Abstract
Adrenal cysts are very rare lesions, especially with parasitic origin. But with the wider application of ultrasonography (US) and computed tomography (CT) more adrenal cysts are detected incidentally. To gain more insight into this entity, the records of nine patients with hydatid cysts of adrenal gland seen at our department from January 1980 till January 2002 are reviewed. There were four men and five women, and their ages ranged from 15 to 80 years (median: 41 years). All of the patients had unilateral cysts. Seven cysts were located on the right and two on the left side. Five of the cysts were primary and four were secondary. In three patients the cysts were found incidentally. The most common presenting symptom was pain, which was present in six patients. An indirect hemagglutination (IHA) test was positive in six cases. In all patients, US and CT successfully imaged all cysts, but the definitive diagnosis was made by macroscopic and microscopic examination of the cyst’s content. The patients were treated surgically. In all patients adrenal glands with the cystic masses were removed. The median follow-up period was 16 months (range: 6–64 months). No evidence of recurrence was found in any patient. It should not be forgotten that cystic masses of the upper abdomen might also originate from the adrenal gland. The etiology and nature of the cyst should be well researched, and appropriate treatment should be given as soon as possible. Surgical excision of the gland, including the cyst is the treatment of choice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydatid disease (echinococcosis) is a parasitic infection caused by several species of the cestode Echinococcus. The most common form is E. granulosus; much less common is E. multilocularis. Hydatid cysts occur throughout the world, but are endemic in pastoral and farming regions of the Mediterranean, Eastern Eurpoe, the Middle East, South America, Australia, and South Africa [1, 2, 3, 4, 5]. Infestation with Echinococcus is quite common in Turkey, especially in the Eastern region [6, 7]. The adult worm is present in the small intestine of canine animals. The echinococcal eggs excreted in the feces of canines are ingested by intermediate hosts like sheep, cattle, or human. The ingested eggs hatch in the intestine of the intermediate host and the oncospheres penetrate the mucosa into the blood and lymphatic circulation from where they are transported to liver, lungs, and other organs. The larvae produce cysts, which grow until discovered incidentally or until they cause the symptoms of a mass lesion [4, 5, 8].
Of hydatid cysts, 60% to 70% are found in the liver, 5% to 15% in the lungs, and only 0.5% in the adrenals [5]. Hydatid cysts in organs other than the liver or the lungs are usually part of generalized echinococcosis, and only rarely are they primary cysts [8].
Here we present nine cases of parasitic cysts of adrenal gland due to E. granulosus, and a review the literature.
Patients and Methods
The records of nine patients with hydatid cysts of adrenal gland seen at the Department of General Surgery, Atatürk University, School of Medicine, Erzurum, Turkey, from January 1980 until January 2002 were reviewed (Table 1). There were four men and five women, and their ages ranged from 15 to 80 years (median: 41 years). All of the patients had unilateral cysts. Seven cysts were located on the right and two on the left side. Five of the cysts were primary and four were secondary. One patient, an 80-year-old man, had undergone operation for pulmonary and hepatic hydatid disease; a 48-year-old woman and an 18-year-old woman had also been operated on for hepatic hydatid disease before. A 41-year-old man had coexistent hepatic and peritoneal hydatid cysts. In three patients the cysts were found incidentally; in two others the cysts were found during sonography for flank pain. Four patients had a history of a bloating and moderate pain in the right upper quadrant, often radiating to the right lumbar region.
Laboratory tests for all patients included urinalysis, complete blood count, eosinophil count, serum biochemistry, and indirect hemagglutinin (IHA) test for E. granulosus. Plain abdominal X-ray films, ultrasound (US), and computed tomography (CT) were obtained in every case. All of the patients underwent operation, and the diagnosis of hydatid cyst was histologically proved. When peritoneal spillage was suspected, and when patients had a coexistent peritoneal cyst, antihelminthic drugs were administered.
Results
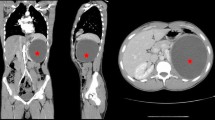
The most common presenting symptom was pain, and it was present in six patients. The IHA test was positive in six cases. Routine laboratory tests were normal in all patients. In the plain abdominal X-ray films, calcifications were seen at the level of 12th thoracic vertebra and the 1st lumbar vertebra in all patients. In every case, US and CT scans successfully imaged all cysts (CT scan of the one patient with a hydatid adrenal cyst is shown in Figure 1), but the definitive diagnosis was made by a macroscopic and microscopic examination of the cyst’s content. In the patients with hydatid adrenal cysts, a macroscopic examination revealed a germinate membrane and daughter cysts. A microscopic examination revealed scolices in the cyst fluid. All of the patients underwent operation via a transabdominal approach. In all patients, adrenal glands with the cystic masses were removed. In one patient who had coexistent hepatic and peritoneal cysts, the peritoneal cysts were totally removed, and partial cystectomy and omentoplasty were performed for the cyst located in the liver in the same operation. Before the dissection of these nine cysts, the cyst fluid was aspirated and hypertonic saline solution was injected into the sac. The area was irrigated with the same solution after the excision. Broad-spectrum antibiotics were started 1 day before the operation until the patient was discharged home. In two patients suspected of having peritoneal spillage and in one patient who had coexistent peritoneal cyst, antihelminthic drugs were administered (mebendazole, 50 mg/kg body weight or albendazole, 10 mg/kg body weight) perioperatively for 1 month. Histological examination confirmed the preoperative diagnosis in all cases. The patients’ postoperative course was uneventful. There were no deaths or major instances of morbidity in this series. The median hospital stay was 14 days (range: 8–34 days).
All patients underwent repeat US and IHA tests 4 weeks after operation,and the patients were then followed by 3-month intervals. The median follow up period was 16 months (range: 6–64 months). No evidence of recurrence was found, and the IHA titer returned to normal in all cases.
Discussion
Adrenal cysts are rare, with an incidence at autopsy of 0.06%–0.18% [5, 8, 9, 10]. The first case of adrenal cyst was reported in 1670 by Greiselius (see de Bree et al. [10]). Hydatid disease accounts for only 6%–7% of all adrenal cysts [5, 8, 9, 10]. Other types of adrenal cysts include the following: (1) endothelial cysts (45%), including lymphangiomatous (42%) and angiomatous cysts (3%); (2) pseudocysts (39%), due to infarction or hemorrhage in the adrenal gland (32%), or to cystic degeneration of benign or malignant adrenal neoplasms (7%); (3) epithelial cysts (9%), including true glandular retention cysts, embryonal cysts, and cystic adenomas [5, 8, 10]. But in endemic regions hydatid cysts constitute most of the adrenal cysts requiring operation. In our series 15 patients had the diagnosis of adrenal cyst, and nine of them were histologically proved to be hydatid cysts.
Adrenal cysts may occur at any age, but are most commonly seen in the fifth and sixth decades, and they occur more frequently in women than in men (ratio: 2:1). They are usually (92%) unilateral and show no special predilection for either side [10, 11].
Parasitic cysts involving the adrenals are usually secondary and are part of generalized echinococcosis. Rarely, the echinococcal infection is limited to an adrenal gland [8]. Of our patients, five had a primary hydatid cyst of the adrenal gland and four had coexistent hepatic, pulmonary, and peritoneal cysts. To date, only case reports about primary hydatid cyst of the adrenal gland have been reported [5, 8, 10]. In presenting one case and reviewing the literature, el İdrissi Dafali et al. [12] found a total of only eleven such cases reported. So, our series is the largest series, with five cases of primary hydatid cyst of the adrenal gland.
There are a wide variety of symptoms associated with an adrenal cyst, although often it will be asymptomatic or will cause vague, nonspecific symptoms. The most prominent clinical features consist of dull pain in the renal area, gastrointestinal symptoms (belching, bloating, fullness, nausea, vomiting, flatulence, constipation, and anorexia), and a palpable mass [5]. Rarely hydatid cyst causes hypertension which is described by the Goldblatt phenomenon [11]. Among the cases presented here, the most common presenting symptom was pain, which was present in six patients.
Because most adrenal cysts are asymptomatic, they are usually found as incidental findings on imaging studies or incidentally during surgery performed for other abdominal pathologies [11]. In our series three cysts were found incidentally at US.
Acute abdominal pain or a tender mass may accompany intracystic hemorrhage, rupture, or infection. Anaphylactic shock may be caused by rupture of a hydatid cyst [8]. Adrenal cysts may be fatal if they hemorrhage and are not rapidly diagnosed. It is thought that hemorrhage occurs secondary to trauma or some toxic or infectious process [11, 13]. None of our patients had a complication.
To identify a hydatid cyst in the adrenal gland, US, CT, and magnetic resonance imaging (MRI) can demonstrate cystic lesions and reveal the presence of daughter cysts. The diagnostic sensitivity of US in abdominal echinococcosis ranges from 93% to 98% [14]. In our series all cysts were demonstrated by US. In our opinion US must be the first choice in imaging abdominal echinococcosis, because it is easy to perform and is inexpensive. The sensitivity of CT scan in abdominal echinococcosis is 97%. On CT scans, concentric areas of septation and calcification indicate that a cyst is of parasitic origin [15]. However, when the lesion is large and located in the right upper quadrant, it can be difficult to define its origin accurately. Such lesions are often incorrectly thought to be intrahepatic. At sonography the reflection produced by the retroperitoneal fat is displaced posteriorly by hepatic and subhepatic masses, and anteriorly by renal and adrenal masses. Additionally, wedging of this reflecting surface by a mass situated near the upper pole of the kidney suggests an adrenal origin [8]. In our series all cysts were demonstrated by CT.
Laboratory tests such as eosinophilia, Casoni skin test, and IHA are not diagnostic [7, 8]. There are many new sensitive and specific serological tests available, such as complement fixation, enzyme-linked immunosorbent assay, ARC 5 precipitation, and specific hydatid IgE tests [4, 5, 8, 9]. We used the IHA test for our patients, and it was positive in six cases. Schoretsanitis et al. [8] and Bastounis et al. [5] reported negative serologic tests in their cases. The presence of calcification on plain abdominal films is strongly suggestive of a hydatid cyst or pseudocyst [4, 5, 8, 9].
Indications for surgery on cystic lesions of the adrenal gland include large and complicated cysts, as well as parasitic, functioning, and malignant cysts [10]. Percutaneous drainage should be avoided, especially in areas endemic for hydatid disease. The surgical approach through a laparotomy incision has the advantage of allowing the surgeon to explore the peritoneal cavity [5]. We used subcostal, paramedian or median incisions for the removal of adrenal cysts. Recently, transabdominal laparoscopic and endoscopic retroperitoneal adrenalectomies have been performed with less morbidity and good results [16]. Endoscopic removal of an adrenal hydatid cyst has been reported by Defechereux et al. [17].
Prior to puncture, injection of hypertonic saline solution into the cyst can render scolices and daughter cysts inactive [8]. In our patients, before the dissection of cystic lesions cyst fluid was aspirated and hypertonic saline solution was injected into the sac.
Most authors recommend adrenalectomy for the treatment of hydatid disease [5, 7, 8, 18, 19]. However, el İdrissi Dafali et al. [12] performed simple cystectomy for the treatment of a patient with a hydatid cyst of the adrenal gland. They stated that among the various suggested surgical methods, simple resection of the cyst is the best treatment that allows preservation of the gland. Balık et al. [14] reported their experience of four cases of hydatid cyst of the adrenal gland. They performed adrenalectomy because of the destruction of the organs by a large cyst. They recommended that surgery with either partial or total excision of the cyst with or without preservation of the adrenal gland is the treatment of choice.
As a result, it should not be forgotten that cystic masses of the upper abdomen might also originate from the adrenal gland. Etiology and nature of the cyst should be well researched, and appropriate treatment should be carried out as soon as possible. Surgical excision of the gland including the cyst is the treatment of choice.
Résumé.
Les kystes de la surrénale sont très rares, surtout les kystes d’origine parasitaire. Cependant, avec l’usage plus répandu de l’échographie (EG) et de la tomodensitométrie (TDM), on découvre davantage de kystes de façon fortuite. Afin d’analyser cette entité plus en détails, nous avons revu les dossiers de neuf patients porteurs de kyste hydatique de la surrénale, vus dans notre département entre jan 1980 et jan 2002. Il y avait quatre hommes et cinq femmes, leurs âges allant de 15 à 80 avec une médiane d’âge de 41 ans. Tous les patients avaient un kyste unilatéral. Sept kystes étaient localisés à droite, deux à gauche. Cinq des kystes étaient primitifs, quatre étaient secondaires. Chez trois patients, les kystes étaient de découverte fortuites. Les symptômes les plus fréquemment rencontrés étaient la douleur (6 patients). Le test indirect d’hémagglutination (IHA) était positif chez les six patients. Chez tous les patients, l’EG et la TDM ont visualisé les kystes, mais le diagnostic définitif a été le fait de l’examen macroscopique et microscopique du contenu du kyste. Toutes les masses kystiques ont été enlevées chirurgicalement. La médiane de suivi a été de 16 mois (extrêmes 6–64 mois). Aucune récidive n’a été observée chez nos patients. En conclusion, il ne faut pas oublier que toute masse de la partie supérieure de l’abdomen peut être en rapport avec la surrénale. L’étiologie et la nature de la masse doivent être recherchées afin de mettre en route un traitement adapté le plus vite possible. L’excision chirurgicale de la surrénale comprenant le kyste est le traitement de choix.
Resumen.
Las lesiones quísticas de las cápsulas suprarrenales son poco frecuentes y muy raras las de origen parasitario. Con la generalización de la ecografía y tomografía abdominales cada vez se detectan más quistes en las cápsulas suprarrenales de manera accidental (incidentalomas). Revisamos 9 pacientes con quistes hidatídicos de cápsula suprarrenal intervenidos en nuestro Servicio entre 1980 y 2002. 4 eran varones y 5 hembras con edades comprendidas entre los 15 y 80 años (media 41 años). En todos los casos el quiste era unilateral; en siete se localizaba en la cápsula suprarrenal derecha y en dos, en la izquierda. En 5 casos se trataba de quistes primarios; en 4 pacientes, la hidatidosis era secundaria; en 3 casos el hallazgo fue accidental. El síntoma más frecuente fue el dolor (en 6 casos). La hemoaglutinación indirecta (IHA) fue positiva en 6 casos; en todos, la imagen quística fue detectada por ecografia o tomografía, pero el diagnóstico definitvo se estableció tras el estudio macro y microscópico del contenido del quiste. El tratamiento fue quirúrgico, extirpándose en todos los casos el quiste “ in toto “ junto con la cápsula suprarrenal. El periodo de seguimiento medio fue de 16 meses (rango 6–64). No se observaron recidivas. Siempre debe tenerse en cuenta que tumores quísticos del abdomen superior pueden tener su origen en las cápsulas suprarrenales. Su etiología ha de establecerse junto a un tratamiento precoz constituyendo, para los autores, el de elección la extirpación de la glándula suprarrenal junto con el quiste.
References
AA Balik M Bas¸oğlu F Çelebi et al. (1999) ArticleTitleSurgical treatment of hydatid disease of the liver: review of 304 cases Arch. Surg. 134 166–169 Occurrence Handle10.1001/archsurg.134.2.166 Occurrence Handle1:STN:280:DyaK1M7kvVehtg%3D%3D Occurrence Handle10025457
SA Barnes KD Lillemoe (1997) Liver abscess and hydatid cyst disease MJ Zinner SI Schwartz H Ellis (Eds) Maingot’s Abdominal Operations Appleton-Lange Norwalk, CT 1513–1547
WC Meyers (1997) Neoplasms of the liver DC Sabiston (Eds) Textbook of Surgery W.B. Saunders Company Philadelphia 825–847
Mö Tan L Emir C Germiyanoğlu et al. (2000) ArticleTitleIsolated renal and retroperitoneal hydatid cysts Int. Urol. Nephrol. 32 41–46 Occurrence Handle10.1023/A:1007191730454 Occurrence Handle1:STN:280:DC%2BD3crgs1Wjug%3D%3D Occurrence Handle11057771
E Bastounis E Pikoulis A Leppanieri et al. (1996) ArticleTitleHydatid disease: a rare cause of adrenal cyst Am. Surg. 62 383–385 Occurrence Handle1:STN:280:BymC1Mngt10%3D Occurrence Handle8615568
MA Çiftçioğlu Mİ Yildirgan MN Akçay et al. (1997) ArticleTitleFine needle aspiration biopsy in hepatic echinococcus multilocularis Acta Cytol. 41 649–652 Occurrence Handle9167677
İ özbey Y Aksoy O Biçgi et al. (2001) ArticleTitleHydatid disease of the urinary tract: review of the management of 9 cases Int. Urol. Nephrol. 33 329–334 Occurrence Handle10.1023/A:1015209106436 Occurrence Handle12092649
G Schoretsanitis E Bee Particlede J Melissas et al. (1998) ArticleTitlePrimary hydatid cyst of the adrenal gland Scand. J. Urol. Nephrol. 32 51–53 Occurrence Handle10.1080/003655998750014693 Occurrence Handle1:STN:280:DyaK1c3it1elsg%3D%3D Occurrence Handle9561575
P Otal G Escourrou C Mazerolles et al. (1999) ArticleTitleImaging features of uncommon adrenal masses with histopathologic correlation Radiographics 19 569–581 Occurrence Handle1:STN:280:DyaK1M3mslagsw%3D%3D Occurrence Handle10336189
E Bree Particlede G Schoretsanitis J Melissas et al. (1998) ArticleTitleCysts of the adrenal gland: diagnosis and management Int. Urol. Nephrol. 30 369–376 Occurrence Handle9821036
Cö Yeniyol S Minareci AR Ayder (2000) ArticleTitlePrimary cyst hydatid of adrenal: a case report Int. Urol. Nephrol. 32 227–229 Occurrence Handle10.1023/A:1007106328997 Occurrence Handle1:STN:280:DC%2BD3MzotFGhuw%3D%3D Occurrence Handle11229636
A el İdrissi Dafali Z Dahami NO Zerouali (2002) ArticleTitleHydatid cyst of the adrenal gland Ann. Urol. 36 99–103 Occurrence Handle10.1016/S0003-4401(01)00081-X
MD Cunningham (1993) ArticleTitleHemorrhagic adrenal cyst J. Am. Osteopath. Assoc. 93 619–622 Occurrence Handle1:STN:280:ByyA3c%2FmvFA%3D Occurrence Handle8314725
AA Balik F Çelebi M Bas¸oğlu et al. (2001) ArticleTitleIntra-abdominal extrahepatic echinococcosis Surg. Today 31 881–884 Occurrence Handle10.1007/s005950170027 Occurrence Handle1:STN:280:DC%2BD38%2FjtFegsA%3D%3D Occurrence Handle11759882
Wegener OH. The adrenal glands. Chapter 18 in Whole Body Computed Tomography, Boston, Blackwell Scientific Publications, 1993;403–415.
S Mercan R Seven S özarmağan et al. (1995) ArticleTitleEndoscopic retroperitoneal adrenalectomy Surgery 118 1071–1076 Occurrence Handle1:STN:280:BymD1cbgt1A%3D Occurrence Handle7491525
T Defechereux J Sauvant L Gramatica et al. (2000) ArticleTitleLaparoscopic resection of an adrenal hydatid cyst Eur. J. Surg. 166 900–902 Occurrence Handle10.1080/110241500447317 Occurrence Handle1:STN:280:DC%2BD3MzgtlKhtQ%3D%3D Occurrence Handle11097159
R Colovic V Kalezic M Ateljevic et al. (1995) ArticleTitleIsolated hydatid cyst of the adrenal gland Acta Chir. Iugosl. 42 167–169
ME Martinez NJ Mora CM Closas et al. (1992) ArticleTitleSolitary adrenal gland hydatid cyst Actas Urol. Esp. 16 333–336 Occurrence Handle1636458
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akçay, M., Akçay, G., Balik, A. et al. Hydatid Cysts of the Adrenal Gland: Review of Nine Patients. World J. Surg. 28, 97–99 (2004). https://doi.org/10.1007/s00268-003-6901-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-003-6901-3