Abstract
The pace-of-life syndrome (POLS) is a framework that attempts to explain empirically observed covariation between physiological, behavioral, and life history traits, whereby individuals fall along slow-fast and shy-bold continuums. The fundamental driver of the position of individuals along these trait axes is thought to be their metabolic rates, with high metabolism leading to faster growth, greater reproductive output, and bolder behavior. However, numerous exceptions to these patterns have been observed in nature, suggesting that crucial components are missing from the classical POLS framework. As many metabolic, physiological, and life history traits are temperature dependent, a growing number of studies have begun to test the role played by the thermal physiology of individuals and the thermal environments in which they live in mediating the trait relationships within POLS. These studies have led to an expansion of classical POLS into what has been called “extended POLS.” Here, we review the recent literature on extended POLS and identify the major themes and patterns that are emerging in this nascent field. We further identify gaps and key outstanding questions in how temperature may drive or modify classical POLS. Finally, we address issues with how temperature and POLS are integrated in empirical studies and suggest pathways by which progress can be made towards a cohesive understanding of the physiology-behavior-life history nexus.
Significance statement
The pace of life syndrome (POLS) is an integrative framework that links life-history, behavioral, and physiological traits into covarying axes that are structured by metabolism. Recent studies have provided only mixed support for the original POLS hypothesis and instead have highlighted the potential importance of thermal physiology in explaining patterns of trait covariation. We review this nascent literature and argue that environmental temperature, the thermal sensitivity of traits, and acclimation to thermal environments can influence the presence and/or direction of trait covariations within individuals or populations. Though some patterns have emerged in the recent POLS literature, important remaining gaps are slowing progress in this field. We suggest avenues by which future investigations can test the proximate and ultimate mechanisms underlying trait covariation in wild animal populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organisms are assemblages of many traits, each of which may be adapted to different aspects of the local environment. Many of these traits do not evolve independently and instead are co-adapted into “complex phenotypes.” For example, historical understanding of life history evolution revolved around the concept of r-K selection, in which life history traits, such as growth rate, age at maturity, and reproductive output, covary along a “slow-fast” axis (Pianka 1970). In other words, individuals that grow quickly and mature at an early age should also produce more offspring per unit time. Although researchers no longer strictly adhere to the r-K selection framework, covariation among life history traits is nevertheless a ubiquitous feature of the natural world (e.g., the well-established trade-off between offspring quantity and quality, Smith et al. 1989; but see Beldade et al. 2012). Other types of traits, such as behavioral and morphological traits, can also occur in co-adapted complexes. Male side-blotched lizards (Uta stansburiana) come in three genetically determined throat color morphs, each of which is associated with distinct behaviors for sexual display and territory guarding (Sinervo et al. 2001). Anolis ecomorphs in the Greater Antilles are distinguished by sets of behavioral and morphological traits which are characteristic of the particular habitats they occupy (Irschick and Losos 1996; Losos 2011). We have a deep understanding of the agents of selection that favor covariation among some well-known life history traits, and between sets of morphological and behavioral traits in specific taxa. However, broader suites of traits (e.g., physiology, behavior, and life history) often covary within populations, and both the proximate and ultimate mechanisms underlying these patterns remain unresolved.
A far-reaching framework has been proposed that links trait covariation across physiology, life history, and behavior (Montiglio et al. 2018). This framework, referred to as the “pace-of-life syndrome” (or POLS), has the potential to be a unifying theory in the field of trait coadaptation and the evolution of complex phenotypes. Interest in POLS has burgeoned in the last several years as a growing number of species have been identified which show patterns consistent with POLS. Regardless, several studies have identified notable exceptions to POLS (see Royauté et al. 2018), and both the proximate and ultimate causes of POLS remain unresolved. In this review, we highlight a recent extension of the classical POLS hypothesis, called “extended POLS,” which expands the model’s framework to include the thermal dependence of life history, behavioral, and physiological traits. Furthermore, we identify emerging patterns and outstanding questions in this literature and suggest avenues of future research that might improve the generality of the POLS framework.
The POLS framework
The POLS framework describes axes of trait covariation among individuals, populations, or species. POLS was first developed as a way to conceptualize correlations between life history and physiology, such that individuals (or populations) were categorized as occurring along a slow-fast axis, whereby “slow” individuals have lower metabolic and growth rates, and reduced reproductive output, relative to “fast” individuals (Ricklefs and Wikelski 2002). On its own, the slow-fast axis is similar to the concept of r-K selection. Subsequently, POLS expanded to include a behavioral “shy-bold” axis (Stamps 2007; Biro and Stamps 2008; Careau et al. 2008; Réale et al. 2010). “Shy” individuals are those that are less aggressive and exploratory, and more social, compared to “bold” individuals. Within POLS, not only do traits vary along these axes but also the slow-fast and shy-bold axes are predicted to covary with each other such that there is an overarching slow/shy to fast/bold continuum (Fig. 1). As with many theories that attempt to explain complex biological phenomena, POLS has been developed piecemeal, with traits added over time and interpretations of the framework varying from study to study. This has led to uncertainty in the literature over exactly which traits should be considered part of the framework or even on how to define POLS. Nevertheless, a common theme among researchers who study POLS-related phenomena is that metabolism is the fundamental lynchpin that links life history with behavior (Careau and Garland 2012). Therefore, we define POLS as a conceptual framework predicated on the idea that physiological, behavioral, and life history traits covary, via their relationships with metabolism, along slow-fast and shy-bold axes.
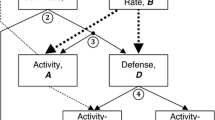
Conceptual links within the extended POLS framework. The classical POLS hypothesis (blue box) links metabolic physiology with life history and behavior, whereas the extended POLS hypothesis (orange box) includes the role of the thermal environment and thermal physiology in modulating animal behavior via the thermal behavioral syndrome (TBS). The arrows represent directions of mechanistic links between trait categories. Finally, a truly comprehensive POLS hypothesis (light gray box) might integrate the roles of a myriad factors (or explain under which ecological contexts data are likely to violate POLS)
The ultimate (evolutionary) mechanism presumably driving POLS is correlational selection favoring phenotypic integration (Réale et al. 2010; Royauté et al. 2018). Correlational selection occurs when particular combinations of trait values are favored in the local environment (Sinervo and Svensson 2002). Thus, an individual’s fitness cannot be predicted based on the value of a single trait, but instead depends on the values of one or more additional traits. In the case of POLS, it is thought that individuals with high metabolic rates and bold behavior (or slow metabolism and shy behavior) are favored over individuals with high metabolic rates and shy behavior (or slow metabolism and bold behavior). One example of this is the olive flounder fish (Paralichthys olivaceus), where bold individuals have higher aerobic scope, maximum metabolic rate, and standard metabolic rate, which permit fighting against or quickly escaping from a threat (which they are more likely to encounter because they are bold; Rupia et al. 2016). By contrast, shy flounders have increased survival if they have lower metabolic rates because they remain still and undetectable until the threat passes and do not waste energy on maintaining a high metabolism. Similar patterns have been observed in other systems ( McKenzie et al. 2015; Myles-Gonzalez et al. 2015; Binder et al. 2016). Over time, correlational selection on these trait combinations might lead to genetic covariance via proximate mechanisms like linkage disequilibrium (physical proximity on a chromosome) and pleiotropy (one gene affecting multiple traits), which could in turn constrain the potential for these populations to evolve away from POLS.
A wide range of studies in diverse taxa have found trait associations that follow at least some predictions of POLS (Biro and Stamps 2008; Careau et al. 2008; Ariyomo and Watt 2011; Pettersen et al. 2016; Auer et al. 2018). For example, recent work on marine gastropods (Littoraria irrorata) showed that metabolic and somatic growth rates were higher in bolder individuals (Cornwell et al. 2020). However, a number of studies have shown only partial support while others have directly contradicted the predictions of POLS. Indeed, a meta-analysis of POLS studies showed only mixed support for the hypothesis (Royauté et al. 2018). Research has found that correlations between traits can differ between the sexes, and evidence for POLS is found more frequently in invertebrate systems compared to vertebrate systems (Royauté et al. 2018). Additionally, some studies have found only weak associations between metabolic, behavioral, and life history traits (Le Galliard et al. 2013) or no associations at all (Royauté et al. 2015). Other investigations have shown that boldness and growth rates covary in the opposite direction from that predicted by POLS (Adriaenssens and Johnsson 2011) or that the direction of trait correlations depend on context (Niemelä et al. 2012; Krams et al. 2014; Mathot et al. 2015). This breadth of prior work on POLS across many species strongly indicates that the hypothesis’s current formulation does not capture the full panoply of trait covariation in nature, indicating that the conceptual framework is incomplete.
Metabolism, temperature, and the thermal behavioral syndrome
POLS posits a crucial role for metabolism as the lynchpin that links physiology with both life history and behavior. However, there are different ways that metabolism can be considered in POLS investigations, and the choice of metabolic trait may affect whether and how trait covariation is detected. POLS studies have tested the role of metabolism by measuring basal metabolic rate, resting metabolic rate, and maximal metabolic rate, or even by integrating resting and maximal metabolic rate to estimate aerobic scope, although rarely are more than one measure of metabolism included in the same study. As such, there is ongoing debate around which measures of metabolism are valid proxies for energetic constraints when linking behavior to energetics ( Careau and Garland 2015; Mathot and Dingemanse 2015a, b). Here, we use the terms “metabolism” and “metabolic rate” as umbrella terms that encompass any estimate of metabolism reported in a given study, although in many cases, we specify which form of metabolism was measured.
Irrespective of the form of metabolism being considered, it is highly thermally sensitive because the enzymatic reactions involved in metabolic pathways have lower activation energy at higher temperatures (Hochachka and Somero 2002). This observation has led to the suggestion that temperature (and variation in thermal physiology among individuals) may be a key missing component to POLS. For example, individuals maintaining higher body temperatures should also have higher metabolic rates which should translate to greater scope for activity or exploration because the latter traits require energetic expenditure (Clarke and Fraser 2004). This is particularly pertinent for ectotherms, as their metabolic rates increase exponentially with body temperature (unlike endotherms that have a thermal neutral zone). It should also be noted that while temperature, through metabolism, might have indirect effects on both life history and behavioral traits (Rocha and Bergallo 1990; Ciota et al. 2014; Forsatkar et al. 2016), temperature can also affect life history and behavior directly, via its effects on processes such as muscle fiber contraction and protein synthesis (Pörtner 2002).
Regardless, the fact that animal physiology, life history, and behavior are all thermally sensitive suggests that an explicit consideration of the relationships between POLS traits and temperature might be needed to explain the plethora of results that are inconsistent with the classical hypothesis. The growing acknowledgement that temperature may play a crucial role in modulating trait relationships within POLS has led to the development of a new concept known as the thermal-behavioral syndrome (TBS). Goulet et al. (2017a) posited that physiological traits covary along a “cold-hot” axis, much as classical POLS suggests that individuals can be classified as slow/fast or shy/bold with respect their life history and behavioral traits, respectively. In TBS, a “cold” individual is one that selects and performs best at lower body temperatures, whereas “hot” individuals select and perform best at higher body temperatures (Fig. 1). Goulet et al. (2017a) suggested that thermal type might remain fixed across internal (e.g., digestion) or external (e.g., phenology) conditions and supported this hypothesis with a laboratory-based experiment on delicate skinks (Lampropholis delicata). They found that the thermal types of individuals stayed consistent after 9 weeks of laboratory acclimation. Cold skinks consistently selected lower body temperatures and had lower thermal optima for performance, while hot skinks selected high body temperatures and had high thermal optima. In a follow-up experiment using the same system, Michelangeli et al. (2017) found that thermal type influenced habitat use such that hot and cold skinks occupied hotter and colder microhabitats, respectively. A third study by this group (Goulet et al. 2017b) demonstrated that the shy-bold axis covaried with the cold-hot axis, such that hot individuals had higher sprint speeds, greater activity levels, and increased exploratory behaviors. Goulet and colleagues termed this expansion of POLS to include thermal considerations “extended POLS,” and a growing number of studies have subsequently explored the role of temperature and thermal physiology in trait covariation across a range of species (Fig. 2).
Emerging patterns in the extended POLS literature
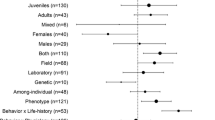
It is widely accepted that metabolic, life history, and behavioral traits are thermally sensitive (Clarke and Fraser 2004; Shine 2005; Abram et al. 2017), but it is less known if and how temperature mediates trait covariation. We conducted a review using Web of Science (years 1965–2022) with the following search term combinations: “pace of life AND temperature,” “temperature AND personality,” “temperature AND life history,” and “temperature AND metabolism AND pace of life.” We then ran a separate set of searches but replaced the term “temperature” with “thermal.” All results were ordered by relevance, and we examined the first 100 results of each search. We included papers if they tested at least one POLS trait in association with either a temperature treatment or a thermophysiological trait, and we only included papers on ectothermic organisms. These searches resulted in a total of 51 papers that we compiled for this review (Table 1; Fig. S1). Below, we outline the major patterns that are emerging from these studies.
Support for covariation between slow-fast, shy-bold, and cold-hot axes is limited, at best
Only 25.5% of the papers considered for this review explicitly examined covariation between POLS traits and thermal traits (e.g., body temperature, thermal tolerance, thermal preference, or a thermal performance curve), and less than half of those studies (6 of 13; Stapley 2006; Rey et al. 2015; Cerqueira et al. 2016; Goulet et al. 2017b; Kashon and Carlson 2017; Michelangeli et al. 2017) found clear support for extended POLS. While these studies showed that individuals who prefer or maintain higher body temperatures are also bolder, the opposite result was found in two additional studies (Goulet et al. 2018; Enders et al. 2019), where bolder individuals selected lower body temperatures. Finally, Horváth et al. (2020) found a weak link between preferred body temperatures and risk-taking, but a strong correlation between preferred body temperatures and activity in Carpetane rock lizards (Iberolacerta cyreni). Within the delicate skink system in which so much of this work has been done, there is limited support for the alignment of the behavioral and thermal trait axes, although Goulet et al. (2018) indicated that a difference in sample sizes may have been the reason why bolder individuals preferred higher temperatures in one study (Goulet et al. 2017b) but not the other (Goulet et al. 2018). Importantly, all the studies referenced in this section tested for covariation between the cold-hot and shy-bold axes but did not consider life history traits or account for the effects of metabolism. In fact, the role of metabolism was only investigated in 2 of 13 studies. These studies contradicted the predictions of extended POLS, finding that (1) there was no relationship between resting metabolism and thermal preference in common lizards (Zootoca vivipara; Artacho et al. 2013) and (2) fast individuals had lower thermal optima for metabolic performance in damselfly larvae (Ischnura elegans; Tüzün and Stoks 2022). Life history traits were only considered in three studies on insects, and these again showed only mixed support for extended POLS depending on the combination of thermal and life history trait examined (Boher et al. 2010; Struelens et al. 2018; Tüzün and Stoks 2022). Most studies testing extended POLS focused on fish (35.3%), followed by insects (23.5%) and reptiles (21.6%). Of the 13 studies that explicitly tested thermal traits, the most commonly assessed trait was thermal preference (69%), with critical thermal limits, voluntary thermal limits, and thermal optima tested in only one study each.
Acclimation to different thermal regimes affects POLS traits, but often in inconsistent ways
Of the studies included in our review, 38 tested whether one or more POLS traits differed across temperature treatments. Of the 20 studies that involved long-term acclimation treatments on adult individuals (e.g., acute, rather than developmental, plasticity), there were seven that supported, ten that partially supported, and three that did not support extended POLS. Several experiments showed that individuals acclimated to higher environmental temperatures had faster life-histories (e.g., higher fecundity, shorter lifetimes, and higher growth rates; Karlsson and Wiklund 2005; Ciota et al. 2014; Wenjie et al. 2019; Günter et al. 2020) or were bolder (e.g. increased activity and exploration; Forsatkar et al. 2016; Magellan et al. 2019; Culumber 2020). Other analyses provided partial support for the role of thermal acclimation, reporting evidence of trait covariation along POLS axes but with some behaviors (Segev et al. 2017; White et al. 2020; Le Roy et al. 2021), life history traits (Junge-Berberović 1996; Frost et al. 2013; Rutschmann et al. 2016), and metabolism (Moffett et al. 2022) deviating from the predictions of extended POLS. For example, ants (Temnothorax longispinosus) acclimated to warm temperatures were not only more exploratory but also less aggressive than those that were acclimated to cool conditions (Segev et al. 2017). Three studies that detected partial support highlighted factors that may have influenced why trait covariations deviated from POLS predictions including how effects of colony composition may influence behavioral types (Goulet et al. 2016) and how behavioral types may fluctuate in repeatability or converge at an intermediate type with increasing temperatures (Maskrey et al. 2020, 2021). A few studies on fish either showed no support for an association between acclimation temperature and behavior (Poecilia reticulata, Lukas et al. 2021; Zacco platypus, Tang and Fu 2021) or they found that populations acclimated to cold environments had faster life histories, higher metabolism, and higher activity (Fundulus heteroclitus, Chung et al. 2018).
In addition to studies of acclimation in adult animals, some researchers have examined developmental plasticity, focusing on the effect of temperature during incubation and rearing. Growth rate was frequently lower when individuals were incubated or reared in cold environments (Gangloff et al. 2015; Brans and De Meester 2018; Debecker and Stoks 2019; Betini et al. 2020; Carbonell and Stoks 2020; Carbonell et al. 2021). However, higher rearing temperatures were associated with higher metabolic rates but slower growth in lake trout (Salvelinus namaycush, Hébert and Dunlop 2020). Another study found that the highest growth rates of jacky dragons (Amphibolurus muricatus) were at an intermediate temperature and that extreme temperatures resulted in slower growth (Warner et al. 2010). The studies that looked at the effects of rearing temperature on behavior had mixed results, with some showing higher exploration and sociability at higher temperatures (Niemelä et al. 2019; Pilakouta et al. 2023), while others showed higher exploration but similar latencies to emerge amongst treatment groups (Li et al. 2021), higher activity or exploration at lower temperatures (Trnik et al. 2011; Závorka et al. 2020), or rearing temperature of juveniles having little effect on the behavior of adults (Dalesman and Rundle 2010).
The thermal sensitivity of POLS traits frequently varies among individuals in a population, and the position of an individual along a given POLS axis is often repeatable
Within a population of damselfish (Pomacentrus bankanensis), activity rates of some individuals dramatically increased with temperature, while activity rates were not plastic in others. Moreover, fish that were more active at a given temperature were also quicker to emerge after a simulated predation attempt (Biro et al. 2009). Similarly, inter-individual differences in behavioral responses to temperature were observed in a marine crab (Ozius truncates, Biro et al. 2013) and a hermit crab (Pagurus bernhardus, Briffa et al. 2013). Activity, aggression, and latency to respond to a simulated threat were correlated across temperatures (albeit not always in the direction predicted by POLS) in red swamp crawfish (Procambarus clarkii; Zhao and Feng 2015). The authors in many of these studies speculate that metabolism is the proximate mechanism that explains individual differences in thermal sensitivity of behavior.
Metabolism is regularly proposed as a crucial driver of trait covariation, but this has rarely been tested
The majority of studies highlight metabolism as the likely mechanism behind the thermal sensitivity of behavior and life history traits and thus the position of individuals along the different trait covariation axes. Nevertheless, very few studies have explicitly tested this hypothesis. Only 10 of the studies reviewed here included a measure of metabolism in their study design. Of these, four found that higher mean metabolic rates were associated with acclimation to lower temperatures (a process known as “metabolic compensation”; Gangloff et al. 2015; Chung et al. 2018; Carbonell et al. 2021; Moffett et al. 2022) and one found no association between metabolism and thermal traits (Artacho et al. 2013). While five studies found that metabolism covaried with the cold-hot axis (Debecker and Stoks 2019; Hébert and Dunlop 2020; Závorka et al. 2020; Le Roy et al. 2021; Tüzün and Stoks 2022), none of these represented strong support for extended POLS as the correlations between metabolic rates, life history, and behavioral traits were weak or in the opposite direction to that predicted by the hypothesis. For example, hot damselfly larvae had higher thermal optima for metabolic rate and displayed bolder behavior compared to cold larvae (Tüzün and Stoks 2022), suggesting that the thermal sensitivity of metabolic rate might be the primary driver of the position of larvae on the shy-bold axis. However, in the same study, the cold-hot and slow-fast axes were negatively correlated. Faster growing larvae had lower thermal optima for swimming speed, which the authors suggest may have resulted from a trade-off between growth and thermal performance (Tüzün and Stoks 2022). This implies that metabolism may be the underlying mechanism of some trait correlations, but not others, or that extended POLS may not apply when trade-offs are present between life history or behavioral traits and thermal performance.
Outstanding questions and future directions
While the extended POLS literature has matured in recent years, there are several outstanding questions, the pursuit of which may represent a fruitful path for improving our knowledge of the proximate and ultimate mechanisms that generate trait covariation in nature.
How does extended POLS arise in nature?
The fact that traits often covary across disparate categories (life history, behavior, etc.) begs the question of what forces generated these covariances in the first place. These patterns are usually assumed to be adaptive, but this is a hypothesis that must be tested (Gould and Lewontin 1979). When traits covary along axes, a reasonable hypothesis is that correlational selection favoring particular combinations of trait values has occurred (or continues to occur). Despite the existence of well-established methods to estimate correlational selection in the wild (Lande and Arnold 1983), to our knowledge, no one has yet attempted to measure selection gradients of any kind in the context of extended POLS. Indeed, of the 51 studies reviewed here that tested aspects of extended POLS, only one was field-based. Future work should examine the relative survival probabilities and reproductive success of wild individuals as a function of variation in POLS traits. In principle, these sorts of studies are straightforward. For example, one could measure variation across individuals in the thermal sensitivities of both behavior (e.g., boldness) and metabolic rate in the lab, mark these individuals, and then release them into the wild. Mark-recapture can then be used over the breeding season to evaluate relative survival probabilities (to estimate viability selection) or over multiple generations in combination with a pedigree to estimate fitness via lifetime reproductive success. Standard regression and spline techniques (Schluter 1988) could then be used to quantify and visualize fitness landscapes, with an emphasis on calculating correlational selection surfaces for the pairs of traits that are predicted by extended POLS to covary. A similar but even more powerful approach would be to manipulate aspects of the environment to change local fitness optima. Predators could be added or removed, or the strength of competition could be manipulated (with respect to a control), and then the same set of mark-recapture and analytical techniques could be used with the prediction that fitness surfaces should differ between the manipulated environment and the control. Of course, these sorts of studies are challenging in practice because they require large sample sizes, measuring behavioral and physiological traits on large numbers of individuals can be prohibitive, and manipulating predation and competition in natural environments can be logistically insurmountable. Regardless, we think this approach may be feasible in some systems and would garner much insight into the evolutionary forces that lead to trait covariation within POLS.
Even if selection studies determine that POLS traits are rarely under selection in contemporary environments, this does not mean that past selection has not shaped trait covariation. In fact, we know virtually nothing about the proximate basis of extended POLS. If correlational selection has been an important force generating trait covariation along slow-fast, shy-bold, and cold-hot axes, then we might predict that the associated traits would be correlated at the genetic level via mechanisms like linkage disequilibrium or pleiotropy. This is because persistent correlational selection should build genetic correlations over time (Sinervo and Svensson 2002; Immonen et al. 2018). Controlled breeding studies can elucidate the quantitative genetic basis of POLS traits, including the degree to which they are genetically correlated and thus constrained from evolving away from POLS. In combination with selection gradients estimated from mark-recapture studies, this information can be used to model future evolutionary trajectories in changing environments.
What is the role, if any, of metabolism in extended POLS?
Metabolism is assumed to be the underlying mechanism that links all traits within the classical POLS framework, and since it is itself a thermally sensitive trait, it should be integral to extended POLS as well. However, as with the assumption that trait covariation is adaptive, the assertion that metabolism underlies covariation among trait categories is a hypothesis that should be explicitly tested. Indeed, contrasting models for how metabolic processes should influence higher order traits have been proposed. The “independent model” states that there should be no mechanistic link between resting metabolic rate and higher level organismal performance (e.g., life history, behavior) and the “allocation model” assumes a negative association between resting metabolic rate and performance caused by a trade-off in allocating available energy to competing functions (Careau and Garland 2012; Careau 2017). Each of these models has received empirical support in the literature, providing evidence for positive, negative, or lack of correlations between basal or resting metabolic rates and different energy-demanding performance and behavioral traits (Burton et al. 2011; Careau and Garland 2012). Unfortunately, in the context of extended POLS, very few studies have included measurements of metabolic rates under any condition, let alone across a thermal gradient (Table 1). Progress in understanding the generality of extended POLS and evidence supporting the hypothesized role of metabolism will require the measurement of the thermal sensitivity of metabolic rate (and its plasticity) in many taxa and across a broad range of ecologically relevant temperatures. Furthermore, deep consideration should be given to the specific measure of metabolism (e.g., resting, maximal, aerobic scope) that researchers choose to quantify given their study system and the types of trait covariations being investigated.
What is the role of plasticity in extended POLS?
It is unlikely that the majority of traits normally considered within the extended POLS framework are genetically canalized. Instead, many of these traits respond to changes in the environment within the lifespan of individual organisms, that is, they are plastic. It is well documented that behavioral, life history, and physiological traits, as well as their thermal sensitivities, can all shift via short-term, reversible plasticity or fixed, developmental plasticity (Snell-Rood 2013; Baker et al. 2015; Gunderson et al. 2017; Montiglio et al. 2018; Cloyed et al. 2019). Indeed, the assumed driver of trait covariation within extended POLS, the thermal sensitivity of metabolic rate, has been shown to be plastic when individuals are exposed to different thermal regimes (Artacho et al. 2013; Cloyed et al. 2019; Réveillon et al. 2019). Nevertheless, to our knowledge, the role of plasticity in generating, maintaining, or eroding extended POLS has not been studied. Studies where POLS trait associations are measured in individuals that are recaptured across seasons might reveal a role for plasticity in generating or eroding extended POLS over time. Similarly, individuals can be exposed to varying environmental conditions in the laboratory and both the magnitude of plasticity and extended POLS trait associations can be remeasured after exposure. Additionally, the potential role of intergenerational plasticity (e.g., maternal effects) has not been considered and might play a role in modulating trait correlations. Studies like these might begin to reveal a role for plasticity in generating the exceptions to both classical and extended POLS that are so common in the literature.
How should we define the ends of the shy-bold continuum?
In the POLS literature, bold individuals are usually characterized by having higher aggression, shorter response latency, less risk aversion, and lower sociality. But the interpretation of a particular behavior as shy or bold often varies by system. This seems due to fundamental differences in the way that behaviors are perceived by researchers who study different taxa. For example, sociality and exploration are traits often used to assess behavior with the baseline assumption that bolder individuals are less social and more exploratory, yet these traits may not be accurate proxies for the position of individuals on the shy-bold axis. Some experiments have found that bold individuals are more social or that shy individuals are more exploratory (Rey et al. 2015). A study on leopard lizards (Gambelia wislizenii, Crowley and Pietruszka 1983) scored individuals that fled from predators as bold and those that stood their ground were scored as shy. These differences in interpretation of behavior across taxa are crucial because they result in individuals being placed at different positions along the shy-bold axis and therefore change the way that associations between the axes of extended POLS are interpreted. Beckmann and Biro (2013) make the case that a single behavioral assay may not be sufficient to describe the boldness or shyness of an individual. If possible, researchers should incorporate multiple behavioral assays and justify why particular traits are chosen as proxies for boldness based on the biology of their particular study organism. While we recognize that the diverse sets of behaviors displayed by organisms across the tree of life will always lead to some inconsistencies in this literature, we urge researchers to critically assess which traits are used to define positions along the shy-bold axis in their systems, as a lack of clarity here is likely to cause confusion over whether the study supports the extended POLS hypothesis.
How much do trends and inconsistencies in study design and methodology prevent us from judging the generality of extended POLS?
Given that the classical POLS framework was created piecemeal over many years, and that extended POLS was proposed recently, there is understandably a wide range of methods and approaches that biologists have taken to test aspects of these ideas. Nevertheless, inconsistencies and tendencies in approach, analysis, and interpretation have made it challenging to understand the contexts in which extended POLS applies and those in which it does not, in wild populations. Few studies have incorporated multiple trait categories into their design, making it difficult to understand the larger patterns of covariation between life history, behavior, metabolism, and thermal traits either within or across systems. Evaluating the role of temperature in mediating trait covariation is admittedly a complex and challenging task, but growth in this field will require experimental designs that test the role of temperature in detailed and multi-faceted ways. We suggest that careful thought be put into experimental design, including which types of temperature (e.g., internal body temperature, body surface temperature, environmental temperature, operative temperature) will be measured, how these variables will be measured and manipulated (e.g., static or fluctuating), and at what life stages the organism will be exposed to experimental treatments. Most studies incorporate a thermal element by using static environmental temperature treatments or providing a thermal preference gradient for adult-stage individuals, and they usually only consider the effect of temperature on one or a few traits (e.g., Brodie and Russell 1999; Stapley 2006; Goulet et al. 2017b). By its nature, extended POLS involves the thermal dependence of many different types of traits, and thus, future work should consider the thermal dependence of multiple traits (physiological, life history, and behavior) across ecologically realistic temperature regimes.
In addition to the varied ways in which researchers have integrated thermal biology into the study of POLS, the taxonomic scope of these investigations has been relatively limited (Table 1; Fig. S1). Outside of several studies on insects, much work to date has focused on vertebrates (primarily fish and squamate reptiles). Tests of trait associations and their drivers across a wider range of taxa would deepen our understanding of extended POLS.
Towards a comprehensive POLS framework
In the deep pantheon of theories in ecology and evolutionary biology, extended POLS has only recently emerged. Nevertheless, the incorporation of thermal physiology into the classical POLS hypothesis has revived hope that a general explanation for trait covariation, and why it may be adaptive, exists. The realization that temperature plays an outsized role in mediating physiology, life history, and behavior has led to a surge of studies aimed at exploring links between the slow-fast and shy-bold axes, and the newly discovered cold-hot axis, of trait covariation. However, progress has been slow due to inconsistencies in experimental design, analysis and interpretation, assumptions that metabolism plays a central role and that trait correlations are adaptive, and a complete lack of field studies. We thus have a growing literature with some common patterns emerging but many notable deviations from these patterns. If we are to develop a comprehensive and generalizable POLS framework, it will likely require re-thinking how we categorize behavioral traits across taxa, a concerted effort to test the role of metabolism (and other potential traits) as the fundamental mechanism linking POLS trait categories, an exploration of the role of phenotypic plasticity in generating or eroding trait correlations, and an understanding of how selection and genetic variation give rise to, and maintain, trait covariation in nature. If this effort ultimately fails, we should attempt to understand why by studying the ecological conditions (e.g., predation, competition, food availability, habitat structure) over which it failed. Ultimately, this endeavor will move us closer to understanding how and why animals behave, grow, and function as they do.
References
Abram PK, Boivin G, Moiroux J, Brodeur J (2017) Behavioural effects of temperature on ectothermic animals: unifying thermal physiology and behavioural plasticity. Biol Rev 92:1859–1876. https://doi.org/10.1111/brv.12312
Adriaenssens B, Johnsson JI (2011) Shy trout grow faster: exploring links between personality and fitness-related traits in the wild. Behav Ecol 22:135–143. https://doi.org/10.1093/beheco/arq185
Ariyomo TO, Watt PJ (2011) The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim Behav 83:41–46. https://doi.org/10.1016/j.anbehav.2011.10.004
Artacho P, Jouanneau I, Le Galliard J-F (2013) Interindividual variation in thermal sensitivity of maximal sprint speed, thermal behavior, and resting metabolic rate in a lizard. Physiol Biochem Zool 86:458–469. https://doi.org/10.1086/671376
Auer SK, Dick CA, Metcalfe NB, Reznick DN (2018) Metabolic rate evolves rapidly and in parallel with the pace of life history. Nat Commun 9:14. https://doi.org/10.1038/s41467-017-02514-z
Baker JA, Wund MA, Heins DC, King RW, Reyes ML, Foster SA (2015) Life-history plasticity in female threespine stickleback. Heredity 115:322–334. https://doi.org/10.1038/hdy.2015.65
Beckmann C, Biro PA (2013) On the validity of a single (boldness) assay in personality research. J Ethol 119:937–947. https://doi.org/10.1111/eth.12137
Beldade R, Holbrook SJ, Schmitt RJ, Planes S, Malone D, Bernardi G (2012) Larger female fish contribute disproportionately more to self-replenishment. Proc R Soc Lond B 279:2116–2121. https://doi.org/10.1098/rspb.2011.2433
Betini GS, Wang X, Avgar T, Guzzo MM, Fryxell JM (2020) Food availability modulates temperature-dependent effects on growth, reproduction, and survival in Daphnia magna. Ecol Evol 10:756–762. https://doi.org/10.1002/ece3.5925
Binder TR, Wilson ADM, Wilson SM, Suski CD, Godin J-GJ, Cooke SJ (2016) Is there a pace-of-life syndrome linking boldness and metabolic capacity for locomotion in bluegill sunfish? Anim Behav 121:175–183. https://doi.org/10.1016/j.anbehav.2016.09.006
Biro PA, Stamps JA (2008) Are animal personality traits linked to life-history productivity? Trends Ecol Evol 23:361–368. https://doi.org/10.1016/j.tree.2008.04.003
Biro PA, Beckmann C, Stamps JA (2009) Small within-day increases in temperature affects boldness and alters personality in coral reef fish. Proc R Soc Lond B 277:71–77
Biro PA, O’Connor J, Pedini L, Gribben PE (2013) Personality and plasticity: consistent responses within-, but not across-temperature situations in crabs. Behaviour 150:799–811
Boher F, Godoy-Herrera R, Bozinovic F (2010) The interplay between thermal tolerance and life history is associated with the biogeography of Drosophila species. Evol Ecol Res 12:973–986
Brans KI, De Meester L (2018) City life on fast lanes: Urbanization induces an evolutionary shift towards a faster lifestyle in the water flea Daphnia. Funct Ecol 32:2225–2240. https://doi.org/10.1111/1365-2435.13184
Briffa M, Bridger D, Biro PA (2013) How does temperature affect behaviour? Multilevel analysis of plasticity, personality and predictability in hermit crabs. Anim Behav 86:47–54. https://doi.org/10.1016/j.anbehav.2013.04.009
Brodie ED III, Russell NH (1999) The consistency of individual differences in behaviour: Temperature effects on antipredator behaviour in garter snakes. Anim Behav 57:445–451. https://doi.org/10.1006/anbe.1998.0990
Burton T, Killen SS, Armstrong JD, Metcalfe NB (2011) What causes intraspecific variation in resting metabolic rate and what are its ecological consequences? Proc Royal Soc Lond B 278:3465–3473. https://doi.org/10.1098/rspb.2011.1778
Carbonell JA, Stoks R (2020) Thermal evolution of life history and heat tolerance during range expansions toward warmer and cooler regions. Ecology 101:e03134. https://doi.org/10.1002/ecy.3134
Carbonell JA, Wang Y-J, Stoks R (2021) Evolution of cold tolerance and thermal plasticity in life history, behaviour and physiology during a poleward range expansion. J Anim Ecol 90:1666–1677. https://doi.org/10.1111/1365-2656.13482
Careau V (2017) Energy intake, basal metabolic rate, and within-individual trade-offs in men and women training for a half marathon: a reanalysis. Physiol Biochem Zool 90:392–398
Careau V, Garland T (2012) Performance, personality, and energetics: correlation, causation, and mechanism. Physiol Biochem Zool 85:543–571. https://doi.org/10.1086/666970
Careau V, Garland T (2015) Energetics and behavior: many paths to understanding. Trends Ecol Evol 30:365–366. https://doi.org/10.1016/j.tree.2015.04.007
Careau V, Thomas D, Humphries MM, Réale D (2008) Energy metabolism and animal personality. Oikos 117:641–653. https://doi.org/10.1111/J.0030-1299.2008.16513.X
Cerqueira M, Rey S, Silva T, Feathersone Z, Crumlish M, MacKenzie S (2016) Thermal preference predicts animal personality in Nile tilapia Oreochromis niloticus. J Anim Ecol 85:1389–1400. https://doi.org/10.1111/1365-2656.12555
Chung DJ, Healy TM, McKenzie JL, Chicco AJ, Sparagna GC, Schulte PM (2018) Mitochondria, temperature, and the pace of life. Integr Comp Biol 58:578–590. https://doi.org/10.1093/icb/icy013
Ciota AT, Matacchiero AC, Kilpatrick AM, Kramer LD (2014) The effect of temperature on life history traits of Culex mosquitoes. J Med Entomol 51:55–62. https://doi.org/10.1603/ME13003
Clarke A, Fraser KPP (2004) Why does metabolism scale with temperature? Funct Ecol 18:243–251. https://doi.org/10.1111/J.0269-8463.2004.00841.X
Cloyed CS, Dell AI, Hayes T, Kordas RL, O’Gorman EJ (2019) Long-term exposure to higher temperature increases the thermal sensitivity of grazer metabolism and movement. J Anim Ecol 88:833–844. https://doi.org/10.1111/1365-2656.12976
Cornwell TO, Mccarthy ID, Biro PA (2020) Integration of physiology, behaviour and life history traits: personality and pace of life in a marine gastropod. Anim Behav 163:155–162. https://doi.org/10.1016/j.anbehav.2020.03.009
Crowley SR, Pietruszka RD (1983) Aggressiveness and vocalization in the leopard lizard (Gambelia wislizennii): the influence of temperature. Anim Behav 31:1055–1060. https://doi.org/10.1016/S0003-3472(83)80012-8
Culumber ZW (2020) Thermal stress increases activity and risk-taking behavior but not anxiety in a livebearing fish. Environ Biol Fish 103:313–317. https://doi.org/10.1007/s10641-020-00966-9
Dalesman S, Rundle SD (2010) Influence of rearing and experimental temperatures on predator avoidance behaviour in a freshwater pulmonate snail. Freshw Biol 55:2107–2113. https://doi.org/10.1111/j.1365-2427.2010.02470.x
Debecker S, Stoks R (2019) Pace of life syndrome under warming and pollution: integrating life history, behavior, and physiology across latitudes. Ecol Monogr 89:e01332. https://doi.org/10.1002/ecm.1332
Enders EC, Wall AJ, Svendsen JC (2019) Hypoxia but not shy-bold phenotype mediates thermal preferences in a threatened freshwater fish, Notropis percobromus. J Therm Biol 84:479–487. https://doi.org/10.1016/j.jtherbio.2019.08.001
Forsatkar MN, Nematollahi MA, Biro PA, Beckmann C (2016) Individual boldness traits influenced by temperature in male Siamese fighting fish. Physiol Behav 165:267–272. https://doi.org/10.1016/j.physbeh.2016.08.007
Frost AJ, Thomson JS, Smith C, Burton HC, Davis B, Watts PC, Sneddon LU (2013) Environmental change alters personality in the rainbow trout, Oncorhynchus mykiss. Anim Behav 85:1199–1207. https://doi.org/10.1016/j.anbehav.2013.03.006
Gangloff EJ, Vleck D, Bronikowski AM (2015) Developmental and immediate thermal environments shape energetic trade-offs, growth efficiency, and metabolic rate in divergent life-history ecotypes of the garter snake Thamnophis elegans. Physiol Biochem Zool 88:550–563. https://doi.org/10.1086/682239
Gould SJ, Lewontin RC (1979) The spandrels of San Marco and the Panglossian paradigm: a critique of the adaptationist programme. Proc R Soc Lond B 205:581–598. https://doi.org/10.1098/rspb.1979.0086
Goulet CT, Ingley SJ, Scharf I, Pruitt JN (2016) Thermal effects on survival and reproductive performance vary according to personality type. Behav Ecol 27:1635–1641. https://doi.org/10.1093/beheco/arw084
Goulet CT, Thompson MB, Chapple DG (2017) Repeatability and correlation of physiological traits: Do ectotherms have a “thermal type”? Ecol Evol 7:710–719. https://doi.org/10.1002/ece3.2632
Goulet CT, Thompson MB, Michelangeli M, Wong BBM, Chapple DG (2017) Thermal physiology: a new dimension of the pace-of-life syndrome. J Anim Ecol 86:1269–1280. https://doi.org/10.1111/1365-2656.12718
Goulet CT, Michelangeli M, Chung M, Riley JL, Wong BBM, Thompson MB, Chapple DG (2018) Evaluating cognition and thermal physiology as components of the pace-of-life syndrome. Evol Ecol 32:469–488. https://doi.org/10.1007/s10682-018-9948-1
Gunderson AR, Dillon ME, Stillman JH (2017) Estimating the benefits of plasticity in ectotherm heat tolerance under natural thermal variability. Funct Ecol 31:1529–1539. https://doi.org/10.1111/1365-2435.12874
Günter F, Beaulieu M, Franke K, Toshkova N, Fischer K (2020) Clinal variation in investment into reproduction versus maintenance suggests a ‘pace-of-life’ syndrome in a widespread butterfly. Oecologia 193:1011–1020. https://doi.org/10.1007/s00442-020-04719-4
Hébert I, Dunlop ES (2020) Temperature response in the physiology and growth of lake trout strains stocked in the Laurentian Great Lakes. J Great Lakes Res 46:366–375. https://doi.org/10.1016/j.jglr.2020.01.012
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanism and process in physiological evolution. Oxford University Press, Oxford
Horváth G, Jiménez-Robles O, Martín J, López P, De la Riva I, Herczeg G (2020) Linking behavioral thermoregulation, boldness, and individual state in male Carpetan rock lizards. Ecol Evol 10:10230–10241. https://doi.org/10.1002/ece3.6685
Immonen E, Hämäläinen A, Schuett W, Tarka M (2018) Evolution of sex-specific pace-of-life syndromes: genetic architecture and physiological mechanisms. Behav Ecol Sociobiol 72:60. https://doi.org/10.1007/s00265-018-2462-1
Irschick DJ, Losos JB (1996) Morphology, ecology, and behavior of the twig anole, Anolis angusticeps. In: Powell R, Henderson RW (eds) Contributions to West Indian herpetology: a tribute to Albert Schwartz. Society for the Study of Amphibians and Reptiles, Ithaca NY, pp 291–301
Junge-Berberović R (1996) Effect of thermal environment on life histories of free living Drosophila melanogaster and D. subobscura. Oecologia 108:262–272. https://doi.org/10.1007/BF00334650
Karlsson B, Wiklund C (2005) Butterfly life history and temperature adaptations; dry open habitats select for increased fecundity and longevity. J Anim Ecol 74:99–104. https://doi.org/10.1111/j.1365-2656.2004.00902.x
Kashon EAF, Carlson BE (2017) Consistently bolder turtles maintain higher body temperatures in the field but may experience greater predation risk. Behav Ecol Sociobiol 72:9. https://doi.org/10.1007/s00265-017-2428-8
Krams I, Kivleniece I, Kuusik A, Krama T, Freeberg TM, Mänd R, Sivacova L, Rantala MJ, Mänd M (2014) High repeatability of anti-predator responses and resting metabolic rate in a beetle. J Insect Behav 27:57–66. https://doi.org/10.1007/s10905-013-9408-2
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226. https://doi.org/10.2307/2408842
Le Galliard JF, Paquet M, Cisel M, Montes-Poloni L (2013) Personality and the pace-of-life syndrome: Variation and selection on exploration, metabolism and locomotor performances. Funct Ecol 27:136–144. https://doi.org/10.1111/1365-2435.12017
Le Roy A, Mazué GPF, Metcalfe NB, Seebacher F (2021) Diet and temperature modify the relationship between energy use and ATP production to influence behavior in zebrafish (Danio rerio). Ecol Evol 11:9791–9803. https://doi.org/10.1002/ece3.7806
Li H, Zhang X, Wu Y, Zhang F, Li C (2021) Environmental temperature during early life affects the personality of mosquitofish in adulthood. Curr Zool 67:481–488. https://doi.org/10.1093/cz/zoab003
Losos JB (2011) Lizards in an evolutionary tree: ecology and adaptive radiation of anoles. Univ Calif Press Berkeley CA. https://doi.org/10.1525/california/9780520255913.001.0001
Lukas J, Kalinkat G, Miesen FW, Landgraf T, Krause J, Bierbach D (2021) Consistent behavioral syndrome across seasons in an invasive freshwater fish. Front Ecol Evol 8:583670
Magellan K, Bonebrake TC, Dudgeon D (2019) Temperature effects on exploratory behaviour and learning ability of invasive mosquitofish. Aquat Invasions 14:502–517. https://doi.org/10.3391/ai.2019.14.3.09
Maskrey DK, Sneddon LU, Arnold KE, Wolfenden DCC, Thomson JS (2020) The impact of personality, morphotype and shore height on temperature-mediated behavioural responses in the beadlet anemone Actinia equina. J Anim Ecol 89:2311–2324. https://doi.org/10.1111/1365-2656.13301
Maskrey DK, Sneddon LU, Arnold KE, Wolfenden DCC, Thomson JS (2021) Temperature-driven changes in behavioural unpredictability and personality in the beadlet sea anemone, Actinia equina. Anim Behav 181:13–27. https://doi.org/10.1016/j.anbehav.2021.08.022
Mathot KJ, Dingemanse NJ (2015) Energetics and behavior: unrequited needs and new directions. Trends Ecol Evol 30:199–206. https://doi.org/10.1016/j.tree.2015.01.010
Mathot KJ, Dingemanse NJ (2015) Energetics and behaviour: a reply to Careau and Garland. Trends Ecol Evol 30:367–368. https://doi.org/10.1016/j.tree.2015.04.009
Mathot KJ, Nicolaus M, Araya-Ajoy YG, Dingemanse NJ, Kempenaers B (2015) Does metabolic rate predict risk-taking behaviour? A field experiment in a wild passerine bird. Ecology 29:239–249. https://doi.org/10.2307/48576962
McKenzie DJ, Belão TC, Killen SS, Rantin FT (2015) To boldly gulp: standard metabolic rate and boldness have context-dependent influences on risk-taking to breathe air in a catfish. J Exp Biol 218:3762–3770. https://doi.org/10.1242/jeb.122903
Michelangeli M, Goulet CT, Kang HS, Wong BBM, Chapple DG (2017) Integrating thermal physiology within a syndrome: locomotion, personality and habitat selection in an ectotherm. Funct Ecol 32:970–981. https://doi.org/10.1111/1365-2435.13034
Moffett ER, Fryxell DC, Simon KS (2022) Multigenerational exposure to increased temperature reduces metabolic rate but increases boldness in Gambusia affinis. Ecol Evol 12:e8853. https://doi.org/10.1002/ece3.8853
Montiglio PO, Dammhahn M, Dubuc Messier G, Réale D (2018) The pace-of-life syndrome revisited: the role of ecological conditions and natural history on the slow-fast continuum. Behav Ecol Sociobiol 72:1–9. https://doi.org/10.1007/s00265-018-2526-2
Myles-Gonzalez E, Burness G, Yavno S, Rooke A, Fox MG (2015) To boldly go where no goby has gone before: boldness, dispersal tendency, and metabolism at the invasion front. Behav Ecol 26:1083–1090. https://doi.org/10.1093/beheco/arv050
Niemelä PT, Niehoff PP, Gasparini C, Dingemanse NJ, Tuni C (2019) Crickets become behaviourally more stable when raised under higher temperatures. Behav Ecol Sociobiol 73:81. https://doi.org/10.1007/s00265-019-2689-5
Niemelä PT, Vainikka A, Hedrick AV, Kortet R (2012) Integrating behaviour with life history: boldness of the field cricket, Gryllus integer, during ontogeny. Funct Ecol 26:450–456. https://doi.org/10.1111/j.1365-2435.2011.01939.x
Pettersen AK, White CR, Marshall DJ (2016) Metabolic rate covaries with fitness and the pace of the life history in the field. Proc R Soc B 283:20160323. https://doi.org/10.1098/RSPB.2016.0323
Pianka ER (1970) On r- and K-selection. Am Nat 104:592–597. https://doi.org/10.1086/282697
Pilakouta N, O’Donnell PJ, Crespel A et al (2023) A warmer environment can reduce sociability in an ectotherm. Glob Change Biol 29:206–214. https://doi.org/10.1111/gcb.16451
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: systemic to molecular hierarchy of thermal tolerance in animals. Comp Biochem Physiol A 132:739–761. https://doi.org/10.1016/S1095-6433(02)00045-4
Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio P-O (2010) Personality and the emergence of the pace-of-life syndrome concept at the population level. Phil Trans R Soc B 365:4051–4063. https://doi.org/10.1098/RSTB.2010.0208
Réveillon T, Rota T, Chauvet É, Lecerf A, Sentis A (2019) Repeatable inter-individual variation in the thermal sensitivity of metabolic rate. Oikos 128:1633–1640. https://doi.org/10.1111/OIK.06392
Rey S, Digka N, Mackenzie S (2015) Animal personality relates to thermal preference in wild-type zebrafish, Danio rerio. Zebrafish 12:243–249. https://doi.org/10.1089/zeb.2014.1076
Ricklefs RE, Wikelski M (2002) The physiology/life-history nexus. Trends Ecol Evol 17:462–468
Rocha CFD, Bergallo HG (1990) Thermal biology and flight distance of Tropidurus oreadicus (Sauria Iguanidae) in an area of Amazonian Brazil. Ethol Ecol Evol 2:263–268. https://doi.org/10.1080/08927014.1990.9525411
Royauté R, Greenlee K, Baldwin M, Dochtermann NA (2015) Behaviour, metabolism and size: phenotypic modularity or integration in Acheta domesticus? Anim Behav 110:163–169. https://doi.org/10.1016/J.ANBEHAV.2015.09.027
Royauté R, Berdal MA, Garrison CR, Dochtermann NA (2018) Paceless life? A meta-analysis of the pace-of-life syndrome hypothesis. Behav Ecol Sociobiol 72:64. https://doi.org/10.1007/s00265-018-2472-z
Rupia EJ, Binning SA, Roche DG, Lu W (2016) Fight-flight or freeze-hide? Personality and metabolic phenotype mediate physiological defence responses in flatfish. J Anim Ecol 85:927–937. https://doi.org/10.1111/1365-2656.12524
Rutschmann A, Miles DB, Clobert J, Richard M (2016) Warmer temperatures attenuate the classic offspring number and reproductive investment trade-off in the common lizard. Zootoca Vivipara Biol Lett 12:20160101. https://doi.org/10.1098/rsbl.2016.0101
Schluter D (1988) Estimating the form of natural selection on a quantitative trait. Evolution 42:849–861. https://doi.org/10.2307/2408904
Segev U, Burkert L, Feldmeyer B, Foitzik S (2017) Pace-of-life in a social insect: behavioral syndromes in ants shift along a climatic gradient. Behav Ecol 28:1149–1159. https://doi.org/10.1093/beheco/arx079
Shine R (2005) Life-history evolution in reptiles. Annu Rev Ecol Evol S 36:23–46
Sinervo B, Svensson E (2002) Correlational selection and the evolution of genomic architecture. Heredity 89:329–338. https://doi.org/10.1038/sj.hdy.6800148
Sinervo B, Bleay C, Adamopoulou C (2001) Social causes of correlational selection and the resolution of a heritable throat color polymorphism in a lizard. Evolution 55:2040–2052. https://doi.org/10.1111/j.0014-3820.2001.tb01320.x
Smith HG, Kallander H, Nilsson J-A (1989) The trade-off between offspring number and quality in the great tit Parus major. J Anim Ecol 58:383–401. https://doi.org/10.2307/4837
Snell-Rood EC (2013) An overview of the evolutionary causes and consequences of behavioural plasticity. Anim Behav 85:1004–1011. https://doi.org/10.1016/j.anbehav.2012.12.031
Stamps JA (2007) Growth-mortality tradeoffs and “personality traits” in animals. Ecol Lett 10:355–363. https://doi.org/10.1111/J.1461-0248.2007.01034.X
Stapley J (2006) Individual variation in preferred body temperature covaries with social behaviours and colour in male lizards. J Therm Biol 31:362–369. https://doi.org/10.1016/j.jtherbio.2006.01.008
Struelens Q, Rebaudo F, Quispe R, Dangles O (2018) Thermal pace-of-life strategies improve phenological predictions in ectotherms. Sci Rep 8:15891. https://doi.org/10.1038/s41598-018-34274-1
Tang Z, Fu S-J (2021) Effects of habitat conditions on the boldness and sociability of wild-caught fish (Zacco platypus) along a river. J Ethol 39:379–391. https://doi.org/10.1007/s10164-021-00715-0
Trnik M, Albrechtová J, Kratochvil L (2011) Persistent effect of incubation temperature on stress-induced behavior in the Yucatan banded gecko (Coleonyx elegans). J Comp Physiol 125:22–30. https://doi.org/10.1037/a0021186
Tüzün N, Stoks R (2022) A fast pace-of-life is traded off against a high thermal performance. Proc R Soc B 289:20212414
Warner DA, Woo KL, Van Dyk DA, Evans CS, Shine R (2010) Egg incubation temperature affects male reproductive success but not display behaviors in lizards. Behav Ecol Sociobiol 64:803–813. https://doi.org/10.1007/s00265-009-0897-0
Wenjie L, Binxia L, Cuijuan N (2019) Effects of temperature on life history strategy of the rotifer Euchlanis dilatata. Zool Sci 36:52–57. https://doi.org/10.2108/zs170096
White DP, Nannini MA, Wahl DH (2020) Examining the effects of chronic, lake-wide elevated temperatures on behavioural expression in largemouth bass, Micropterus salmoides. J Fish Biol 97:39–50. https://doi.org/10.1111/jfb.14313
Závorka L, Koeck B, Armstrong TA, Soğanci M, Crespel A, Killen SS (2020) Reduced exploration capacity despite brain volume increase in warm-acclimated common minnow. J Exp Biol 223:jeb223453. https://doi.org/10.1242/jeb.223453
Zhao D, Feng P (2015) Temperature increase impacts personality traits in aquatic non-native species: implications for biological invasion under climate change. Curr Zool 61:966–971. https://doi.org/10.1093/czoolo/61.6.966
Acknowledgements
The authors would like to acknowledge Guillermo Garcia Costoya, Claire E. Williams, and Jennifer J. Heppner for their thoughtful inputs on this article. The authors would also like to thank the reviewers for their valuable insights.
Author information
Authors and Affiliations
Contributions
ACG, KA, and MLL contributed to conceptualizing the idea. ACG performed the literature search and drafted the article. ACG and KA revised the literature and created the figures. KA and MLL contributed to manuscript editing and revision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by J. Lindström.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gopal, A.C., Alujević, K. & Logan, M.L. Temperature and the pace of life. Behav Ecol Sociobiol 77, 59 (2023). https://doi.org/10.1007/s00265-023-03333-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-023-03333-7