Abstract
Background
Insight into the physical processes of aging can be gained by comparing the loss of facial volume that occurs during aging with the dramatic fat loss resulting from acquired lipoatrophy, including human immunodeficiency virus (HIV) treatment-associated lipoatrophy. The superficial effects of aging, such as rhytid formation, often are the focus of investigations into this phenomenon. However, age-related volume loss often is ignored.
Methods
A review of the relevant literature was conducted to provide an overview of age-related lipoatrophy and its etiology and to compare it by facial region with HIV-associated facial lipoatrophy.
Results
As a side effect of highly active antiretroviral therapy, HIV-associated lipoatrophy results in fat lipodystrophy (including both lipoatrophy and lipohypertrophy) and progresses toward nearly complete subdermal facial fat loss. Aging is accompanied by changes in the soft tissues of the face, leaving atrophic regions of generalized tissue ptosis. Some facial regions are affected differently by fat loss, depending on its cause. In the aging patient, certain parts of the face display only minimal fat loss.
Conclusions
The role of fat loss in facial aging is slight compared with its considerable role in HIV-associated lipoatrophy. The losses of various facial tissues and the ptosis of some soft tissues are strong contributors to the appearance of the aged face. This regional anatomic assessment of the face engenders a more thorough understanding of the progression that characterizes volume changes associated with aging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The physical consequences of aging to the skin, particularly the face, have received considerable attention in the past decade. However, because facial aging often is trivialized and because it has a minimal adverse impact on the health of the individual, it is not considered a target for scientific research or medical treatment. Despite this, an entire industry has developed to improve the outward appearance of individuals.
Patients with human immunodeficiency virus (HIV) who are receiving highly active antiretroviral therapy (HAART) have been observed to experience lipodystrophy, including facial lipoatrophy [10]. In particular, the loss of facial fat, including the buccal fat and temporal fat pads, leads to facial skeletonization with concave cheeks, prominent nasolabial folds, periorbital hollowing, and visible facial musculature [8, 17, 26]. These volume deficits alter the contours of the face from youthful, healthy, convex curves to aged, pathologic, concave contours [15, 19, 23, 50]. Insight into the physical process of aging can be obtained by comparing the loss of facial volume that occurs during aging with the dramatic fat loss associated with drug-related facial lipoatrophy [12].
The effects of facial aging often are narrowly focused on consideration of superficial wrinkles and sagging skin. The majority of products attempting to rejuvenate the face are aimed at the amelioration of wrinkles, with the issue of sagging skin reserved for surgical tightening procedures such as face-lifts. However, this approach to the treatment of facial aging ignores one of its most important factors: the loss of volume beneath the wrinkled sagging skin [4]. We know that photodamage, specifically, seems to cause significant collagen deterioration and elastin reorganization [21, 25, 46] as well as water barrier depletion in the stratum corneum [44, 47]. Volume depletion appears to be the major contributor not only to wrinkles but also to the descent or sagging of the tissues [12, 56].
Little research has been undertaken to determine how age influences facial volume in different anatomic regions. Such research, however, may help us better elucidate the mechanisms of facial aging and refine our treatment choices.
The American Society for Aesthetic Plastic Surgery has reported that more than 9 million nonsurgical cosmetic procedures were performed in 2006, compared with approximately 2 million surgical procedures the same year [51]. Indeed, compared with 1997, the number of nonsurgical procedures reportedly has increased by 747%, compared with only a 98% increase in surgical procedures. Approximately 50% of the nonsurgical procedures involved soft-tissue injections (e.g., botulinum toxin type A, stabilized hyaluronic acid). Despite these trends, the physiologic and anatomic cause of facial aging remains elusive, and consensus about these has yet to be reached.
Contributing to the staggering increase in nonsurgical rejuvenating facial procedures has been the introduction of many new cosmetic injection products that have met high levels of consumer acceptance. Modern soft tissue augmentation dates back to the late 1800s when Neuber [41] first used autologous fat from the arm to correct depressed facial scars. Currently, popular compounds include botulinum toxin type A, collagen (e.g., bovine, human), stabilized hyaluronic acid, hydrophilic polyacrylamide, calcium hydroxylapatite and polymethylmethacrylate. These products help to enhance facial appearance either by relaxing hyperdynamic lines, in the case of botulinum toxin type A, or by filling rhytids, grooves, and areas of soft tissue loss.
At the same time, knowledge of the underlying physical and physiologic processes of facial aging has not expanded at the same rate as the rise in products and procedures. When applied to facial rejuvenation, many of these new devices are used off-label, beyond their current indication. Such usage, coupled with a lack of clinical trial data, can lead to unrealistic expectations and an increased incidence of adverse outcomes [32].
Facial lipoatrophy has been observed to occur in more than 80% of HIV patients treated with HAART [45], frequently within the first year of HAART treatment [38], and most commonly within the first 5 years of treatment. The HAART approach typically involves the combination of several drugs including nucleoside reverse transcriptase inhibitors (NRTIs), protease inhibitors (PIs), and non-nucleoside reverse transcriptase inhibitors (NNRTIs).
Several hypotheses have been proposed to explain the mechanism of lipodystrophy action in HAART patients, including mitochondrial damage from NRTIs [42] and an interaction between PIs and the enzymes involved in lipid metabolism [7, 9]. However, the precise mechanism of lipoatrophy associated with HAART still is not understood. Facial lipoatrophy can leave patients stigmatized because they may be unable to hide their HIV status, which has a negative impact on self-esteem and quality of life [11, 30, 39]. Even more disturbing, the results of lipoatrophy in these patients can lead to compliance issues, seriously reducing the effectiveness of HAART and consequently the individual’s health [1]. By comparing HIV-associated lipoatrophy with generalized facial aging, patterns of volume depletion can be elucidated such that treatment for each can be better directed.
Aging, Lipoatrophy, and Quality of Life
Even without physical debilitation, negative facial changes can have an impact on quality of life. Generalized facial aging and HIV-associated facial lipoatrophy can have a profound psychological effect and, in more extreme forms, may draw undesired reactions from others [30]. It is not difficult to understand how aging or lipoatrophy can result in lowered self-esteem and social withdrawal. With more extreme psychological discomfort, an individual’s displeasure with his or her appearance may reduce interaction with family, friends, and health providers, potentially damaging both physical and mental health [22].
Returning patients to a state of comfort with their appearance can improve their quality of life and should therefore be considered of paramount importance. It can be argued that when lipoatrophy is a side effect of medical treatment, such as HAART, addressing the aesthetic condition can become as important as treating the underlying disease [39]. In one study, two-thirds of HIV-infected patients stated that they would prefer to lose 1 year of their life rather than experience the facial changes associated with lipoatrophy [33]. This finding is less shocking when considered in light of the overwhelmingly large aesthetic industry that caters to healthy individuals with neither life-threatening illness nor aesthetic defects that could in any way be perceived as “disfiguring.”
In addition, the lipoatrophy associated with HAART is like a “scarlet letter” announcing the disease to all who recognize the typical facial features. Studies have shown that treatments providing aesthetic enhancement significantly contribute to returning satisfaction with physical appearance and even improving marital relations [11, 16, 30].
Overview of Lipoatrophy
Lipoatrophy presents in several lipodystrophy conditions, which can be organized as being either inherited or acquired [17]. Although rare, three types of inherited lipodystrophy exist: congenital generalized lipodystrophy, mandibuloacral dysplasia, or familial partial lipodystrophy. Facial lipoatrophy, specifically, can be present in congenital generalized lipodystrophy such as Perry-Romberg syndrome or mandibuloacral dysplasia, but rarely presents in familial partial lipodystrophy, the only autosomal dominant form [17].
Four types of acquired lipodystrophy exist: generalized, partial (Barraquer-Simons syndrome), localized, or HIV-treatment associated lipodystrophy [17]. Generalized lipodystrophy typically presents in childhood as a result of immune-mediated disease, such as juvenile dermatomyositis, and can cause fat loss throughout the body, with the most severe depletion occurring in the face, arms, and legs. Partial lipodystrophy is thought to be the most common autoimmune-mediated fat-wasting condition in childhood or adolescence. The associated fat loss in this case, however, is limited to the upper body, with fat sparing in the lower limbs. Localized lipodystrophy typically presents as small indentations of fat loss related to a local insult (e.g., corticosteroid injections, trauma). Human immunodeficiency virus treatment associated lipodystrophy is the most prevalent acquired type, causing hypertrophy of central visceral and dorsocervical fat, wasting of peripheral body fat and, most dramatically, facial fat loss (Fig. 1) [17].
Etiology of Human Immunodeficiency Virus Lipoatrophy
Initiated in the mid-1990s, HAART still is considered the mainstay treatment option for HIV. The first observations of metabolic and morphologic alterations resulting from HAART were noted in the late 1990s [10]. In addition to the observed hyperglycemia, hyperinsulinemia, hyperlipidemia, and hypertension, HIV patients treated with HAART were experiencing irregular fat deposits and deficits [34]. Specifically, accumulations of central visceral fat and dorsocervical fat (lipohypertrophy) were noted, together with the loss of peripheral fat (lipoatrophy) in the upper and lower limbs, buttocks, and face. Together, these fat and metabolic changes are known as lipodystrophy syndrome [10].
Certain factors are observed as contributing to the presentation of HIV-related lipoatrophy, such as the use and duration of antiretrovirals, CD4 count, viral load, race, sex, exercise level, and age at the start of the therapy [35]. Although the underlying causes of lipodystrophy remain elusive, research has implicated PIs, NRTIs, and even drug-naïve HIV involvement. For example, the PIs responsible for cellular retinoic acid-binding protein type 1 (CRABP-1) inhibition, may be accountable for the apoptosis of peripheral adipocytes [10]. The interplay between CRABP-1 and peroxisome proliferators-activated receptors, preferentially expressed peripherally, is postulated to be responsible for regional adipose apoptosis [52]. Because of their mitochondrial toxicity, NRTIs have been associated with lipoatrophy. Mitochondrial DNA polymerase, which is necessary for mitochondrial DNA replication, has a high sensitivity to NRTIs [6, 28]. The interference of NRTIs affects the ability of mitochondria to carry out oxidative phosphorylation, leading to an overabundance of pyruvate and lactic acid, which destroys adipocytes [20].
Regional Anatomy of Volume Depletion: Human Immunodeficiency Virus-Associated Facial Lipoatrophy Compared With Facial Aging
Cutaneous Layers
Located immediately beneath the skin is the hypodermis, or subcutaneous layer, composed of adipose tissue, a robust capillary plexus, dermal papillae, and eccrine ducts. In addition to the fat in this layer, adipose tissue can be found as dense loculations known as “fat pads” in the cheeks (buccal fat pads), orbit (periorbital fat pads), and temples (superficial temporal fat pads; Fig. 2). Moreover, the cheeks tend to have a greater supply of dispersed subcutaneous fat than other regions. Eccrine glands, hair bulbs, blood vessels, lymphatic channels, and nerves also are located in this superficial adipose layer. The muscles of facial expression are separated from the skin by this superficial fatty layer. The facial musculature attaches to the skin of the face via the superficial musculoaponeurotic system, which encompasses the muscle with fascia, orchestrating facial movement and causing facial expression with even the subtlest muscle contraction [5, 15, 40].
Patients with HIV-associated lipoatrophy progress toward subtotal loss of hypodermal fat in the face that is disproportionate to the loss of adnexal tissue [54]. Skin thickness also is observed to become somewhat atrophic [3, 23, 50, 54]. This fat depletion can occur within the first year of treatment [38] and is 45% more likely to occur with every additional 6 months of HAART [37].
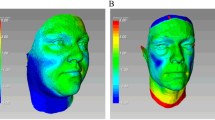
Patients with HIV-associated lipoatrophy have a fairly rapid and localized loss of facial soft tissue, unlike the slower loss of facial fullness associated with generalized aging (Fig. 3). In both cases, however, the resulting atrophy and deflation of the soft tissues causes skin laxity and descent of the previously inflated skin. The muscles lying juxtaposed to subcutaneous tissue and attached to the overlying skin become more apparent. With a decrease in skin thickness and hypodermal fat, each muscle contraction can result in exaggerated facial expressions. Some of these resulting rhytids and folds can project negative emotions such as anger, disdain, and surprise, which may be discordant with the actual feelings of the patient. In both patient populations, the loss of adnexal structures and fullness reduces tissue elasticity, resulting in collapse and subsequent descent of the skin.
Generally, beginning at the age of 20 years, individuals naturally succumb to a progressive decrease in cutaneous thickness in the cheeks, temples, and orbits [15, 19, 23, 50], with noticeable signs of aging becoming apparent at 30 years. It is the speed and the degree to which this happens in specific areas of the face that differentiates generalized aging from HIV-related lipoatrophy [14].
Periorbital Region
Volume in the periorbital region is due to the underlying bone, muscle, and fat. The orbicularis oculi muscle provides fullness within and beyond the orbital bones and is responsible for the wrinkling at the lateral margin of the orbit, or the “crow’s feet.” The periorbital fat pads in the upper and lower eyelids also contribute significantly to periorbital volume.
In both HIV-associated lipoatrophy and generalized facial aging, the orbit exhibits nearly uniform volume loss, exaggerating the orbital rims superiorly, medially, and laterally. Considerable hollowing can be seen along the infraorbital rim, consistent with the loss of fat pads in this region. In the authors’ experience, the glabella tends to be spared from the effects of fat loss in HIV-associated lipoatrophy but not in generalized aging. The loss of volume in the superior aspect of the orbit results in deflation of the previously full tissues and the descent of the now apparently excess upper eyelid skin. This skin tends either to fall forward toward the ciliary margin or to fall posteriorly, accentuating a deep, hollow crease.
Temple
The majority of volume in the temple is contributed by the temporalis muscle and the deep and superficial temporal fat pads. Concavity of the temples is highly consistent with the effects of both HIV-associated lipoatrophy and aging. At an advanced stage of either aging or HIV-related atrophy, the frontal process of the zygomatic bone and the superior border of the zygomatic arch can become visible, giving a sharply angulated appearance to the forehead.
Perioral Region
The perioral region has a relative absence of fat, with the lip volume composed predominantly of the obicularis oris muscle and skin. The depletion of lip volume with subsequent flattening of the white roll/vermillion border and philtral columns is typical of generalized aging, but in HIV-related lipoatrophy patients, no noticeable change occurs in this region. Typical of generalized aging, the upper lip lengthens. There is less show of the maxillary incisors, and the lip inverts. The lower lip loses submucosal fullness, inverts, and has less of a pout. Other aging changes include the development of radial lip lines and descent of the mouth angles, contributing to marionette lines.
Midface and Cheek
The midface and cheek portion of the face exhibit the most dramatic contrast in generalized aging- and HIV-related lipoatrophy. The HIV-associated lipoatrophy patient tends to sustain complete fat loss in the preauricular, buccal, and malar areas, leading to pathognomonic concavities where there were once convex, full facial features. The patient treated with HAART typically has this concavity as well as nasolabial banding, or a redundancy of nasolabial skin that occurs as the volume of fat inflating the buccal and malar regions is lost. Additionally, the zygomaticus and masseter muscle striations can become visible through the atrophic skin. In the most severe cases, a skeletonized appearance results, presenting as an obvious sign of the disease.
Certain elements of the aging face are similar to the facial characteristics displayed by the HIV patient with lipoatrophy, including loss of the convex contours of youth. In the aging face, however, the degree of hollowing in the cheek, especially in the anterior malar and buccal cheek, is not as pronounced as it is with HIV-related lipoatrophy. In fact, in most healthy aging faces, the anterior malar fat is retained even into extreme old age. Atrophy in this area usually is associated with illness, starvation, or reactions to drugs.
Whereas almost no change to the perioral region occurs with age-related lipoatrophy, the extreme atrophy of the anterior cheek seen with HIV-related lipoatrophy can allow the emptied skin of the cheek to “pleat” over the nasolabial fold, thereby accentuating the fold. Restoring fullness to the cheek can correct this apparent deepening of the fold, even without filling the fold itself.
In a recent clinical trial by Hanke and Redbord [24], the difference between age- and HIV-related facial lipoatrophy of the midface/cheek was aptly demonstrated [24]. The average severity of lipoatrophy at baseline in this population, as assessed using the 5-point Facial Lipoatrophy Grading Scale (range, 0 [no lipoatrophy] to 5 [severe depression of one or more facial regions, severe prominence of bony landmarks, and clear visibility of the underlying musculature]) [2] was 4.0 for HIV patients compared with 2.1 for patients undergoing treatment for age-related facial lipoatrophy. These patients then were treated with injectable poly-L-lactic acid (PLLA), a biocompatible, biodegradable synthetic polymer device that elicits an increase in dermal volume after injection [18].
Injectable PLLA currently is approved in Europe, Canada, and the United States for the restoration or correction of lipoatrophy signs in people with HIV. It also is approved in Europe, Brazil, Australia, and Canada for the cosmetic correction of facial volume defects, and currently is under review by the Food and Drug Administration (FDA) in the United States for volume restoration or correction of facial wrinkles and folds, such as nasolabial lines or folds. All other uses should be considered off-label.
In the trial by Hanke and Redbord [24], due to the greater severity of lipoatrophy in HIV patients, the mean total number of injectable PLLA treatments undertaken was greater (n = 4.6) than in the HIV-negative population (n = 2.7). Furthermore, HIV-lipoatrophy patients demonstrated a longer time until full correction (23.9 weeks; average improvement of −2.3 in the lipoatrophy score) than patients undergoing treatment for age-related facial lipoatrophy (17.4 weeks; average improvement of −1.2 in the lipoatrophy score).
Jaw Line
The angle and body of the mandible, with its overlying masseter and platysma muscles, define the inferior border of the lower face and create the jaw line. The jaw line can be exaggerated in HAART patients depleted of facial fat, allowing the skin to sheath the musculoskeletal features more closely. This also occurs in the aged face, but there often is remaining fat that descends below and obscures the edge of the mandible as the facial fullness deflates.
General Features
Although HIV-associated lipoatrophy is not age dependent, it can be exacerbated with chronologic aging and is more severe when HAART is initiated at an advanced age. Because the disease can affect people at any age, the reorganization that occurs is not necessarily reflected in the condition [43]. Compared with the experience of chronologically aged patients, visual protrusion of the maxilla and mandible is apparent with HIV-associated lipoatrophy as the mid-face is depleted of fat volume and develops the lateral concavities that typify the condition. The aging patient undergoes bony resorption and reorganization, with some retrusion of the maxilla and mandible and reduction in the underlying surface area for soft tissue support that allows the skin to sag [36, 43, 56]. On the other hand, the patient with HIV-associated lipoatrophy can have an apparent increase in prominence of the maxilla and mandible as the surrounding soft tissues recede.
Conclusion
Recently, a variety of substances have been used safely to correct signs of aging or facial fat loss including fat, collagen, polymethylmethacrylate, calcium hydroxylapatite, and injectable PLLA [27, 29, 31, 48, 49, 53, 55]. A desirable treatment option involves filling the deficits with autologous fat [13], but this is not always an option for patients without much central body fat, such as those with HIV-related lipodystrophy. Additionally, not all patients are prepared or medically able to undergo a surgical procedure. As the etiology of the manifestations of facial aging is increasingly understood, physicians are better able to recommend and administer the most appropriate corrective strategy for the subtly different signs of facial aging.
The recent recognition of HIV-associated facial lipoatrophy as a side effect of HAART treatment presents the aesthetically oriented physician with a new model for comparison with the aging face. The concavities and volume distortions that result from HIV-associated facial lipoatrophy present certain similarities and differences compared with the aging face, providing an opportunity for better understanding the aesthetic effects of volume changes in the face. The regional anatomic comparisons herein suggest a lesser role of fat loss in facial aging, in contrast to its considerable role in HIV-associated lipoatrophy. Instead, with aging, skin and subcutaneous atrophy (including loss of collagen, elastin, hyaluronic acid, bony skeleton, and water), and the subsequent ptosis of soft tissues contribute to the appearance of the aged face. Through this regional anatomic assessment of the face, we have a more thorough understanding of the characteristic progression of volume changes associated with aging and HIV-associated facial atrophy.
References
Ammassari A, Antinori A, Cozzi-Lepri A, Trotta MP, Nasti G, Ridolfo AL, Mazzotta F, Wu AW, d’Arminio Monforte A, Galli M (2002) Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr 31(Suppl 3):S140–S144
Ascher B, Coleman S, Alster T, Bauer U, Burgess C, Butterwick K, Donofrio L, Engelhard P, Goldman MP, Katz P, Vleggaar D (2006) Full scope of effect of facial lipoatrophy: a framework of disease understanding. Dermatol Surg 32:1058–1069
Bansevicius D, Pareja JA, Sjaastad O (1997) “Skin roll” (“pinch and roll”) test: skinfold thickness and tenderness. Headache 37:281–285
Bartlett SP, Grossman R, Whitaker LA (1992) Age-related changes of the craniofacial skeleton: an anthropometric and histologic analysis. Plast Reconstr Surg 90:592–600
Bolognia JL (1993) Dermatologic and cosmetic concerns of the older woman. Clin Geriatr Med 9:209–229
Brinkman K, Smeitink JA, Romijn JA, Reiss P (1999) Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet 354:1112–1115
Carr A (2000) HIV protease inhibitor-related lipodystrophy syndrome. Clin Infect Dis 30(Suppl 2):S135–S142
Carr A, Law M (2003) An objective lipodystrophy severity grading scale derived from the lipodystrophy case definition score. J Acquir Immune Defic Syndr 33:571–576
Carr A, Miller J, Law M, Cooper DA (2000) A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 14:F25–F32
Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA (1998) A syndrome of peripheral lipodystrophy, hyperlipidaemia, and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51–F58
Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ (1996) Skindex, a quality-of-life measure for patients with skin disease: Reliability, validity, and responsiveness. J Invest Dermatol 107:707–713
Coleman SR (2004) Structural fat grafting. Quality Medical Publishing, St. Louis, MO
Coleman SR (2004) Treatment of facial fat atrophy related to treatment with protease inhibitors by autologous fat injection in patients with human immunodeficiency virus infection (discussion). Plast Reconstr Surg 114:556–558
Donofrio LM (2000) Fat distribution: a morphologic study of the aging face. Dermatol Surg 26:1107–1112
Fenske NA, Lober CW (1986) Structural and functional changes of normal aging skin. J Am Acad Dermatol 15:571–585
Fried RG, Cash TF (1998) Cutaneous and psychosocial benefits of alpha hydroxy acid use. Percept Mot Skills 86:137–138
Garg A (2004) Acquired and inherited lipodystrophies. N Engl J Med 350:1220–1234
Gogolewski S, Jovanovic M, Perren SM, Dillon JG, Hughes MK (1993) Tissue response and in vivo degradation of selected polyhydroxyacids: polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). J Biomed Mater Res 27:1135–1148
Gonzalez-Ulloa M, Simonin F, Flores E (1971) The anatomy of the aging face. In: Hueston JT (ed) Transactions of the fifth international congress of plastic and reconstructive surgery. London, Butterworth and Co Ltd, pp 1059–1066
Gopinath R, Hutcheon M, Cheema-Dhadli S, Halperin M (1992) Chronic lactic acidosis in a patient with acquired immunodeficiency syndrome and mitochondrial myopathy: biochemical studies. J Am Soc Nephrol 3:1212–1219
Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ (1993) Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med 329:530–535
Grossbart TA, Sarwer DB (1999) Cosmetic surgery: surgical tools—psychosocial goals. Semin Cutan Med Surg 18:101–111
Hall DA, Blackett AD, Zajac AR, Switala S, Airey CM (1981) Changes in skinfold thickness with increasing age. Age Ageing 10:19–23
Hanke CW, Redbord KP (2007) Safety and efficacy of poly-L-lactic acid in HIV lipoatrophy and lipoatrophy of aging. J Drugs Dermatol 6:123–128
Imayama S, Braverman IM (1989) A hypothetical explanation for the aging of skin: chronologic alteration of the three-dimensional arrangement of collagen and elastic fibers in connective tissue. Am J Pathol 134:1019–1025
James J, Carruthers A, Carruthers J (2002) HIV-associated facial lipoatrophy. Dermatol Surg 28:979–986
Jones DH, Carruthers A, Orentreich D, Brody HJ, Lai MY, Azen S, Van Dyke GS (2004) Highly purified 1000-cSt silicone oil for treatment of human immunodeficiency virus-associated facial lipoatrophy: an open pilot trial. Dermatol Surg 30:1279–1286
Kakuda TN (2000) Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther 22:685–708
Klein AW (2001) Collagen substances. Facial Plast Surg Clin North Am 9:205–218, viii
Kligman AM (1989) Psychological aspects of skin disorders in the elderly. Cutis 43:498–501
Lemperle G, Gauthier-Hazan N, Lemperle M (1998) PMMA-microspheres (Artecoll) for long-lasting correction of wrinkles: refinements and statistical results. Aesth Plast Surg 22:356–365
Lemperle G, Morhenn V, Charrier U (2003) Human histology and persistence of various injectable filler substances for soft tissue augmentation. Aesth Plast Surg 27:354–366 (discussion 367)
Lenert LA, Feddersen M, Sturley A, Lee D (2002) Adverse effects of medications and trade-offs between length of life and quality of life in human immunodeficiency virus infection. Am J Med 113:229–232
Leow MK, Addy CL, Mantzoros CS (2003) Clinical review 159: human immunodeficiency virus/highly active antiretroviral therapy-associated metabolic syndrome: clinical presentation, pathophysiology, and therapeutic strategies. J Clin Endocrinol Metab 88:1961–1976
Lichtenstein KA, Ward DJ, Moorman AC, Delaney KM, Young B, Palella FJ Jr, Rhodes PH, Wood KC, Holmberg SD (2001) Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS 15:1389–1398
Little JW (2000) Volumetric perceptions in midfacial aging with altered priorities for rejuvenation. Plast Reconstr Surg 105:252–266 (discussion 286–259)
Martinez E, Mocroft A, Garcia-Viejo MA, Perez-Cuevas JB, Blanco JL, Mallolas J, Bianchi L, Conget I, Blanch J, Phillips A, Gatell JM (2001) Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet 357:592–598
Miller J, Carr A, Smith D, Emery S, Law MG, Grey P, Cooper DA (2000) Lipodystrophy following antiretroviral therapy of primary HIV infection. AIDS 14:2406–2407
Mirmirani P, Maurer TA, Berger TG, Sands LP, Chren MM (2002) Skin-related quality of life in HIV-infected patients on highly active antiretroviral therapy. J Cutan Med Surg 6:10–15
Moore K (1992) Clinically oriented anatomy, 3rd edn. Baltimore, Williams and Wilkins
Neuber F (1893) Fettransplantation. Verh Dtsch Ges Chir 22:66
Nolan D, Hammond E, Martin A, Taylor L, Herrmann S, McKinnon E, Metcalf C, Latham B, Mallal S (2003) Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS 17:1329–1338
Pessa JE, Zadoo VP, Mutimer KL, Haffner C, Yuan C, DeWitt AI, Garza JR (1998) Relative maxillary retrusion as a natural consequence of aging: combining skeletal and soft-tissue changes into an integrated model of midfacial aging. Plast Reconstr Surg 102:205–212
Rogers J, Harding C, Mayo A, Banks J, Rawlings A (1996) Stratum corneum lipids: the effect of ageing and the seasons. Arch Dermatol Res 288:765–770
Rozenbaum W, Gharakhanian S, Salhi Y, Adda N, Ngyuyen T, Vigouroux C, Capeau J (1999) Clinical and laboratory characteristics of lipodystrophy in a French cohort of HIV-infected patients treated with protease inhibitors. In: First international workshop on adverse drug reactions and lipodystrophy in HIV. San Diego, CA, p 20
Sakuraoka K, Tajima S, Seyama Y, Teramoto K, Ishibashi M (1996) Analysis of connective tissue macromolecular components in Ishibashi rat skin: role of collagen and elastin in cutaneous aging. J Dermatol Sci 12:232–237
Scott IR, Harding CR (1986) Filaggrin breakdown to water binding compounds during development of the rat stratum corneum is controlled by the water activity of the environment. Dev Biol 115:84–92
Sengelmann R, Tull S, Pollack S (2005) Soft tissue augmentation. In: Robinson J, Hanke C, Sengelmann R, Siegel D, Bhatia A, Rohrer T (eds) Surgery of the skin. Elsevier Science, Inc., St. Louis, MO
Sklar J, White S (2004) Radiance FN: a new soft tissue filler. Dermatol Surg 30:764–768
Tan CY, Statham B, Marks R, Payne PA (1982) Skin thickness measurement by pulsed ultrasound: its reproducibility, validation, and variability. Br J Dermatol 106:657–667
The American Society for Aesthetic Plastic Surgery (2006) Quick Facts: highlights of the ASAPS 2006 statistics on cosmetic surgery. American Society for Aesthetic Plastic Surgery. Retrieved 20 November 2007 at http://www.surgery.org/download/2006QFacts.pdf
Tontonoz P, Hu E, Spiegelman BM (1995) Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev 5:571–576
Tzikas TL (2004) Evaluation of the Radiance FN soft tissue filler for facial soft tissue augmentation. Arch Facial Plast Surg 6:234–239
Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoen H, Bousquet R, Katz P, Costagliola D, Katlama C (2003) Polylactic acid implants (New-Fill) to correct facial lipoatrophy in HIV-infected patients: results of the open-label study VEGA. AIDS 17:2471–2477
Vleggaar D, Bauer U (2004) Facial enhancement and the European experience with poly-L-lactic acid. J Drugs Dermatol 3:526–530
Zimbler MS, Kokoska MS, Thomas JR (2001) Anatomy and pathophysiology of facial aging. Facial Plast Surg Clin North Am 9:179–187, vii
Acknowledgments
The authors thank Andrew Owen, MSc, Medicus International, for his editorial assistance. Editorial support for this article was provided by Dermik Laboratories, a business of Sanofi-Aventis U.S., LLC. The opinions expressed in the current article are those of the authors. The authors received no honoraria or other form of financial support related to the development of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coleman, S., Saboeiro, A. & Sengelmann, R. A Comparison of Lipoatrophy and Aging: Volume Deficits in the Face. Aesth Plast Surg 33, 14–21 (2009). https://doi.org/10.1007/s00266-008-9258-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9258-z