Abstract
Introduction
Perceived age is defined as how old a person looks to external evaluators. It reflects the underlying biological age, which is a measure based on physical and physiological parameters reflecting a person’s aging process more accurately than chronological age. People with a higher biological age have shorter lives compared to those with a lower biological age with the same chronological age. Our review aims to find whether increased perceived age is a risk factor for overall mortality risk or comorbidities.
Methods
A literature search of three databases was conducted following the PRISMA guidelines for studies analyzing perceived age or isolated facial characteristics of old age and their relationship to mortality risk or comorbidity outcomes. Data on the number of patients, type and characteristics of evaluation methods, evaluator characteristics, mean chronologic age, facial characteristics studied, measured outcomes, and study results were collected.
Results
Out of 977 studies, 15 fulfilled the inclusion criteria. These studies found an increase in mortality risk of 6–51% in older-looking people compared to controls (HR 1.06–1.51, p < 0.05). In addition, perceived age and some facial characteristics of old age were also associated with cardiovascular risk and myocardial infarction, cognitive function, bone mineral density, and chronic obstructive pulmonary disease (COPD).
Conclusion
Perceived age promises to be a clinically useful predictor of overall mortality and cardiovascular, pulmonary, cognitive, and osseous comorbidities.
Level of Evidence III
This journal requires that authors assign a level of evidence to each article. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors www.springer.com/00266.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1980, Borkan and Norris [1] found that perceived age, defined as how old a person looks to external evaluators [2], could reflect the underlying biological age, a measure based on physical and physiological parameters (e.g., forced expiratory volume, basal metabolic rate). Additionally, they found that people with higher biological ages (i.e., worse physiological status) died sooner than their chronologic age-matched counterparts with lower biological ages. Since measuring biological age requires extensive patient testing and considering that facial skin is the most accessible organ to evaluate [3], it could be inferred that perceived age could be used to predict mortality. Therefore, our review aims to find whether a high perceived age is a risk factor for overall mortality and comorbidities.
Methods
Studies were identified by searching PubMed, CINAHL and Embase from inception to July 27, 2020. MeSH (Medical Subject Headings) terms for “perceived age,” “facial aging,” “mortality,” “survival,” “heart disease,” “cancer,” “lower pulmonary disease,” “stroke,” “diabetes,” “Alzheimer’s disease,” “kidney disease,” “bone status,” and “hypertension” were used. See Table 1, which demonstrates the complete inquiry input.
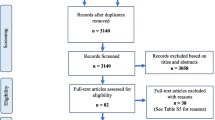
Studies were included if they (1) measured perceived age or isolated facial characteristics of old age, (2) measured mortality risk, mortality rate, or comorbidity outcomes, and (3) were in English. No particular time frame or publication status was considered. The study selection process was performed following the PRISMA Guidelines. This process, along with reasons for exclusion, is detailed in Fig. 1.
Eligibility assessment was performed by one reviewer, starting with the title of the studies and followed by abstracts and full-text evaluations. If there were doubts in selecting an article, a second author reviewed the article according to the inclusion criteria and both reviewers came to a consensus for the final decision. Data extraction was performed by one reviewer. Risk of bias of the included studies was assessed using the ROBINS-I (Risk Of Bias In Non-Randomized Studies–of Interventions) tool of the Cochrane Library for nonrandomized studies. A summary and graph were created using RevMan 5.3 (Cochrane Collaboration), which allowed for bias stratification in several domains (Figs. 2 and 3).
Results
The inquiry identified 977 studies, of which 11 fulfilled the inclusion criteria. Four more studies that fulfilled inclusion criteria were later added by searching the reference list of included studies and high impact journals in the related field for a total of 15 included studies (see Table 2). Four studies evaluated patients face to face, while 11 evaluated the patients with photographs. The included studies evaluated the association of perceived age with mortality (six studies), cardiovascular disease (four studies), chronic obstructive pulmonary disease (COPD) (three studies), cognitive function (one study), and bone mineral density (one study). All studies that included facial wrinkling or photoaging assessment along with their perceived age evaluations used either standardized evaluation criteria or photographic computerized assessments. The following paragraphs describe the most relevant information regarding each study’s outcome.
Mortality
In 1982, by assessing patients from the Baltimore Longitudinal Study of the Gerontology Research Center, Borkan et al. [4] found that the majority of older-appearing men of ages 45–75 years were more likely to die at the end of 15 years’ follow-up (p < .001). Almost 30 years later, Schnohr et al. [5], using patients from the Copenhagen City Heart Study, found that men with no gray hair had a significantly lower mortality rate than the rest (p < .05). On the other hand, there was a significant correlation (p < .01) between half and complete arcus senilis and mortality in women. Facial wrinkle severity, however, was not found to be correlated with mortality.
A few years later, Christensen et al. [6] photographed a group of twins from the Longitudinal Study of Aging Danish Twins and found that among the pairs in which a twin had died 2 years after the first evaluation, the longest surviving twin’s perceived age was considered to be approximately 1.15 years younger. Additionally, the oldest-looking twin died first in 73% of cases in the subgroup in which perceived age differed by ≥ 2 years. Subsequently, Christensen and colleagues [7] found that 7-year mortality risk increased by 8–19% per standard deviation (SD) increase in perceived age (p < .001). Consistent with these findings, Gunn et al. [2] found a 17% increase in 7-year mortality risk and a 6% increase in 12-year mortality risk per SD increase in perceived age (p < .05). Additionally, Dykiert et al. [8] found a 51% increase in 7-year mortality risk per SD increase in perceived age in women (p < .02).
Cardiovascular Disease
In 1995, Schnohr et al. [9] found that completely gray hair increased the probability of myocardial infarction (MI) in men (p < .01). In addition, facial wrinkling was associated with an increased risk of MI in men ≤ 55 years (p < .05). Gunn et al. [10] found that women with the lowest cardiovascular risk looked > 2 years younger than women with higher risk (p < .002). Among cardiovascular risk components, blood pressure was found to have a significant positive correlation with perceived age (p = .004).
In 2012, Kido et al. [11] found that younger-looking patients had a lower age-dependent increase in carotid intima-media thickness (CIMT) (p < .0054). Additionally, CIMT had a negative impact on looking young (p < .05). Miyawaki et al. [12] found that facial pigmentation was significantly and positively associated with CIMT (p < .03) and brachial-ankle pulse wave velocity (p < .02). Interestingly, obesity-related parameters were significantly associated with pigmentation (p < .0003).
Chronic Obstructive Pulmonary Disease
In 1994, Lange and Schnohr [13] found that in current and past smokers, patients with higher wrinkle scores had a significantly lower percent of forced vital capacity exhaled in the first second (FEV1/FVC) than patients with lower wrinkle scores (p < .05). The association was stronger in women than in men. The authors also concluded that the presence of a significant interaction between smoking and wrinkling implied that patients with wrinkles were more susceptible to the effects of tobacco on lung function. However, the weak association did not allow for risk stratification of airflow obstruction by facial wrinkle assessment.
In 2006, Patel et al. [14] found that independently of cumulative tobacco exposure, facial wrinkling was strongly associated with the risk of airflow obstruction (p < .05). Facial wrinkling in smokers was also associated with an increased risk of COPD (p < .02) and with the presence and extension of emphysema on computed tomography (p≤.05). Contrastingly, O’Brien et al. [15] did not find the same association but instead found that facial wrinkling scores were significantly correlated with the diffusing capacity of lung for carbon monoxide (p < .05). In addition, O’Brien et al. [15] found that skin elasticity, as measured by the skin viscoelastic modulus of the forearm, was inversely and significantly correlated with FEV1/FVC (p = .001) and emphysema quantified from computed tomographic images (p < .001).
Cognitive Function
As part of their previous studies, Christensen et al. [7] also measured patients’ cognitive function with the Mini-Mental State Examination (MMSE) and found that perceived age had a significant inverse correlation with MMSE scores (p < .001). Recently, Umeda-Kameyama et al. [3] found that perceived age was more strongly correlated with MMSE scores than chronologic age in women (p < .00003). Additionally, perceived age showed a better correlation with the Vitality Index in the total population (p < .00000003) and in women (p < .0000000009) than did chronologic age.
Bone Mineral Density
In 2015, Nielsen et al. [16] used whole body pictures of women to assess perceived age based on the hypothesis that physicians might misjudge a patient’s age based on her posture since this reflects the severity of spinal osteoporosis. The authors found that an increased perceived age was significantly associated with a lower bone mineral density (p < .04).
Discussion
To our knowledge, the first article discussing the association between perceived age and mortality was published by Borkan et al. [4] in 1982. Their study initiated in 1958 with data collection, and patients were reexamined at 18-month intervals until the time of death. Analysis of covariance and multiple classification analysis demonstrated that patients who survived until 1977 were perceived as significantly younger, with an estimated age of 1.04 years lower on average than their chronologic age. This study’s critical bias was its age evaluation method, which was done face to face by a physician. This evaluation was subject to external cues that might have influenced the evaluator’s estimate [8].
Three other studies suffered from the same limitation: two by Schnohr et al. [5, 9] and one one by Lange and Schnor [13]. These three studies were performed before the year 2000 when digital photography was not widely available. Until 1998, 35-mm film photography was the standard method for documenting patients’ physical appearance [17].
Among all studies, Christensen et al. [6] were the first to use photography assessments. The studies that came after theirs all used photography; however, they sometimes differed in the way pictures were taken. Facial photography, particularly in plastic surgery, should follow some general recommendations to increase perceived skin detail. There are contrasting viewpoints on background color, with some advocating for the use of dark blue [18] and others for medium to light blue [19]. These blue shades seem like good complements for all skin tones, yet darker shades might diminish the three-dimensional quality of the picture [19]. Additionally, backgrounds should be free of folds and creases and composed of nonreflective materials such as matte paint, wallpaper, or cloth [20]. Except for Patel et al. [14], who took their pictures in the medical photography department of Addenbrooke’s Hospital of Cambridge University Hospitals, the articles included in this review described the background as “neutral” [6] or did not describe it at all [2, 3, 7, 8, 10,11,12, 15, 16].
Appropriate lighting is another crucial characteristic of good quality facial photography. It is recommended that two light sources be arranged at 45° angles and above the patient [21,22,23]. Also, multiple flash units and softboxes or umbrellas should be used as diffusers to eliminate shadows and provide an accurate depiction of facial redness and pigmentation [24]. The articles in this review did not describe in detail the lighting conditions they used for obtaining the patients’ pictures, with some only claiming to use “the same” or “standardized” lighting conditions for all patients [8, 15, 16] and others not describing them at all [2, 6, 7, 10]. Excluding Patel et al. [14], only three articles were more specific. One stated the use of a shadowless lamp [11], one used a 400–600 lux light source [3], and one used a device that provided a scattered light source [12]. Therefore, most of the articles lacked precision in describing their photography-taking technique, posing a substantial risk of bias for identifying facial aging cues.
Several studies have searched for the specific facial cues that influence perceived age. Nkengne et al. [25] found that the eyes and lips areas and skin color uniformity were the most critical characteristics influencing perceived age. Subsequently, their results were replicated by Kwart et al. [26] and expanded on by Forte et al. [27], who found that crow’s feet and lips’ vertical rhytides were some of the essential factors influencing perceived age in lateral and frontal pictures, respectively. The included studies that evaluated facial wrinkling always used lateral pictures of the patients’ temporal regions, either at 45° [10] or 60° [12] angles. Although some studies did not specify the angle from which the picture was taken [14, 15], periorbital skin wrinkling was evaluated, contributing to a more reliable assessment of patients’ perceived age. Of note, although O’Brien et al. [15] obtained photographs from patients’ perioral region, the authors did not specify if they included the evaluations in the facial wrinkling score.
In 1993, Sherertz and Hess [28] concluded that perceived age is a subjective estimation and that it might not be clinically useful as a marker, mainly because of the influence of environmental factors on skin characteristics. After this, it has been said that the association between perceived age and mortality risk might be a consequence of exposure to harmful factors [8]. However, since the study by Borkan et al. [4] in 1982, which evaluated perceived age as a mortality predictor, five more studies evaluated this association, with results pointing to the existence of a significant positive association between increased perceived age and mortality risk, even when controlling for factors that influence skin aging [2, 4, 6,7,8]. Only one of these studies looked for specific facial aging characteristics associated with mortality [5]. Interestingly, although the study found gray hair and arcus senilis to be mortality predictors in men and women, respectively, it did not find an association with facial wrinkling, which was the only facial skin aging characteristic evaluated [5]. Since the rest of the studies evaluating perceived age found either a significant difference between the estimated ages of the deceased and survivors [4] or an increased mortality rate [6] or mortality risk [2, 7, 8] in older-looking patients, the set of facial aging characteristics as a whole may be what drives the association with mortality, instead of an isolated component. Nevertheless, considering that the study by Schnohr et al. [5] was conducted more than 20 years ago, new prospective studies evaluating the association between specific facial skin aging characteristics and mortality risk are warranted.
Information was scarce regarding the association between perceived age and comorbidities. For cardiovascular diseases, the relationship with MI risk [9], carotid atherosclerosis [11, 12], and general cardiovascular risk [10] was studied. Schnohr et al. [9] found that gray hair and facial wrinkling were associated with a significant increase in probability of MI in men. The latter held only for patients younger than 55 years. Although this relationship might seem intuitive, the associations were significant even after including chronologic age and other age-related parameters as covariates. In another study, CIMT was significantly and positively associated with perceived age [11], while in a second study, it was associated, along with brachial-ankle pulse wave velocity, with facial pigmentation [12]. Additionally, Miyawaki et al. [12] found that facial pigmentation was also significantly and positively correlated with obesity measures, concluding that atherosclerosis indices were correlated with perceived age in women through increased fat.
Studies have demonstrated that a higher body mass index is associated with a decrease in perceived age in men, with a tendency for significance in women [29]. Furthermore, Guinot et al. [30] found that skin age scores were lower than chronologic age in overweight premenopausal women but not in overweight postmenopausal women or nonoverweight women regardless of menopausal status. The link between increased adipose tissue and perceived age might, therefore, be correlated with the effect of estrogens, whose levels have also been found to be inversely correlated with perceived age [31]. Menopausal status should be considered a potential confounder when establishing associations with perceived age or aging facial characteristics, such as in the study by Miyawaki et al. [12]
Gunn et al. [10] found that women with lower cardiovascular risk looked significantly younger than those with higher cardiovascular risk. Additionally, they found a significant positive correlation between blood pressure and perceived age in women. Previous studies have found a significant inverse association of microvascular skin function with cardiovascular risk [32] and blood pressure [33]. Therefore, the altered microvascular function in patients with high cardiovascular risk or essential hypertension may promote premature aging of specific areas of facial skin. Although the literature has mostly focused on histopathologic analyses of aging skin [34, 35], functional evaluation of the microvasculature and its repercussions on aging characteristics is still lacking.
No author studied the direct association of COPD with perceived age. Instead, due to its relationship with smoking, facial wrinkling was studied. The first study done on this matter by Lange and Schnohr [13] initially used a group of patients containing nonsmokers and found a significant inverse association between facial wrinkle severity and spirometry measures related to COPD. However, after group stratification, the association disappeared for lifetime nonsmokers and remained significant for past and current smokers. Two studies identified a similar pattern of outcomes [14, 15], with one finding a significant positive association between facial wrinkle scores and extensive emphysema on computed tomography [14]. Therefore, the evidence included in our review suggest that facial wrinkling severity, measured by the Daniell scoring system, warrants systematic investigation as a COPD severity predictor.
In addition to evaluating the association between perceived age and mortality and in their attempt to prove that perceived age was a suitable aging biomarker, Christensen et al. [7] also found perceived age to have a significant inverse correlation with MMSE scores. The recent findings by Umeda-Kameyama et al. [3] corroborated this association, with the difference that their results only held for women. Additionally, Umeda-Kameyama and colleagues [3] also found a significant inverse correlation between perceived age and vitality measures. Despite the reduced number of studies, the results demonstrate that perceived age is a good predictor of cognitive decline. Lastly, as to bone mineral density, though the results of a study by Nielsen et al. [16] seem promising, studies following a similar methodology are required to confirm the association found by the authors.
A biomarker is defined as a specific analyte, anatomic feature, or physiological characteristic that is measured [36]. A more specific definition by the Institute of Medicine states that biomarkers are indicators of normal biological processes, pathogenic processes, or pharmacologic responses to an intervention [37]. Researchers have been looking for a reliable and reproducible biomarker of aging that can substitute chronological age for decades since not everyone ages at the same rate. As a result, a wide array of aging biomarkers have been proposed, primarily biochemical, such as interleukin-6 and other inflammatory cytokines [38], testosterone [39], mitochondrial DNA [40], and telomere length [41], among many others involved in metabolic processes [42]. The American Federation of Aging Research proposed a set of criteria specific for aging biomarkers. They stated that these biomarkers should (1) predict the rate of aging (i.e., identify where a person is in their lifespan), (2) monitor a primary mechanism underlying the aging process (not an effect of disease), (3) be able to be tested repeatedly without harming the person, and (4) be something that works in both humans and research animals, so that it can be tested in the laboratory before being validated in humans [43]. In this setting, perceived age might only fulfill criteria 2 and 3, serving to monitor the aging process without harming the evaluated person. Therefore, it would be inappropriate to consider it a proper biomarker of aging. However, its association with mortality and the previously outlined comorbidities warrant its consideration and further study as a risk factor for these outcomes.
Lastly, no individual factors have been identified to explain the association between facial aging and mortality or comorbidities. However, oxidative stress and genetic predisposition have been proposed as possible explanations [8]. The oxidative stress theory of aging states that tissue functionality is lost due to macromolecule damage generated by reactive oxygen species (ROS) [44]. In addition to apoptosis [45], ROS induce cellular senescence and decrease the cells’ replicating capacity [46, 47]. Although it is known that the protective response to ROS decreases with age [48], some people might be genetically predisposed to either increased ROS production or decreased scavenging, increasing the risk of organ damage and disease development [49,50,51]. Moreover, the basis of photoaging is the continuous exposure of the skin to UV radiation and environmental chemicals leading to ROS formation and skin damage [52,53,54]. Ultimately, it could be possible that by overwhelming the protective capacity of the skin to ROS in genetically susceptible individuals, environmental insults could make a person look older than they are. In short, looking older for one’s age might reflect an already defective ROS clearance, with more underlying organ damage compared to same-aged peers.
Conclusion
Our review demonstrates that perceived age promises to be a useful predictor of overall mortality and cardiovascular, pulmonary, cognitive, and osseous comorbidities. The relative absence of studies evaluating the association between perceived age and different comorbidities is a topic that must be addressed to support the eventual use of perceived age in the clinical setting. Additionally, our review also highlighted that authors do not always follow the photography recommendations that ensure optimal visualization of facial skin characteristics, and even if they do, they often do not adequately record it in their methodology.
Limitations
This study has several limitations. Although some studies specified asking patients not to use any hairstyle or facial products when taking the photographs [10], others did not. This poses a substantial bias, since using these products can artificially decrease a subject’s perceived age. Since only studies published in English were included in this review, some relevant studies may have been missed. Other limitations include the scarcity of studies reporting on this topic, the potential bias of misinterpreting data and results, and the study selection process, the latter being a potential source of bias common to systematic reviews.
References
Borkan GA, Norris AH (1980) Assessment of biological age using a profile of physical parameters. J Gerontol 35:177–184
Gunn DA, Larsen LA, Lall JS, Rexbye H, Christensen K (2016) Mortality is written on the face. J Gerontol A Biol Sci Med Sci 71:72–77
Umeda-Kameyama Y, Kameyama M, Kojima T, Ishii M, Kidana K, Yakabe M, Ishii S, Urano T, Ogawa S, Akishita M (2020) Cognitive function has a stronger correlation with perceived age than with chronological age. Geriatr Gerontol Int. https://doi.org/10.1002/alz.037133
Borkan GA, Bachman SS, Norris AH (1982) Comparison of visually estimated age with physiologically predicted age as indicators of rates of aging. Soc Sci Med 16:197–204
Schnohr P, Nyboe J, Lange P, Jensen G (1998) Longevity and gray hair, baldness, facial wrinkles, and arcus senilis in 13,000 men and women: the Copenhagen City Heart Study. J Gerontol A Biol Sci Med Sci 53:M347-350
Christensen K, Iachina M, Rexbye H, Tomassini C, Frederiksen H, McGue M, Vaupel JW (2004) “Looking old for your age”: genetics and mortality. Epidemiology 15:251–252
Christensen K, Thinggaard M, McGue M, Rexbye H, Hjelmborg JV, Aviv A, Gunn D, van der Ouderaa F, Vaupel JW (2009) Perceived age as clinically useful biomarker of ageing: cohort study. BMJ 339:b5262
Dykiert D, Bates TC, Gow AJ, Penke L, Starr JM, Deary IJ, Dykiert D, Bates TC, Gow AJ, Penke L, Starr JM, Deary IJ (2012) Predicting mortality from human faces. Psychosom Med 74:560–566
Schnohr P, Lange P, Nyboe J, Appleyard M, Jensen G (1995) Gray hair, baldness, and wrinkles in relation to myocardial infarction: the Copenhagen City Heart Study. Am Heart J 130:1003–1010
Gunn DA, de Craen AJ, Dick JL, Tomlin CC, van Heemst D, Catt SD, Griffiths T, Ogden S, Maier AB, Murray PG, Griffiths CE, Slagboom PE, Westendorp RG (2013) Facial appearance reflects human familial longevity and cardiovascular disease risk in healthy individuals. J Gerontol A Biol Sci Med Sci 68:145–152
Kido M, Kohara K, Miyawaki S, Tabara Y, Igase M, Miki T (2012) Perceived age of facial features is a significant diagnosis criterion for age-related carotid atherosclerosis in Japanese subjects: J-SHIPP study. Geriatr Gerontol Int 12:733–740
Miyawaki S, Kohara K, Kido T, Tabara Y, Igase M, Miki T, Sayama K (2016) Facial pigmentation as a biomarker of carotid atherosclerosis in middle-aged to elderly healthy Japanese subjects. Skin Res Technol 22:20–24
Lange P, Schnohr P (1994) The relationship between facial wrinkling and airflow obstruction. Int J Dermatol 33:123–126
Patel BD, Loo WJ, Tasker AD, Screaton NJ, Burrows NP, Silverman EK, Lomas DA (2006) Smoking related COPD and facial wrinkling: Is there a common susceptibility? Thorax 61:568–571
O’Brien ME, Chandra D, Wilson RC, Karoleski CM, Fuhrman CR, Leader JK, Pu J, Zhang Y, Morris A, Nouraie S, Bon J, Urban Z, Sciurba FC (2019) Loss of skin elasticity is associated with pulmonary emphysema, biomarkers of inflammation, and matrix metalloproteinase activity in smokers. Respir Res 20:128
Nielsen BR, Linneberg A, Christensen K, Schwarz P (2015) Perceived age is associated with bone status in women aged 25–93 years. Age (Dordr) 37:106
DeLange GS, Diana M (1999) 35 mm film vs. digital photography for patient documentation: is it time to change? Ann Plast Surg 42:15–19 (discussion 19-20)
Prantl L, Brandl D, Ceballos P (2017) A proposal for updated standards of photographic documentation in aesthetic medicine. Plast Reconstr Surg Glob Open 5:e1389
Kontis TC (2002) Photography in facial plastic surgery. Facial plastic and reconstructive surgery: Thieme Medical Publishers, Inc, New York, 116-124
Khavkin J, Ellis DA (2011) Standardized photography for skin surface. Facial Plast Surg Clin North Am 19:241–246
DiBernardo BE, Adams RL, Krause J, Fiorillo MA, Gheradini G (1998) Photographic standards in plastic surgery. Plast Reconstr Surg 102:559–568
Archibald DJ, Carlson ML, Friedman O (2010) Pitfalls of nonstandardized photography. Facial Plast Surg Clin North Am 18:253–266
Persichetti P, Simone P, Langella M, Marangi GF, Carusi C (2007) Digital photography in plastic surgery: how to achieve reasonable standardization outside a photographic studio. Aesthet Plast Surg 31:194–200
Galdino GM, DaSilva GJP (2002) Digital photography for rhinoplasty. Plast Reconstr Surg 109:1421–1434
Nkengne A, Bertin C, Stamatas GN, Giron A, Rossi A, Issachar N, Fertil B (2008) Influence of facial skin attributes on the perceived age of Caucasian women. J Eur Acad Dermatol Venereol 22:982–991
Kwart DG, Foulsham T, Kingstone A (2012) Age and beauty are in the eye of the beholder. Perception 41:925–938
Forte AJ, Andrew TW, Colasante C, Persing JA (2015) Perception of age, attractiveness, and tiredness after isolated and combined facial subunit aging. Aesthet Plast Surg 39:856–869
Sherertz EF, Hess SP (1993) Stated age. N Engl J Med 329:281–282
Rexbye H, Petersen I, Johansens M, Klitkou L, Jeune B, Christensen K (2006) Influence of environmental factors on facial ageing. Age Ageing 35:110–115
Guinot C, Malvy DJ, Ambroisine L, Latreille J, Mauger E, Tenenhaus M, Morizot F, Lopez S, Le Fur I, Tschachler E (2002) Relative contribution of intrinsic vs extrinsic factors to skin aging as determined by a validated skin age score. Arch Dermatol 138:1454–1460
Wildt L, Sir-Petermann T (1999) Oestrogen and age estimations of perimenopausal women. Lancet 354:224
Ij RG, de Jongh RT, Beijk MA, van Weissenbruch MM, Delemarre-van de Waal HA, Serné EH, Stehouwer CD (2003) Individuals at increased coronary heart disease risk are characterized by an impaired microvascular function in skin. Eur J Clin Invest 33:536–542
Serné EH, Gans RO, ter Maaten JC, Tangelder GJ, Donker AJ, Stehouwer CD (2001) Impaired skin capillary recruitment in essential hypertension is caused by both functional and structural capillary rarefaction. Hypertension 38:238–242
Contet-Audonneau JL, Jeanmaire C, Pauly G (1999) A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol 140:1038–1047
Li L, Mac-Mary S, Marsaut D, Sainthillier JM, Nouveau S, Gharbi T, de Lacharriere O, Humbert P (2006) Age-related changes in skin topography and microcirculation. Arch Dermatol Res 297:412–416
BEST (Biomarkers, EndpointS, and other Tools) Resource [Internet]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK326791/. Accessed March 12, 2022
Krumholz HM (2015) Biomarkers, risk factors, and risk: clarifying the controversy about surrogate end points and clinical outcomes. Circ Cardiovasc Qual Outcomes 8:457–459
Maggio M, Guralnik JM, Longo DL, Ferrucci L (2006) Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci 61:575–584
Maggio M, Basaria S, Ceda GP, Ble A, Ling SM, Bandinelli S, Valenti G, Ferrucci L (2005) The relationship between testosterone and molecular markers of inflammation in older men. J Endocrinol Invest 28:116–119
Eshaghian A, Vleugels RA, Canter JA, McDonald MA, Stasko T, Sligh JE (2006) Mitochondrial DNA deletions serve as biomarkers of aging in the skin, but are typically absent in nonmelanoma skin cancers. J Invest Dermatol 126:336–344
Bekaert S, De Meyer T, Van Oostveldt P (2005) Telomere attrition as ageing biomarker. Anticancer Res 25:3011–3021
Engelfriet PM, Jansen EH, Picavet HS, Dollé ME (2013) Biochemical markers of aging for longitudinal studies in humans. Epidemiol Rev 35:132–151
Lara J, Cooper R, Nissan J, Ginty AT, Khaw KT, Deary IJ, Lord JM, Kuh D, Mathers JC (2015) A proposed panel of biomarkers of healthy ageing. BMC Med 13:222
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Chandrasekaran A, Idelchik M, Melendez JA (2017) Redox control of senescence and age-related disease. Redox Biol 11:91–102
Pole A, Dimri M, Dimri GP (2016) Oxidative stress, cellular senescence and ageing. AIMS Mol Sci. https://doi.org/10.3934/molsci.2016.3.300
Ikeyama S, Kokkonen G, Shack S, Wang XT, Holbrook NJ (2002) Loss in oxidative stress tolerance with aging linked to reduced extracellular signal-regulated kinase and Akt kinase activities. Faseb j 16:114–116
Cuevas S, Villar VAM, Jose PA (2019) Genetic polymorphisms associated with reactive oxygen species and blood pressure regulation. Pharmacogenomics J 19:315–336
Hovnik T, Dolžan V, Bratina NU, Podkrajšek KT, Battelino T (2009) Genetic polymorphisms in genes encoding antioxidant enzymes are associated with diabetic retinopathy in type 1 diabetes. Diabetes Care 32:2258
Karahalil B, Orhan G, Ak F (2015) The impact of detoxifying and repair gene polymorphisms and the levels of serum ROS in the susceptibility to multiple sclerosis. Clin Neurol Neurosurg 139:288–294
Bickers DR, Athar M (2006) Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol 126:2565–2575
Mudiyanselage SE, Hamburger M, Elsner P, Thiele JJ (2003) Ultraviolet a induces generation of squalene monohydroperoxide isomers in human sebum and skin surface lipids in vitro and in vivo. J Invest Dermatol 120:915–922
Sander CS, Chang H, Salzmann S, Müller CS, Ekanayake-Mudiyanselage S, Elsner P, Thiele JJ (2002) Photoaging is associated with protein oxidation in human skin in vivo. J Invest Dermatol 118:618–625
Funding
This study was funded in part by the Center for Regenerative Medicine and the Clinical Research Operations Group of Mayo Clinic Florida.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Christopher J. McLeod has provided consultancy services to Biosig Technologies.
Human or Animal Rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animal participants.
Informed Consent
For this type of study, formal consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Part of this work was previously presented at Plastic Surgery The Meeting 2021 in Atlanta, Georgia. It was selected as one of the top 200 abstracts and was published in the meeting’s supplement in Plastic and Reconstructive Surgery Global Open: Verduzco, Francisco Avila MD; Guzmán, Ricardo Torres MD; Huayllani, Maria MD; Maita, Karla MD; Garcia, John MD; Forte, Antonio MD Increased Perceived Age as a Risk Factor for All-cause Mortality and Comorbidities: A Systematic Review, Plastic and Reconstructive Surgery - Global Open: October 2021 - Volume 9 - Issue 10S - p 23-24 doi: 10.1097/01.GOX.0000799204.51519.bc.
Rights and permissions
About this article
Cite this article
Avila, F.R., Torres-Guzman, R.A., Maita, K.C. et al. Perceived Age as a Mortality and Comorbidity Predictor: A Systematic Review. Aesth Plast Surg 47, 442–454 (2023). https://doi.org/10.1007/s00266-022-02932-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-022-02932-5