is similar to that reported in other malignancies.
In conclusion, in the present study we clearly identified preoperative thrombocytosis not only as an independent indicator for the prediction of poor cancer-specific survival in patients with colorectal cancer but also as an independent predictor of poor disease-free survival in patients with stage II colorectal cancer. In the preoperative period, most surgeons carefully check for anemia, leukocytopenia, or thrombocytopenia in the hemogram. In addition to these findings, there is a need to pay special attention to preoperative thrombocytosis, which may significantly affect prognosis. Therefore, we recommend the careful check of the hemogram preoperatively for the presence of thrombocytosis, which may be used as a useful clinical marker informing the decision of whether to recommend additional adjuvant therapy. It is a simple and costless test routinely performed perioperatively, and it should be more appropriately employed.
References
Xu R, Zhou B, Fung PC et al (2006) Recent advances in the treatment of colon cancer. Histol Histopathol 21:867–872
McArdle CS, Hole DJ (2002) Outcome following surgery for colorectal cancer: analysis by hospital after adjustment for case-mix and deprivation. Br J Cancer 86:331–335
O’Connell JB, Maggard MA, Ko CY (2004) Colon cancer survival rates with the new American Joint Committee on Cancer Sixth Edition staging. J Natl Cancer Inst 96:1420–1425
Graziano F, Cascinu S (2003) Prognostic molecular markers for planning adjuvant chemotherapy trials in Dukes B colorectal cancer patients: how much evidence is enough? Ann Oncol 14:1026–1038
McMillan DC, Crozier JE, Canna K et al (2007) Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis 22:881–886
Jones JM, McGonigle NC, McAnespie M et al (2006) Plasma fibrinogen and serum C-reactive protein are associated with non-small cell lung cancer. Lung Cancer 53:97–101
Lee JH, Ryu KW, Kim S et al (2004) Preoperative plasma fibrinogen levels in gastric cancer patients correlate with extent of tumor. Hepatogastroenterology 51:1860–1863
Oya M, Akiyama Y, Okuyama T et al (2001) High preoperative plasma D-dimer level is associated with advanced tumor stage and short survival after curative resection in patients with colorectal cancer. Jpn J Clin Oncol 31:388–394
Levitan N, Dowlati A, Remick SC et al (1999) Rates of initial and recurrent thromboembolic disease among patients with malignancy versus those without malignancy, Risk analysis using Medicare claims data. Medicine (Baltimore) 78:285–291
Pedersen LM, Milman N (1996) Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur Respir J 9:1826–1830
Scholz HS, Petru E, Gucer F et al (2000) Preoperative thrombocytosis is an independent prognostic factor in stage III and IV endometrial cancer. Anticancer Res 20:3983–3985
Shimada H, Oohira G, Okazumi S et al (2004) Thrombocytosis associated with poor prognosis in patients with esophageal carcinoma. J Am Coll Surg 198:737–741
Monreal M, Fernandez-Llamazares J, Pinol M et al (1998) Platelet count and survival in patients with colorectal cancer—a preliminary study. Thromb Haemost 79:916–918
Taucher S, Salat A, Gnant M et al (2003) Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb Haemost 89:1098–1106
Nyasavajjala SM, Runau F, Datta S et al (2010) Is there a role for preoperative thrombocytosis in the management of colorectal cancer? Int J Surg 8:436–438
Qiu MZ, Yuan ZY, Luo HY et al (2010) Impact of pretreatment hematologic profile on survival of colorectal cancer patients. Tumour Biol 31:255–260
Kandemir EG, Mayadagli A, Karagoz B et al (2005) Prognostic significance of thrombocytosis in node-negative colon cancer. J Int Med Res 33:228–235
Amitrano L, Guardascione MA, Brancaccio V et al (2002) Coagulation disorders in liver disease. Semin Liver Dis 22:83–96
Klinger MH, Jelkmann W (2002) Role of blood platelets in infection and inflammation. J Interferon Cytokine Res 22:913–922
American Joint Cancer Commission (1997) AJCC cancer staging manual. In American Joint Committee on Cancer, 5th edn. Lippincott-Raven, Philadelphia
Erdemir F, Kilciler M, Bedir S et al (2007) Clinical significance of platelet count in patients with renal cell carcinoma. Urol Int 79:111–116
Ikeda M, Furukawa H, Imamura H et al (2002) Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol 9:287–291
Ding PR, An X, Zhang RX et al (2010) Elevated preoperative neutrophil to lymphocyte ratio predicts risk of recurrence following curative resection for stage IIA colon cancer. Int J Colorectal Dis 25:1427–1433
Benson AB, Choti MA, Cohen AM et al (2000) NCCN practice guidelines for colorectal cancer. Oncology (Williston Park) 14:203–212
Benson AB, Schrag D, Somerfield MR et al (2004) American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 22:3408–3419
Blay JY, Favrot M, Rossi JF et al (1993) Role of interleukin-6 in paraneoplastic thrombocytosis. Blood 82:2261–2262
Verheul HM, Pinedo HM (2003) The importance of platelet counts and their contents in cancer. Clin Cancer Res 9:3219–3221
Verheul HM, Hoekman K, Lupu F et al (2000) Platelet and coagulation activation with vascular endothelial growth factor generation in soft tissue sarcomas. Clin Cancer Res 6:166–171
Banks RE, Forbes MA, Kinsey SE et al (1998) Release of the angiogenic cytokine vascular endothelial growth factor (VEGF) from platelets: significance for VEGF measurements and cancer biology. Br J Cancer 77:956–964
Folman CC, Linthorst GE, van Mourik J et al (2000) Platelets release thrombopoietin (Tpo) upon activation: another regulatory loop in thrombocytopoiesis? Thromb Haemost 83:923–930
Jain S, Harris J, Ware J (2010) Platelets: linking hemostasis and cancer. Arterioscler Thromb Vasc Biol 30:2362–2367
Janowska-Wieczorek A, Wysoczynski M, Kijowski J et al (2005) Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer 113:752–760
Nieswandt B, Hafner M, Echtenacher B et al (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59:1295–1300
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sasaki, K., Kawai, K., Tsuno, N.H. et al. Impact of Preoperative Thrombocytosis on the Survival of Patients with Primary Colorectal Cancer. World J Surg 36, 192–200 (2012). https://doi.org/10.1007/s00268-011-1329-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-011-1329-7