Abstract
Background
Highly active antiretroviral therapy (HAART) is associated with facial lipoatrophy, which is potentially stigmatizing for HIV-positive patients. We assessed the long-term effects of polyalkylimide gel injections on the severity of lipoatrophy and quality of life of patients on HAART.
Methods
A prospective study was performed of 17 HIV-positive patients with grade 2 and grade 3 facial lipoatrophy. A mean volume of 14 cc of polyalkylimide gel (Bio-Alcamid™) was injected subcutaneously at one or more sites of the face in a single stage. Each patient was seen for follow-up after 3, 12, 24, and 48 weeks. The severity of lipoatrophy and the quality of life was assessed using a self-report questionnaire containing the relevant parts of the SF-36, MOS-HIV, and CES-D.
Results
The severity of facial lipoatrophy decreased significantly from baseline over 48 weeks. Quality of life improved significantly from baseline over 48 weeks for mental health and social functioning. Depression at week 48 was significantly correlated with the extent to which lipoatrophy had disappeared at week 48.
Conclusion
Individualized volumes of polyalkylimide gel injected in one session significantly decreased the subjective severity of lipoatrophy and improved the quality of life of HIV-positive patients with grade 2 and 3 lipoatrophy, even in the four patients who had complications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The introduction of highly active antiretroviral therapy (HAART) for HIV-1 infection has been accompanied by a marked reduction in HIV-related morbidity and mortality [1]. However, prolonged HAART has been associated with the lipodystrophy syndrome [2]. The physical features of this syndrome include peripheral lipoatrophy of the face, limbs, and buttocks and central subcutaneous fat accumulation of the abdomen, breasts, and dorsocervical spine. Lipoatrophy and lipoaccumulation may concomitantly present in the same individual. The overall reported prevalence of at least one physical feature of this syndrome is 50% after 12–18 months of HAART. Of these, facial lipoatrophy in particular is disfiguring and potentially stigmatizing because affected individuals usually have good virologic and immunologic response to HAART and feel healthy but often appear quite the opposite. Previous studies have shown that lipodystrophy syndrome is associated with a reduced well-being and quality of life [3–5]. Moreover, it may hinder adherence to antiretroviral treatment, thereby reducing its effectiveness [4, 5].
No definite cure for lipodystrophy syndrome is available so far [2], but various attempts have been made to diminish the characteristic facial wasting. As such, structured interruption of HAART [6], switching HAART regimens [7–9], other pharmacologic approaches such as glitazones [9, 10], and a growth hormone secretagogue (GHRH) [9, 11] have been tried without adequate results. Other options like dermal fat grafts [12, 13], submalar silicon implants [12], autologous fat injections [13, 14], injections of resorbable filler materials [9, 12, 15, 16], and injections of liquid, permanent filler materials have been used to treat facial lipoatrophy [9, 15, 17–23]. In a previous study we suggested that polyalkylimide gel (PAIG, Bio-Alcamid™, Polymekon Biotech Industry, Milan, Italy) may suit the purpose of correcting HAART-related facial lipoatrophy [24]. Polyalkylimide gel is a nonresorbable, nondegradable filler that may be used in high volumes [22]. To assess our suggestion, we initiated a prospective study on the long-term effects on the severity of lipoatrophy and the quality of life of a single-stage treatment with polyalkylimide gel injections in HIV-positive patients.
Patients and Methods
Between December 2004 and December 2005, we screened 24 consecutive HIV-positive patients who were treated at the outpatient clinic of the Department of Internal Medicine in the Onze Lieve Vrouwe Gasthuis as potential candidates for our study. Eligible patients were older than 18 years, had lipoatrophy of the face, and were on a HAART regimen containing at least three antiretroviral agents from two different classes (triple antiretroviral therapy). Exclusion criteria were grade 1 lipoatrophy according to the grading system proposed by Hillebrand et al. [25]. With these criteria, 1 female and 16 male patients were included in this prospective study (Table 1). Written informed consent was obtained from all patients.
Procedure
Patients on anticoagulation therapy were required to interrupt this therapy 3 days before treatment through 2 days after. Preoperatively, all patients were given a single dose of claritromicin 1000 mg orally (Klacid SR, Abbott BV, Hoofddorp, The Netherlands). The injection sites were infiltrated with lidocaine 2% and epinephrine 1:100.000. Using an 18-gauge, 1.5–in. needle, polyalkylimide gel (Bio-Alcamid™, Polymekon Biotech Industry, Milan, Italy) was bilaterally injected subcutaneously at one or more sites of the face as a single-stage procedure. All injections were done by one physician (LvR) experienced in the use of polyalkylimide to prevent bias. The total amount of injected material ranged from 5 to 31.5 cc (mean = 14 cc) per patient. Patients were instructed not to touch the injected sites for the first 4 hours after injection and to avoid high pressure at these sites for 3 weeks. They were allowed to wash, shave, and use makeup but were advised to avoid the sun for 4 weeks.
Assessment of Outcome
The patients were seen for follow-up at the outpatient clinic after 3, 12, 24, and 48 weeks. At each follow-up visit a clinical evaluation was performed for possible complaints or complications and for the stability of the effect of injections, and quality of life was assessed.
Patients were asked to rate the severity of their current facial lipoatrophy on a six-point scale ranging from “very severe” to “absent,” to rate the extent to which their facial lipoatrophy had disappeared on a five-point scale ranging from “completely” to “not at all,” and to rate their satisfaction with the result of polyalkylimide gel injections on a five-point scale ranging from “very satisfied” to “very dissatisfied.” They were also asked if they considered additional treatment of their facial lipoatrophy necessary (yes/no).
Quality of life (QoL) was assessed using a self-report questionnaire with proven reliability and validity. Because we had anticipated that facial lipoatrophy would predominantly affect the patients’ psychological functioning and their ability to participate in social and occupational activities, the questionnaire included the social functioning, role functioning–physical, role functioning–emotional, and mental health dimension of the Short Form 36-item health survey (SF-36) [26]. In addition, it included the social functioning and the role functioning dimensions of the Medical Outcomes Study health survey for HIV (MOS-HIV) [27]. Each dimension of the SF-36 and the MOS-HIV was scored from 0 to 100, with higher scores indicating better QoL. Possible feelings of depression were measured using the 20-item Center for Epidemiological Studies Depression scale (CES-D) [28]. CES-D scores may range from 0 to 60, with higher scores indicating more severe depression. Scores higher than 16 are indicative of depressive symptoms.
Statistical Analysis
Data analysis was conducted using SPSS software version 11 for Windows (SPSS Inc., Chicago, IL, USA). We compared baseline QoL measurements with QoL measurements after 48 weeks using Wilcoxon tests for paired samples. If a statistically significant effect was found, we additionally conducted post hoc Wilcoxon paired-samples tests comparing baseline QoL with the QoL measurements after 3, 12, and 24 weeks. This was done to avoid type 1 errors resulting from multiple testing. To investigate the impact of complications of polyalkylimide gel injections on QoL, the QoL at week 48 of patients who had an eventful follow-up was compared with that of patients without complications by use of Wilcoxon tests for independent samples. Two-sided p values less than 0.05 were considered to indicate statistical significance.
The correlation between depression and both the severity of self-reported lipoatrophy and the extent to which lipoatrophy had disappeared at week 48 was assessed using Spearman correlation coefficients.
Results
During follow-up, 4 of the 17 patients (23.5%) reported a complication. These complications varied from capsule formation or gel migration that did not need additional intervention (n = 3) to an infection at one injection site necessitating surgical drainage (n = 1). Occurrence of complications was not correlated with age, body mass index, viral load, CD-4 cell count, grade of facial lipoatrophy, location of injection, or volume of injected polyalkylimide gel.
At week 48, 16 patients indicated the extent to which facial lipoatrophy had disappeared. This was regarded as “completely” by three patients (0.19), “largely” by seven patients (0.44), “about half” by three patients (0.19), “a little” by two patients (0.13), and as “hardly to not at all” by one patient (0.06) (Table 2). Nine patients (0.56) were very satisfied or satisfied with the final result, four patients were neither satisfied nor dissatisfied (0.25), two were dissatisfied (0.13), and one was very dissatisfied (0.06). Nine of 14 patients (64.3%) reported that in their opinion additional treatment would still be necessary.
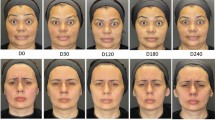
The reported severity of facial lipoatrophy decreased significantly from baseline over 48 weeks (Wilcoxon test, p = 0.002). The median (IQR) reported severity of facial lipoatrophy at week 48 was mild (very mild–moderate). Post hoc tests revealed that the decrease in severity of lipoatrophy from baseline was statistically significant from week 3 onward (Fig. 1). Patients who previously had complications considered their lipoatrophy to be more severe (p = 0.058) and reported their lipoatrophy to have disappeared to a lesser extent (p = 0.058) at week 48.
(a) Facial appearance before (left) and 48 weeks after (right) polyalkylimide injection in a 44-year-old patient with grade 2 lipoatrophy with a total of 14 cc injected apical and distal in the nasolabial fold. (b) A 47-year-old patient with grade 3 lipoatrophy with 24 cc injected in the nasolabial fold and temporal region. (c ) A 48-year-old patient with grade 3 lipoatrophy with 23.5 cc injected in the nasolabial fold and temporal region
Quality of mental health (Wilcoxon test, p = 0.049), and social functioning (Wilcoxon test, p = 0.046) improved significantly from baseline over 48 weeks (Table 3). Post hoc tests revealed that the improvement in mental health became apparent only at week 48, whereas social functioning tended to improve from week 12 onward (p = 0.084).
Depression tended to decline from baseline over 48 weeks (Wilcoxon test, p = 0.069). Post hoc tests revealed that depression tended to decline from baseline to week 3 (Wilcoxon test, p = 0.090), but the decline in depression was most pronounced at week 24 (Wilcoxon test, p = 0.018). Depression at week 48 was significantly correlated with the extent to which lipoatrophy had disappeared at week 48 (Spearman correlation = 0.56, p = 0.031) (Fig. 2) and with the severity of lipoatrophy at week 48 (Spearman correlation = 0.59, p = 0.022).
Correlation between feelings of depression as scored by the CES-D scale (Y axis) [28] and the extent to which the facial lipoatrophy had been corrected after 48 weeks according to the patients (X axis)
Discussion
This study shows that individualized amounts of polyalkylimide gel injected in a single session may significantly decrease the subjective severity of grade 2 and grade 3 facial lipoatrophy in HIV-positive patients on HAART. It improved quality of life with respect to mental health and social functioning, even in the four patients in whom complications occurred.
Other injectable permanent fillers such as liquid silicone [17], polyactic acid (Newfill™, Sculptra™) [15, 18–20], polymethylmethacrylate (Artecoll™) [9], polyacrylamide (Aquamid™) [9, 20], or calcium hydroxylapatite (Radiesse™) [21] have been used to treat facial lipoatrophy. However, because larger quantities of polyalkylimide compared with other fillers can be injected in a single stage and based on vast experience of its use with healthy subjects, we suggested its use to correct lipoatrophy in HIV-positive subjects [24]. Such use has been studied by others [22, 23, 29], but these studies did not feature standardized assessment of the aesthetic and psychosocial outcome or a one-stage setting of injections.
Unlike others [22, 23, 29], we encountered minor complications in three of our patients and a major complication necessitating additional surgery in one. This may be explained by the higher total volumes injected and by our application of a single prophylactic dose of claritromicin instead of a 3–7-day antibiotic course. That complications occur after polyalkylimide gel injections has been reported previously by both our group [24] and others [30].
Autologous fat injection may be a better alternative because it lacks the complications of injectable fillers [13, 14], but this technique is not always available. At the start of our study sufficient clinical experience in the use of fat injections as part of the study was not available to our patients in Amsterdam. Future studies should compare the outcome of such autologous injections with that of alloplastic injections in these patients. Provided that a larger study population would be included, it would then be possible to statistically distinguish between the outcomes in patients with various characteristics and to further assess risk factors for an eventful outcome. Meanwhile, we conclude that polyalkylimide gel injections should be considered an addition to the armamentarium for treatment of facial lipoatrophy in HIV-positive patients.
References
Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860
Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, Cooper DA (1998) A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS 12:F51–F58
Oette M, Juretzko P, Kroidl A, Sagir A, Wettstein M, Siegrist J, Häussinger D (2002) Lipodystrophy syndrome and self-assessment of well-being and physical appearance in HIV-positive patients. AIDS Patient Care STDS 16:413–417
Duran S, Saves M, Spire B, Cailleton V, Sobel A, Carrieri P, Salmon D, Moatti JP, Leport C; APROCO Study Group (2001) Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS 15:2441–2444
Ammassari A, Antinori A, Cozzi-Lepri A, Trotta MP, Nasti G, Ridolfo AL, Mazzotta F, Wu AW, d’Arminio Monforte A, Galli M; AdICoNA Study Group. LipoICoNA Study Group (2002) Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr 31(Suppl 3):S140–S144
Hatano H, Miller KD, Yoder CP, Yanovski JA, Sebring NG, Jones EC, Davey RT Jr (2000) Metabolic and anthropometric consequences of interruption of highly active antiretroviral therapy. AIDS 14:1935–1942
Lafeuillade A, Clumeck N, Mallolas J, Jaeger H, Livrozet JM, Ferreira Mdo S, Johnson M, Cheret A, Antoun Z; European Trizal team (2003) Comparison of metabolic abnormalities and clinical lipodystrophy 48 weeks after switching from HAART to Trizivir versus continued HAART: the Trizal study. HIV Clin Trials 4:37–43
John M, McKinnon EJ, James IR, Nolan DA, Herrmann SE, Moore CB, White AJ, Mallal SA (2003) Randomized, controlled, 48-week study of switching stavudine and/or protease inhibitors to combivir/abacavir to prevent or reverse lipoatrophy in HIV-infected patients. J Acquir Immune Defic Syndr 33:29–33
Jones D (2005) HIV facial lipoatrophy: causes and treatment options. Dermatol Surg 31:1519–1529
Carr A, Workman C, Carey D, Rogers G, Martin A, Baker D, Wand H, Law M, Samaras K, Emery S, Cooper DA; Rosey investigators (2004) No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet 363:429–438
Koutkia P, Canavan B, Breu J, Torriani M, Kissko J, Grinspoon S (2004) Growth hormone-releasing hormone in HIV-infected men with lipodystrophy: a randomized controlled trial. JAMA 292:210–218
Talmor M, Hoffman LA, LaTrenta GS (2002) Facial atrophy in HIV-related fat redistribution syndrome: anatomic evaluation and surgical reconstruction. Ann Plast Surg 49:11–18
Strauch B, Baum T, Robbins N (2004) Treatment of human immunodeficiency virus-associated lipodystrophy with dermafat graft transfer to the malar area. Plast Reconstr Surg 113:363–370; discussion 371–372
Levan P, Nguyen TH, Lallemand F, Mazetier L, Mimoun M, Rozenbaum W, Girard PM (2002) Correction of facial lipoatrophy in HIV-infected patients on highly active antiretroviral therapy by injection of autologous fatty tissue. AIDS 16:1985–1987
Moyle GJ, Lysakova L, Brown S, Sibtain N, Healy J, Priest C, Mandalia S, Barton SE (2004) A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med 5:82–87
Walther RA (2002) [Facial lipodystrophy in patients with HIV infections troublesome to treat]. Lakartidningen 99:3826–3829
Orentreich D, Leone AS (2004) A case of HIV-associated facial lipoatrophy treated with 1000-cs liquid injectable silicone. Dermatol Surg 30:548–551
Valantin MA, Aubron-Olivier C, Ghosn J, Laglenne E, Pauchard M, Schoen H, Bousquet R, Katz P, Costagliola D, Katlama C (2003) Polylactic acid implants (New-Fill) to correct facial lipoatrophy in HIV-infected patients: results of the open-label study VEGA. AIDS 17:2471–2477
Onesti MG, Renzi LF, Paoletti F, Scuderi N (2004) Use of polylactic acid in face lipodystrophy in HIV positive patients undergoing treatment with antiretroviral drugs (HAART). Acta Chir Plast 46:12–15
Negrado E, Higueras C, Adell X, Martinez JC, Martinez E, Puig J, Fumaz CR, Muñoz-Moreno JA, Perez-Alvarez N, Videla S, Estany C, Cinquegrana D, Gonzalez-Mestre V, Clotet B (2006) Reconstructive treatment for antiviral-associated facial lipoatrophy: a prospective study comparing autologous fat and synthetic substances. AIDS Patient Care STDS 20:829–837
Comite SL, Liu JF, Balasubramanian S, Christian MA (2004) Treatment of HIV-associated facial lipoatrophy with Radiance FN (Radiesse). Dermatol Online J 10:2
Protopapa C, Sito G, Caporale D, Cammarota N (2003) Bio-Alcamid in drug-induced lipodystrophy. J Cosmet Laser Ther 5:226–230
Loutfy MR, Raboud JM, Antoniou T, Kovacs C, Shen S, Halpenny R, Ellenor D, Ezekiel D, Zhao A, Beninger F (2007) Immediate versus delayed polyalkylimide gel injections to correct facial lipoatrophy in HIV-positive patients. AIDS 21:1147–1155
Karim RB, Hage JJ, van Rozelaar L, Lange CA, Raaijmakers J (2006) Complications of polyalkylimide 4% injections (Bio-Alcamid): a report of 18 cases. J Plast Reconstr Aesthet Surg 59:1409–1414
Hillebrand ME, Strackee SD, van Ulden BJG, ten Veen JH, van Dalen J (2002) Behandeling van lipoatrofie in het gelaat met L-polymelkzuur: de PLA-studie. Nederl Tijdschr Geneeskd 146:2274–2275
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 30:473–483
Wu AW, Rubin HR, Mathews WC, Ware JE Jr, Brysk LT, Hardy WD, Bozzette SA, Spector SA, Richman DD (1991) A health status questionnaire using 30 items from the Medical Outcomes Study. Preliminary validation in persons with early HIV infection. Med Care 29:786–798
Radloff LS (1977) The Ces-D scale: a self-report depression scale for research in the general population. Appl Psychol Measures 1:385–401
Ramon Y, Fodor L, Ullmann Y (2007) Preliminary experiences with Bio-Alcamid in HIV facial lipoatrophy. Dermatosurgery 214:151–154
Christensen L, Breiting V, Janssen M, Vuust J, Hogdall E (2005) Adverse reactions to injectable soft tissue permanent fillers. Aesthetic Plast Surg 29:34–48
Acknowledgments
We are grateful to Rosa Regez and Lucy Schrijnders at the Department of Clinical Research of Internal Medicine for their enthusiastic assistance in selecting the patients and providing the clinical information, and to Nicole Receveur-van Zanten at our outpatient clinic for her voluntary help and dedication. We thank Polymekon, Italy, for providing Bio-Alcamid™ free of charge to the participants of our study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karim, R.B., de Lint, C.A., van Galen, S.R. et al. Long-Term Effect of Polyalkylimide Gel Injections on Severity of Facial Lipoatrophy and Quality of Life of HIV-Positive Patients. Aesth Plast Surg 32, 873–878 (2008). https://doi.org/10.1007/s00266-008-9189-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00266-008-9189-8