Abstract
When resources are limited and defensible, inter-group encounters in animals are often of aggressive nature. Individuals can participate in inter-group encounters to defend mates, infants, and food resources, but also to attract out-group individuals for additional mating opportunities. Since inter-group conflicts have mainly been studied in group-living species, we examined the mate, infant, and food resource defense and mate attraction hypotheses in pair-living Javan gibbons (Hylobates moloch) in Gunung Halimun-Salak National Park, Indonesia. To this end, we investigated factors influencing male and female participation and outcome of encounters (i.e., win vs. lose). We observed 234 complete encounters between three habituated and five unhabituated gibbon groups over 43 months, of which 72% were aggressive. Males were the main participants and they were more likely to participate when cycling females or dependent infants were present, supporting the mate and infant defense hypotheses. Males were also more likely to participate when more fruits were available, contradicting the food resource defense hypothesis. Females participated by singing more often when they were cycling and when there were singing opponents, suggesting an advertisement function of their reproductive status through songs. The probability of winning an inter-group encounter was only higher when cycling females were present, supporting the mate defense hypothesis. The intensity of space use or aggression level had no effect on the outcome of inter-group encounters. Our results highlight that mate and infant defense are crucial for male Javan gibbons, especially in view of their pair-living system, long interbirth intervals, and slow infant development.
Significance statement
While animal groups interact aggressively with each other to defend valuable resources, they can also interact to increase additional mating opportunities. Here, we examined male and female participation and the outcomes of inter-group encounters in a wild pair-living primate, the Javan gibbon. Crucially, we found that the presence of cycling females had a significant impact on male participation, female singing, and the outcome of encounters. Our findings suggest that Javan gibbon females may advertise their reproductive status through singing during inter-group encounters while Javan gibbon males rather participate to defend their mates and infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When resources are limited and defensible, inter-group encounters in animals are often of aggressive nature and can affect individual fitness (Kelly 2005; Harris 2010; Koch et al. 2016a). In non-human primates, three main explanations have been accredited to understand the participation of individuals in inter-group encounters. Because female fitness is limited by access to food, females are expected to mostly defend food resources (food resource defense hypothesis; Trivers 1972; Korstjens et al. 2005). Male fitness, however, is limited by access to females; therefore, males are expected to mainly defend females (mate defense hypothesis; Emlen and Oring 1977; Kitchen and Beehner 2007) or food resources for females (food resource defense hypothesis). Males also defend infants from potential infanticides by out-group males (infant defense hypothesis; van Schaik 1996; Steenbeek 1999).
Although these three hypotheses are not mutually exclusive, numerous studies supported the mate defense hypothesis. Males were more aggressive during inter-group encounters in the mating season compared to the non-mating season (bonnet macaques, Macaca radiata: Cooper 2004; moustached tamarins, Saguinus mystax: Garber et al. 1993; Samango monkeys, Cercopithecus mitis erythrarchus: Payne et al. 2003) or when estrous/cycling females were present in comparison to when no estrous/cycling females were present (chacma baboons, Papio cynocephalus ursinus: Kitchen et al. 2003; white-faced sakis, Pithecia pithecia: Thompson et al. 2012). In contrast, in other studies, the food resource defense hypothesis predicted best participation of individuals in inter-group conflicts (Reichard and Sommer 1997; Cooper et al. 2004; Korstjens et al. 2005; Thompson et al. 2012). For example, female vervet monkeys (Chlorocebus aethiops pygerythrus) actively defended access to valuable food resources (Arseneau-Robar et al. 2016) and female Western black-and-white colobus (Colobus polykomos polykomos) were more likely to be aggressive in inter-group encounters during months in which they heavily relied on a specific high-quality fruit species (Korstjens et al. 2005). However, other studies revealed that food availability or feeding patch quality had no effect on participation in inter-group encounters (Cowlishaw 1995; Steenbeek 1999; Koch et al. 2016a; Mirville et al. 2018). Finally, infant defense may function differently between sexes (Hrdy 1979). Male may actively defend infants from infanticidal males (Palombit et al. 2000; Wich et al. 2002). In contrast, female Verreaux’s sifakas (Propithecus verreauxi) participated less often when vulnerable infants were present, potentially to defend infant from injury or infanticide (Koch et al. 2016a).
Inter-group encounters have mainly been studied in primates living in larger groups (Sicotte 1993; Saito et al. 1998; Fashing 2001; Harris 2006; Crofoot et al. 2008; Koch et al. 2016a, 2016b; Lucchesi et al. 2020; Samuni et al. 2020). Yet, only a handful of studies have investigated inter-group encounters in pair-living primates (Leontopithecus rosalia: Peres 1989; S. mystax: Garber 1993; Hylobates lar: Sommer and Reichard 1997; Callicebus brunneus: Lawrence 2007; P. pithecia: Thompson et al. 2012; Indri indri: Bonadonna et al. 2020; H. moloch: Yi et al. 2020a). While the food resource defense hypothesis is widely supported in species with diverse social structures (reviewed in Fashing 2001), mate defense might be particularly important for males in pair-living species. The risk that females mate with out-group males during inter-group conflicts may have higher costs for pair-living than group-living males, because pair-living males can sire only a limited number of offspring in comparison to group-living males, which have the potential to mate with several females. Although infanticide occurs more frequently in animals living in larger groups (Lukas and Huchard 2014), infanticides have also been witnessed in pair-living species and may, hence, predict participation in inter-group conflicts (Alfred and Sati 1991; Palombit 1999; Rasoloharijaona et al. 2000; Borries et al. 2011). Because the risk of infanticide has been suggested to be a major selective force for the evolution and maintenance of pair-living in primates (van Schaik and Dunbar 1990; van Schaik and Kappeler 1997; Opie et al. 2013; Kappeler and Pozzi 2019), the mate and infant defense hypotheses are of particular relevance to predict participation in inter-group conflicts.
In this study, we investigated inter-group encounters of Javan gibbons (Hylobates moloch), a pair-living primate species living in tropical rainforests in Indonesia. For gibbon males, mate defense during inter-group encounters may be critical, as extra-pair copulations occur exclusively during inter-group encounters (Symphalangus syndactylus: Palombit 1994; H. lar: Bartlett 2003; Nomascus concolor jingdongensis: Huang et al. 2013). Moreover, take-over or replacement by extra-group members during inter-group encounters have been reported in several gibbon species, potentially resulting in the permanent loss of opportunities to reproduce (H. lar: Brockelman et al. 1998; N. concolor: Hu et al. 2018). Gibbon females participate in inter-group encounters mainly by singing and seldom by chasing, whereas males participate exclusively by chasing, even though female gibbons have a similar body and canine size as males, and, hence, a similar fighting ability (Frisch 1963). In contrast, in other species lacking a sexual size dimorphism, as in lemurs, females participate equally often or even more often in inter-group conflicts than males (reviewed in Koch et al. 2016a). Furthermore, Javan gibbon pairs do not duet and mainly females sing solos, while in most other gibbon species, pairs duet to strengthen and advertise pair-bond but also to defend their territory (Ellefson 1968; Raemaekers and Raemaekers 1985b; Geissmann 1993,2002). Hence, female solo songs of the non-duetting Javan gibbons may not have pair-bond related functions (Ham et al. 2017), instead solos may serve to attract males from other groups, especially by advertising their reproductive status (i.e., when they are cycling, mate attraction hypothesis; Seiler et al. 2019). Hence, understanding which factors influence female participation in inter-group conflicts in Javan gibbons is of particular interest.
Because the probability of winning an encounter often depends on “the asymmetry in fighting ability” and “pay-off asymmetry” (Smith and Parker 1976), we here also examined factors potentially affecting the outcome of inter-group encounters. In many species, differences in group size can result in asymmetries in fighting abilities during inter-group encounters leading larger groups to win over smaller groups (Sillero-Zubiri and Macdonald 1998; Kitchen et al. 2004; Palmer 2004; Crofoot et al. 2008; Furrer et al. 2011; Majolo et al. 2020). Moreover, if an encounter location has been intensively used by a group, it might be of a higher value than other locations resulting potentially in a higher motivation to defend the area (Kitchen et al. 2004; Crofoot et al. 2008; Wilson et al. 2012; Brown 2013; Koch et al. 2016b). Thus, the encounter location (i.e., location-based pay-off asymmetry) may affect the outcome of inter-group encounters as already indicated in some primate species (Schradin 2004; Crofoot et al. 2008; Markham et al. 2012; Koch et al. 2016b; Roth and Cords 2016). Given gibbons’ high territoriality and small group size, the encounter location rather than asymmetries in fighting ability might affect the outcome of inter-group encounters in Javan gibbons.
In this study, we specifically tested the mate, infant, and food resource hypotheses and the mate attraction hypothesis by investigating which factors predict the male and female participation as well the outcome of inter-group encounters in Javan gibbons (Table 1). For the mate defense hypothesis (1), we predicted that Javan gibbon males participate more often during inter-group encounters when cycling females are present. We also predicted that groups win more often when cycling females are present. For the infant defense hypothesis (2), we predicted that males participate more often during inter-group encounters when dependent infants are present. We predicted that females participate less often during inter-group encounters to protect dependent infants. We also predicted that groups win more often when dependent infants are present. For the food resource defense hypothesis (3), we predicted that both males and females will participate more often during inter-group encounters when food availability is lower. Furthermore, we assumed that the encounter location predicts the probability of winning an inter-group encounter, with groups being more likely to win an inter-group encounter when the encounter takes place in an area that have been intensively used the months before the inter-group encounter. Finally, for the mate attraction hypothesis (4), we predicted that females sing more often during inter-group encounters when they are cycling.
Methods and materials
Study subjects and site
The local Javan gibbon population in the primary forest of Citalahab area, Gunung Halimun-Salak National Park (6.74° S, 106.53° E), West Java, Indonesia, has been studied regularly since the establishment of the Javan Gibbon Research & Conservation Project in 2007 (Kim et al. 2011, 2012; Ham et al. 2016, 2017; Oktaviani et al. 2018; Yi et al. 2020a, 2020b). This study focused on three habituated adjacent gibbon groups (A, B, and S) and five surrounding unhabituated groups (C, D, E, O, and W). We collected data on inter-group encounters for a total of 43 months over 7 years from 2009 to 2016 (2696.25 h of total observation, see Table S1 in Supplementary Information). During the study period, each group was composed of an adult male-female pair, up to three offspring resulting in a group size between two to five individuals (see Table S2 in Supplementary Information).
Data collection during inter-group encounters
We defined an inter-group encounter when two different groups were observed within 50 m of each other, following the definition from previous studies on arboreal primates inhabiting dense tropical forests including gibbons (Sommer and Reichard 1997; Steenbeek 1999; Fashing 2001; Korstjens et al. 2005). We collected data on inter-group encounters between two habituated groups as well as between a habituated and an unhabituated group. We defined participation in an encounter when an individual was chasing an opponent group member for both females and males, and also when females were singing. We included female songs only as male Javan gibbons rarely sing (Kappeler 1984; Geissmann and Nijman 2006) and sang only few times during inter-group encounters during our study period. For each inter-group encounter, we recorded the encounter duration (min), interactive behaviors between all individuals from the focal and opponent groups (i.e., chasing, singing, hitting, grooming, playing, or copulation), initiators and targets of interactions (i.e., chases initiated by and involving whom), and GPS coordinates of encounter locations (see Table S1 in Supplementary Information for detailed data collection). When the opponent group was impossible to identify, we recorded the opponent group as “unknown.”
We used the number of actively participating opponents to operationalize the fighting ability of an opponent group during an encounter. In addition, we recorded the presence of a singing opponent (yes/no), instead of the exact number of singing opponents, as it was difficult to distinguish singing between adult and sub-adult females from unhabituated groups.
Female reproductive status and infancy
To investigate the mate and infant defense hypotheses, we subdivided female reproductive status into three mutually exclusive phases: (1) cycling females: females who have given birth at least 2 years prior and who were not pregnant, determined a posteriori based on observations on the same population after the study period, (2) dependent infants: females with infants younger than 1 year old, and (3) others: when females were neither cycling or had dependent infants (e.g., a pregnant female or a female who lactates infants older than 1 year). We estimated the pregnancy of females by considering the last birth of infants from our observations and the putative gestation period of 7 months (Ardito 1976; Geissmann 1991). As weaning occurs gradually over a period of 22 months in gibbons (Treesucon 1984; Reichard and Barelli 2008), it would have been inappropriate to consider that the whole lactating period reflects a period of higher infanticide risk. In addition, infant gibbons start consuming solid food by themselves at an age of about 4 months (Berkson 1966) and spent about 70% of time independent from their mothers at an age of about 1 year (Yi 2020). Therefore, we considered infants younger than 1 year as most vulnerable to infanticide and categorized them as dependent infants.
Fruit availability
Fruit availability was estimated since 2007 during monthly phenology transects of Javan gibbons’ feeding trees with a diameter at breast height (dbh) ≥ 10 cm and lianas with dbh ≥ 7 cm, in 25 plots (10 × 50 m) within the home range of the habituated groups. The plots were randomly selected at the crossroads of grid trails (200 × 200 m intervals) and also randomly oriented along the trail intersections (Kim 2012). We collected phenology data at the end of each month and considered it to represent fruit availability during the elapsed month. We scored the relative abundance of fruits on a 4-level scale (0: no fruits, 1: present but few, 2: moderately present, 3: abundant; Kim 2012). We added the scores obtained and divided the sum by the total number of trees to represent the fruit availability for each month.
Home range size and encounter location
The home range size was estimated by collecting GPS coordinates of adult females and males at 15-min intervals during animal focal observations, using kernel density estimations (95 %). To investigate the effect of the encounter location on the probability of winning a conflict, we measured the size of the overlapping area between the encounter location and core areas for three timescales: 1, 3, and 6 months preceding to each encounter event (Markham et al. 2012). Thereby, the encounter location was estimated by drawing a circle of 50-m radius around the GPS coordinate that was taken at the beginning of an encounter to represent the “encounter location.” Although groups could move during encounters, we considered the location where the encounter started as biologically more relevant than any other locations during or at the end of the conflict. The “core area” of home ranges of each focal group was calculated using kernel density estimations (50%). The size of the overlapping area between the encounter location and the core area for each of the three timescales was defined as the intensity of space use. All the GPS-data were analyzed using ArcGIS Pro (version 2.0.1; Esri 2018). Finally, we defined the winner of an encounter as the group which stayed longer in the encounter location, and the loser as the group that left the encounter area first (Fashing 2001; Harris 2010). When it was not clear which group left the encounter location first, we recorded it as “draw.” Theoretically, unhabituated groups may have left the encounter location earlier because they were not habituated to the presence of human observers. However, unhabituated groups won or lost in almost all dyads equally often against habituated groups and one unhabituated group even won more often over a habituated group (see results), making it unlikely that the presence of human observers might have influenced the outcome of conflicts between unhabituated and habituated groups.
Statistical analyses
First, to describe general characteristics of inter-group encounters in Javan gibbons (Table 2), we investigated factors influencing the inter-group encounter frequency. We used a generalized linear mixed model (GLMM; Bolker et al. 2009) with a Poisson distribution to fit the monthly encounter frequency (Nmonth = 108) as a response variable, and female reproductive status and fruit availability as predictor variables. Monthly observation day was set as offset to control the difference in observation days in each month. Focal group identity was set as a random factor to control for possible group differences. For this model, we included encounters we could not observe from the beginning until the end (Nencounter = 286). To examine whether there was a sex difference in participation (Nencounter = 174), we used a chi-squared test. Finally, we examined whether gibbon groups exhibit dominance relationships among groups by using binomial tests.
Next, we examined factors predicting the probability of participation in inter-group encounters and winning an inter-group encounter. Since our hypotheses were not mutually exclusive, we tested several hypotheses in our models so that all predictors could be simultaneously investigated. In model I (Table 2, male participation model), we ran a binomial GLMM with male chasing (yes/no) for each encounter (Nencounter = 243) as a response variable. We included fruit availability, female reproductive status (cycling female, dependent infant, others), and the number of actively participating opponents as predictors. Focal group ID was set as a random factor, and encounter duration as offset to control for the possible effect of group differences and encounter duration.
For model II (Table 2, female participation model) and model III (Table 2, female singing model), we included data collected on female participation during between 2013 and 2016 (Nencounter = 142). We fitted separate models for female participation and singing because females participated by chasing and singing, whereas males participated by chasing only. For model II (female participation model), we ran a binomial GLMM with female chasing (yes/no) for each encounter (Nencounter = 142) as a response variable and the same predictors, random factor, and offset as in the model I (male participation model). For model III (female singing model), we ran a binomial GLMM with female singing (yes/no, Nencounter = 142) as a response variable and fruit availability, female reproductive status, presence of singing opponent as predictors, focal group ID as a random factor, and encounter duration as offset. For all these models, we included random slopes for fruit availability and number of actively participating opponents within gibbon groups in order to decrease type I error (Schielzeth and Forstmeier 2008; Barr et al. 2013).
For model IV (Table 2, outcome model), we collected data on the outcome of encounters between 2014 and 2016 and included only encounters which had a clear winner or loser to examine factors influencing encounter outcomes (Nencounter = 86). For model IV, we ran a binomial GLMM by including winning (yes/no) as a response variable and female reproductive status and intensity of space use in 1 month, number of actively participating opponents, proportion of chasing frequency (i.e., “focal chasing frequency” divided by “focal and non-focal chasing frequency”) as predictors, focal group ID as a random factor, all predictors as random slopes within focal group ID, and encounter duration as offset. We also fitted two additional binomial GLMMs using the two other timescales for the core area: intensity of space use in 3 months and 6 months.
For all models, quantitative predictors were z-transformed to a mean of 0 and standard deviation of 1 before fitting the models. Only main effects were included when there were no significant effects of interactions between predictors. We also controlled for collinearity among predictors using the package ‘car’ (Fox et al. 2012). Since all variance inflation factors were below or around 1, collinearity was not an issue. We compared full and null models including only random factors and offsets using likelihood ratio tests. We discussed the results of the models with respect to test predictors only when a full-null comparison revealed significance. All p values were two-tailed. We discussed the results of a model only when a full-null model comparison revealed a significance or a trend (Forstmeier and Schielzeth 2011; Mundry 2014). All data were analyzed using R (version 3.4.3; R Development Core Team 2018). As we did focal animal observations in the field, blind methods were not applicable.
Results
General characteristics of inter-group encounters in Javan gibbons
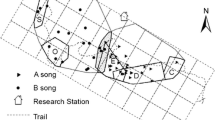
We observed 234 completely observed and 52 incompletely observed encounters during the 581 days of observations. Gibbons encountered other groups exclusively in the overlapping areas of their home ranges (Fig. 1) with a mean encounter frequency of 0.49 per day (SD = 0.63, range 0–2). The inter-group encounter frequency was not predicted by female reproductive status or fruit availability (full-null model comparison: χ2 = 0.47, df = 3, p = 0.925). Mean duration of complete encounters was 80 min (SD = 52, range 3–293). In the 234 completely observed encounters, chasing occurred in 72% of cases, and was mostly observed between adult males (87%). In general, males participated more often than females in inter-group encounters (chi-squared test; χ2 = 43.16, p < 0.001). During each encounter, we observed on average 3.9 chases by both sexes (SD = 4.7, range 0–25). We did not observe males preventing females from approaching another group by forcing them to stay away from opponent groups (i.e., herding). However, when females were chased by an opponent male, males almost always chased immediately the opponent male back within a minute (14 out of 20 times). Between 2013 and 2016, females participated by chasing in 18 out of 142 encounters, and only after the male partner initiated chasing. Females from either focal or non-focal groups sang in 32% of inter-group encounters (Nencounters = 46). Female songs lasted on average for 12 min (SD = 7, range 1–32). In seven encounters, we observed 16 cases of gibbons hitting each other with their fist. In all cases, an adult male hit an opponent male except in a single case in which an adult male hit a sub-adult female. Nonetheless, no serious injuries or lethal attacks during encounters were observed in this study, while it occurs, albeit very rarely, in other gibbon species (H. lar: Palombit 1993; H. albibarbis: Cheyne et al. 2010). We did not observe any affiliative interactions between adult individuals from different groups, neither grooming nor copulation. We observed playing twice between juveniles from two different groups (group B and S), which lasted only for a few seconds as they were immediately chased by the parents from the opponent group. The average home range size of three habituated groups was 38.3 ± 14 ha. The size of area overlapping was both 6 ha between group A and B, and between group B and S. Finally, gibbon groups did not exhibit clear dominance relationships, except one group dyad (group A and C; see Table 3, Fig. S1 in Supplementary Information).
Model I: male participation
Male participation was predicted by fruit availability, female reproductive status (cycling female/dependent infant), and the number of actively chasing opponents (full and null model comparison: χ2 = 19.12, df = 5, p = 0.002; Table 4). Males were significantly more likely to participate in inter-group encounters when the female was cycling and when a dependent infant was present (Fig. 2(a)), when fruit availability was higher (Fig. 2(b)) and when more individuals of the opponent group participated in the inter-group encounters (Fig. 2(c)).
Effects of (a) female reproductive status (other period, cycling, or dependent infant), (b) fruit availability, and (c) the number of actively participating opponents on probability of male participation in Javan gibbons (Hylobates moloch) in Gunung Halimun-Salak National Park, Indonesia, between 2009 and 2016. The bars indicate mean values of the probability of male participation for each female reproductive status when male participation absence and presence were coded into 0 and 1 respectively. The bubble size indicates the sample size for each data point (b) N = 3 to 22, (c) N = 3 to 142) and the shaded areas represent the 95% confidence intervals
Model II: female participation and model III: female singing
Neither female reproductive status, fruit availability, nor number of chasing opponents predicted female participation in inter-group encounters (full and null model comparison: χ2 = 4.74, df = 3, p = 0.192). The probability of females singing during inter-group encounters was predicted by the female reproductive status and presence of singing opponents (full and null model comparison: χ2 = 9.75, df = 4, p = 0.045; Table 5; Fig. 3(a), (b)), with females being more likely to sing when they were cycling and when individuals in the opponent group also sang.
Effect of (a) female reproductive status (other period, cycling, or dependent infant) and (b) presence of a singing opponent female (absence or presence) on the presence of female song during inter-group encounters in Javan gibbons (Hylobates moloch) in Gunung Halimun-Salak National Park, Indonesia, between 2013 and 2016. The bars indicate mean values of the probability of female singing. Presence and absence of singing opponent female were coded into 0 and 1 respectively
Model IV: outcome
In all three models examining the influence of space use for each time category (1, 3, and 6 months) on the probability of winning an encounter, only female reproductive status predicted the probability of winning an encounter. Focal groups were more likely to win an encounter when females were cycling compared to when females were not cycling (full and null model comparison: 1-month overlap: χ2 = 12.64, df = 5, p = 0.027; 3-month overlap: χ2 = 13.26, df = 5, p = 0.021; 6-month overlap: χ2 = 13.28, df = 5, p = 0.021). The intensity of space use (1, 3, and 6 months), number of actively participating opponents, or proportion of chasing frequency did not predict the probability of winning an encounter (Table 6).
Discussion
Inter-group encounters in Javan gibbons occurred every other day and were mostly aggressive, without any affiliative interactions between groups such as grooming or extra-pair copulation, which have been frequently reported in other gibbon species (S. syndactylus: Palombit 1994; N. gabriellae: Kenyon et al. 2011; H. lar: Bartlett 2003; Barelli et al. 2013; N. concolor jingdongensis: Huang et al. 2013). The frequency of inter-group encounters did not co-vary with any socio-ecological factors investigated and we did not find any dominance relationships among groups. Male Javan gibbons participated more often in inter-group encounters than females, especially when females were cycling or dependent infants were present, supporting both the mate and infant defense hypotheses. In addition, males also participated more often when more opponents were actively participating in the inter-group encounters. They also participated more when food availability was high, contradicting the food resource hypothesis. In contrast to males, female participation by chasing in inter-group encounters was not predicted by any socio-ecological factors investigated in this study, probably because female chasing was too rare to draw a significant conclusion. Females participated more often in singing when they were cycling and when females in the opponent group also sang, supporting the mate attraction hypothesis. The probability of winning an encounter was best predicted by the presence of cycling females but not by the intensity of space use, providing additional support for the mate defense hypothesis. Hence, in pair-living Javan gibbons the mate and infant defense hypotheses best predicted male participation in inter-group encounters.
Mate defense hypothesis
Javan gibbon males participated more often when cycling females were present, supporting the mate defense hypothesis. Moreover, Javan gibbon males immediately chased opponent males back when their pair-mates were chased, also supporting the mate defense hypothesis. This appears to be a highly effective strategy to defend females and/or to prevent potential extra-pair copulations, considering that extra-pair copulations occur exclusively during inter-group encounters in other gibbon species (S. syndactylus: Palombit 1994; H. lar: Bartlett 2003; N. c. jingdongensis: Huang et al. 2013). Males also participated more often when in the opponent group more individuals were actively participating in the inter-group encounters, suggesting that they actively adjust their fighting power to that of the opponent group, a phenomenon that has also been observed in Verreaux’s sifakas (Koch et al. 2016b).
The probability of winning an encounter was only predicted by the presence of cycling females in a group, which again supports the mate defense hypothesis. Interestingly, winning in Javan gibbons was not achieved by higher levels of aggression (i.e., chasing frequency). Inter-group encounters in Javan gibbons lasted on average for 80 min and often ended long after the last aggressive interaction took place. In contrast, in blue monkeys (Cercopithecus mitis), a losing group usually retreated almost immediately after the last aggressive interaction (Roth and Cords 2016). Hence, Javan gibbons may use a different tactic to win a conflict, and probably to defend their mate, by withstanding and not giving up on the area by moving away. The mate defense hypothesis might be of particular importance for male gibbons, because gibbons have a relatively slow development with long interbirth intervals (ca. 41 months: H. lar: Reichard and Barelli 2008; about 43 months: Javan gibbons in the study population), and males may face high reproductive costs when females copulate with extra-pair males.
Infant defense hypothesis
Because male Javan gibbons also participated more often in inter-group encounters when their own infants were dependent, participation may also serve to defend dependent infants from a potential risk of infanticide. Losing an infant might be especially costly for Javan gibbons due to the slow development and long interbirth intervals. However, the presence of infants did not influence the probability of participating in an inter-group encounter in females. Since female Javan gibbons mainly participate by singing and not by chasing, infants might not be exposed to potential injuries or infanticide during inter-group encounters in contrast to other primates such as vervet monkeys or Verreaux’s sifakas (Arseneau-Robar et al. 2016; Koch et al. 2016a).
Food resource defense hypothesis
Javan gibbon males participated more often in inter-group encounters, when fruit availability was higher, contradicting prediction of the food resource defense hypothesis (Reichard and Sommer 1997; Cooper et al. 2004; Korstjens et al. 2005; Thompson et al. 2012). Since participation might be energetically costly, males may participate more often when more food is available. Similarly, Taî chimpanzees were engaged more often in territorial activities during periods of high food availability because individuals might have been in a better physical condition, a direct correlate of fighting abilities (Pan troglodytes verus: Herbinger et al. 2001).
Moreover, figs, the preferred food item for Javan gibbons (Kim et al. 2012), are difficult to defend because they are fruiting asynchronously (Janzen 1979; Kinnaird et al. 1999). In contrast, other gibbon species usually defend their main fruiting trees of non-fig species, whereas figs serve only as fallback food (Leighton 1983; Reichard and Sommer 1997; Harrison and Marshall 2011). Therefore, the food defense may have a different mechanism for Javan gibbons in comparison to other gibbon species (H. lar: Reichard and Sommer 1997). For instance, male Javan gibbons may participate in inter-group encounters more often when food availability is high because they might be in better condition. When less food is available, they may focus more on foraging and saving energy, rather than competing with other groups over limited food sources which anyway may not be defensible.
While fruit availability can be a temporal index to test the food resource defense hypothesis, the intensity of space use prior to an encounter can represent a spatial index to test the (food) resource defense hypothesis. Considering that the universally used definition of outcome of inter-group encounters (i.e., a winning group stays longer in the encounter location) is location-based, the intensity of space use prior to an encounter can well reflect the spatial perspective of food resources for animals. However, the intensity of space use at the encounter location in any time period (i.e., 1, 3, and 6 months preceding encounter events) did not predict the outcome of inter-group encounters in Javan gibbons in this study. On the contrary, in other territorial primates such as Verreaux’s sifakas (Koch et al. 2016b), yellow baboons (Papio cynocephalus: Markham et al. 2012), and blue monkeys (Cercopithecus mitis: Roth and Cords 2016), the relative intensity of space use predicted the outcome of inter-group conflicts, with groups using the area more intensively being more likely to win the encounter because these areas might have been more valuable for them (i.e., probably more feeding occurred in the area). In addition, in Verreaux’s sifakas and yellow baboons winning groups used the encountered area after an encounter more often than losing groups, indicating that losing an encounter can result in longer-term disadvantages (Markham et al. 2012; Koch et al. 2016b). It is possible that the intensity of space use may not represent a good proxy to assess the potential value of the used area in Javan gibbons. However, the fact that Javan gibbons did not win conflicts more often in intensively used areas may suggest that the outcome of encounters might not be associated with critical dis/advantages in Javan gibbons. For instance, regardless of the outcome of inter-group encounters, Javan gibbons rather avoid potential inter-group encounters by sleeping further away from aggressive encounter locations (Yi et al. 2020a). Given the frequent inter-group encounters in overlapping areas, winning Javan gibbon groups might not be expected to monopolize the area for long-term because they share a large portion of their home ranges with other groups’ and are able to cross their home range multiple times a day (mean home range 38.8 ha (range 25.6–53.5 ha); this study, mean daily path lengths 1488 m (range 638–3300 m); Ham et al. 2017). Hence, the (food) resource defense hypothesis may not explain participation in inter-group encounters in Javan gibbons.
Mate attraction hypothesis
Javan gibbon females participated more often in inter-group encounters by singing, when they were cycling and when the female of the opponent group also sang. Hence, female songs of this non-duetting species might function to attract extra-group males when they are cycling, which in turn may trigger opponent females to follow singing to advertise their presence. Since singing, especially the long-distance calls that female gibbons produce, is costly, it might have evolved to signal female’s physical condition and, hence, fighting ability (Vehrencamp 2000; Terleph et al. 2016). In other gibbon species, female-female replacements have been observed several times (H. lar: Reichard et al. 2012; Terleph et al. 2016), suggesting that females may face strong intra-sexual competition over territories (Sommer and Reichard 1997). In addition, the song of females during inter-group encounters has been suggested for some gibbon species to function in intra-sexual competition (Raemaekers et al. 1984; Mitani 1985; Raemaekers and Raemaekers 1985a; Cowlishaw 1992). Hence, the song of female Javan gibbons during inter-group encounters may have a dual function: to attract extra-group males when females are cycling but also in intra-sexual competition.
Conclusion
In conclusion, our results indicate that in Javan gibbons, socio-ecological factors affected individual participation in inter-group conflicts, differently in males and females. Males participate mainly by chasing opponents to defend mates and infants but not food resources. Females, in contrast, participate mainly by singing, most likely to advertise their reproductive status. The probability to win an encounter was predicted by female’s reproductive status but not by the relative intensity of space use providing additional support for the mate defense hypothesis for males but no support for the food resource defense hypothesis. Since Javan gibbons frequently encounter neighboring groups and easily cross their home ranges several times a day, they may rely on a strategy of frequently checking overlapping areas and exhibit moderate levels of aggression such as non-lethal chasing of their “dear enemy” (Fisher 1954) during inter-group encounters. The mate and infant defense appear to predict best participation during inter-group encounters in pair-living species because costs of extra-group copulations and infanticide risk might be relatively higher in pair-living than in group-living species.
Data availability
The data used for this study are available from the corresponding author on request.
References
Alfred J, Sati J (1991) On the first record of infanticide in the hoolock gibbon Hylobates hoolock in the wild. Rec Zool Surv India 89:319–321
Ardito G (1976) Check-list of the data on the gestation length of primates. J Hum Evol 5:213–222
Barelli C, Matsudaira K, Wolf T, Roos C, Heistermann M, Hodges K, Ishida T, Malaivijitnond S, Reichard UH (2013) Extra-pair paternity confirmed in wild white-handed gibbons. Am J Primatol 75:1185–1195
Barr DJ, Levy R, Scheepers C, Tily HJ (2013) Random effects structure for confirmatory hypothesis testing: keep it maximal. J Mem Lang 68:255–278
Bartlett TQ (2003) Intragroup and intergroup social interactions in white-handed gibbons. Int J Primatol 24:239–259
Berkson G (1966) Development of an infant in a captive gibbon group. J Genet Psychol 108:311–325
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bonadonna G, Zaccagno M, Torti V, Valente D, De Gregorio C, Randrianarison RM, Tan C, Gamba M, Giacoma C (2020) Intra- and intergroup spatial dynamics of a pair-living singing primate, Indri indri: a multiannual study of three indri groups in Maromizaha Forest, Madagascar. Int J Primatol 41:224–245
Borries C, Savini T, Koenig A (2011) Social monogamy and the threat of infanticide in larger mammals. Behav Ecol Sociobiol 65:685–693
Brockelman WY, Reichard U, Treesucon U, Raemaekers JJ (1998) Dispersal, pair formation and social structure in gibbons (Hylobates lar). Behav Ecol Sociobiol 42:329–339
Brown M (2013) Food and range defence in group-living primates. Anim Behav 85:807–816
Cheyne SM, Monks EM, Kuswanto Y (2010) An observation of lethal aggression in Bornean white-bearded gibbons Hylobates albibarbis. Gibbon J 6:1–6
Cooper MA, Aureli F, Singh M (2004) Between-group encounters among bonnet macaques (Macaca radiata). Behav Ecol Sociobiol 56:217–227
Cowlishaw G (1992) Song function in gibbons. Behaviour 121:131–153
Cowlishaw G (1995) Behavioural patterns in baboon group encounters: the role of resource competition and male reproductive strategies. Behaviour 132:75–86
Crofoot MC, Gilby IC, Wikelski MC, Kays RW (2008) Interaction location outweighs the competitive advantage of numerical superiority in Cebus capucinus intergroup contests. P Natl Acad Sci USA 105:577–581
Ellefson JO (1968) Territorial behavior in the common white-handed gibbon (Hylobates lar). Holt, Rinehart and Winston, New York
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Fashing PJ (2001) Male and female strategies during intergroup encounters in guerezas (Colobus guereza): evidence for resource defense mediated through males and a comparison with other primates. Behav Ecol Sociobiol 50:219–230
Fisher J (1954) Evolution and bird sociality. In: Huxley J, Hardy AC, Ford EB (eds) Evolution as a process. Allen & Unwin, London, pp 71–83
Forstmeier W, Schielzeth H (2011) Cryptic multiple hypotheses testing in linear models: overestimated effect sizes and the winner’s curse. Behav Ecol Sociobiol 65:47–55
Fox J, Weisberg S, Adler D, Bates D, Baud-Bovy G, Ellison S, Firth D, Friendly M, Gorjanc G, Graves S (2012) Package ‘car’. R Foundation for Statistical Computing, Vienna https://cran.rproject.org/web/packages/car/index.html
Frisch JE (1963) Sex-differences in the canines of the gibbon (Hylobates lar). Primates 4:1–10
Furrer RD, Kyabulima S, Willems EP, Cant MA, Manser MB (2011) Location and group size influence decisions in simulated intergroup encounters in banded mongooses. Behav Ecol 22:493–500
Garber PA, Pruetz J, Isaacson J (1993) Patterns of range use, range defense, and intergroup spacing in moustached tamarin monkeys (Saguinus mystax). Primates 34:11–25
Geissmann T (1991) Reassessment of age of sexual maturity in gibbons (Hylobates spp.). Am J Primatol 23:11–22
Geissmann T (1993) Evolution of communication in gibbons (Hylobatidae). PhD thesis, Anthropological Institute, Zürich University
Geissmann T (2002) Duet-splitting and the evolution of gibbon songs. Biol Rev 77:57–76
Geissmann T, Nijman V (2006) Calling in wild silvery gibbons (Hylobates moloch) in Java (Indonesia): behavior, phylogeny, and conservation. Am J Primatol 68:1–19
Ham S, Hedwig D, Lappan S, Choe JC (2016) Song functions in nonduetting gibbons: evidence from playback experiments on Javan gibbons (Hylobates moloch). Int J Primatol 37:225–240
Ham S, Lappan S, Hedwig D, Choe JC (2017) Female songs of the nonduetting Javan gibbons (Hylobates moloch) function for territorial defense. Int J Primatol 38:533–552
Harris TR (2006) Between-group contest competition for food in a highly folivorous population of black and white colobus monkeys (Colobus guereza). Behav Ecol Sociobiol 61:317–329
Harris TR (2010) Multiple resource values and fighting ability measures influence intergroup conflict in guerezas (Colobus guereza). Anim Behav 79:89–98
Harrison ME, Marshall AJ (2011) Strategies for the use of fallback foods in apes. Int J Primatol 32:531–565
Herbinger I, Boesch C, Rothe H (2001) Territory characteristics among three neighboring chimpanzee communities in the Taï National Park, Côte d'Ivoire. Int J Primatol 22:143–167
Hrdy SB (1979) Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40
Hu N, Guan Z, Huang B, Ning W, He K, Fan PF, Jiang X (2018) Dispersal and female philopatry in a long-term, stable, polygynous gibbon population: evidence from 16 years field observation and genetics. Am J Primatol 80:e22922
Huang B, Guan Z, Ni Q, Orkin JD, Fan P, Jiang X (2013) Observation of intra-group and extra-group copulation and reproductive characters in free ranging groups of western black crested gibbon (Nomascus concolor jingdongensis). Integr Zool 8:427–440
Janzen DH (1979) How to be a fig. Annu Rev Ecol Evol S 10:13–51
Kappeler M (1984) Vocal bouts and territorial maintenance in the moloch gibbon. In: Preuschoft H, Chivers DJ, Brockelman WY, Creel N (eds) The lesser apes: evolutionary and behavioral biology. Edinburgh University Press, Edinburgh, pp 376–389
Kappeler PM, Pozzi L (2019) Evolutionary transitions toward pair living in nonhuman primates as stepping stones toward more complex societies. Sci Adv 5:eaay1276
Kelly RC (2005) The evolution of lethal intergroup violence. P Natl Acad Sci USA 102:15294–15298
Kenyon M, Roos C, Binh VT, Chivers D (2011) Extrapair paternity in golden-cheeked gibbons (Nomascus gabriellae) in the secondary lowland forest of Cat Tien National Park, Vietnam. Folia Primatol 82:154–164
Kim S (2012) Feeding ecology and foraging strategies of the endangered Javan gibbon (Hylobates moloch) in a submontane forest of West Java. Phd thesis, Department of Life Science, Seoul National University
Kim S, Lappan S, Choe JC (2011) Diet and ranging behavior of the endangered Javan gibbon (Hylobates moloch) in a submontane tropical rainforest. Am J Primatol 73:270–280
Kim S, Lappan S, Choe JC (2012) Responses of Javan gibbon (Hylobates moloch) groups in submontane forest to monthly variation in food availability: evidence for variation on a fine spatial scale. Am J Primatol 74:1154–1167
Kinnaird MF, O’Brien TG, Suryadi S (1999) The importance of figs to Sulawesi’s imperiled wildlife. Trop Biodivers 6:5–18
Kitchen DM, Beehner JC (2007) Factors affecting individual participation in group-level aggression among non-human primates. Behaviour 144:1551–1581
Kitchen DM, Cheney DL, Seyfarth RM (2004) Factors mediating inter-group encounters in savannah baboons (Papio cynocephalus ursinus). Behaviour 141:197–218
Koch F, Signer J, Kappeler PM, Fichtel C (2016a) Intergroup encounters in Verreaux’s sifakas (Propithecus verreauxi): who fights and why? Behav Ecol Sociobiol 70:797–808
Koch F, Signer J, Kappeler PM, Fichtel C (2016b) The role of the residence-effect on the outcome of intergroup encounters in Verreaux’s sifakas. Sci Rep 6:28457
Korstjens AH, Nijssen EC, Noë R (2005) Intergroup relationships in western black-and-white colobus, Colobus polykomos polykomos. Int J Primatol 26:1267–1289
Lawrence JM (2007) Understanding the pair bond in brown titi monkeys (Callicebus brunneus): male and female reproductive interests. PhD thesis, Columbia University
Leighton M (1983) Vertebrate responses to fruiting seasonality within a Bornean rain forest. In: Whitmore STC, Chadwick AC (eds) Tropical rain forest: ecology and management, vol 2. Blackwell Scientific Publications, Palo, pp 181–195
Lucchesi S, Cheng L, Janmaat K, Mundry R, Pisor A, Surbeck M (2020) Beyond the group: how food, mates, and group size influence intergroup encounters in wild bonobos. Behav Ecol 31:519–532
Lukas D, Huchard E (2014) The evolution of infanticide by males in mammalian societies. Science 346:841–844
Majolo B, Vizioli AD, Martínez-Íñigo L, Lehmann J (2020) Effect of group size and individual characteristics on intergroup encounters in primates. Int J Primatol 41:325–341
Markham AC, Alberts SC, Altmann J (2012) Intergroup conflict: ecological predictors of winning and consequences of defeat in a wild primate population. Anim Behav 84:399–403
Mirville MO, Ridley AR, Samedi J, Vecellio V, Ndagijimana F, Stoinski TS, Grueter CC (2018) Factors influencing individual participation during intergroup interactions in mountain gorillas. Anim Behav 144:75–86
Mitani JC (1985) Gibbon song duets and intergroup spacing. Behaviour 92:59–96
Mundry R (2014) Statistical issues and assumptions of phylogenetic generalized least squares. In: Garamszegi LZ (ed) Modern phylogenetic comparative methods and their application in evolutionary biology: concepts and practice. Springer, Berlin, pp 131–153
Oktaviani R, Kim S, Cahyana A, Choe J (2018) Nutrient composition of the diets of Javan gibbons (Hylobates moloch). IOP Conf Ser: Earth Environ Sci 197:012048
Opie C, Atkinson QD, Dunbar RI, Shultz S (2013) Male infanticide leads to social monogamy in primates. P Nat Acad Sci USA 110:13328–13332
Palmer TM (2004) Wars of attrition: colony size determines competitive outcomes in a guild of African acacia ants. Anim Behav 68:993–1004
Palombit RA (1993) Lethal territorial aggression in a white-handed gibbon. Am J Primatol 31:311–318
Palombit RA (1994) Extra-pair copulations in a monogamous ape. Anim Behav 47:721–723
Palombit RA (1999) Infanticide and the evolution of pair bonds in nonhuman primates. Evol Anthropol 7:117–129
Palombit RA, Cheney DL, Fischer J, Johnson S, Rendall D, Seyfarth R, Silk J (2000) Male infanticide and defense of infants in chacma baboons. In: van Schaik C, Janson C (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 123–152
Payne HF, Lawes MJ, Henzi SP (2003) Competition and the exchange of grooming among female samango monkeys (Cercopithecus mitis erythrarchus). Behaviour 140:453–471
Peres CA (1989) Costs and benefits of territorial defense in wild golden lion tamarins, Leontopithecus rosalia. Behav Ecol Sociobiol 25:227–233
Raemaekers JJ, Raemaekers PM (1985a) Field playback of loud calls to gibbons (Hylobates lar): territorial, sex-specific and species-specific responses. Anim Behav 33:481–493
Raemaekers PM, Raemaekers JJ (1985b) Long-range vocal interactions between groups of gibbons (Hylobates lar). Behaviour 95:26–44
Raemaekers JJ, Raemaekers PM, Haimoff EH (1984) Loud calls of the gibbon (Hylobates lar): repertoire, organisation and context. Behaviour 91:146–189
Rasoloharijaona S, Rakotosamimanana B, Zimmermann E (2000) Infanticide by a male Milne-Edwards’ sportive lemur (Lepilemur edwardsi) in Ampijoroa, NW-Madagascar. Int J Primatol 21:41–45
Reichard UH, Barelli C (2008) Life history and reproductive strategies of Khao Yai Hylobates lar: implications for social evolution in apes. Int J Primatol 29:823–844
Reichard U, Sommer V (1997) Group encounters in wild gibbons (Hylobates lar): agonism, affiliation, and the concept of infanticide. Behaviour 134:1135–1174
Reichard UH, Ganpanakngan M, Barelli C (2012) White-handed gibbons of Khao Yai: social flexibility, complex reproductive strategies, and a slow life history. In: Kappeler PM, Watts DP (eds) Long-term field studies of primates. Springer, Berlin, pp 237–258
Roth AM, Cords M (2016) Effects of group size and contest location on the outcome and intensity of intergroup contests in wild blue monkeys. Anim Behav 113:49–58
Saito C, Sato S, Suzuki S, Sugiura H, Agetsuma N, Takahata Y, Sasaki C, Takahashi H, Tanaka T, Yamagiwa J (1998) Aggressive intergroup encounters in two populations of Japanese macaques (Macaca fuscata). Primates 39:303–312
Samuni L, Mielke A, Preis A, Crockford C, Wittig RM (2020) Intergroup competition enhances chimpanzee (Pan troglodytes verus) in-group cohesion. Int J Primatol 41:342–362
Schielzeth H, Forstmeier W (2008) Conclusions beyond support: overconfident estimates in mixed models. Behav Ecol 20:416–420
Schradin C (2004) Territorial defense in a group-living solitary forager: who, where, against whom? Behav Ecol Sociobiol 55:439–446
Seiler M, Hending D, Stanger-Hall KF (2019) Spacing and mate attraction: sex-specific function of advertisement calls in the northern giant mouse lemur (Mirza zaza). Folia Primatol 90:361–378
Sicotte P (1993) Inter-group encounters and female transfer in mountain gorillas: influence of group composition on male behavior. Am J Primatol 30:21–36
Sillero-Zubiri C, Macdonald DW (1998) Scent-marking and territorial behaviour of Ethiopian wolves Canis simensis. J Zool 245:351–361
Smith JM, Parker GA (1976) The logic of asymmetric contests. Anim Behav 24:159–175
Steenbeek R (1999) Tenure related changes in wild Thomas's langurs I: between-group interactions. Behaviour 136:595–625
Terleph TA, Malaivijitnond S, Reichard UH (2016) Age related decline in female lar gibbon great call performance suggests that call features correlate with physical condition. BMC Evol Biol 16:4
Thompson CL, Norconk MA, Whitten PL (2012) Why fight? Selective forces favoring between-group aggression in a variably pair-living primate, the white-faced saki (Pithecia pithecia). Behaviour 149:795–820
Treesucon U (1984) Social development of young gibbons (Hylobates lar) in Khao Yai National Park. MSc thesis, Mahidol University, Bangkok
Trivers R (1972) Parental investment and sexual selection. In: Houck LD, Drickamer LC (eds) Foundations of animal behavior: classic papers with commentaries. Biological Laboratories, Harvard University Cambridge, MA, Chicago, pp 795–838
van Schaik CP (1996) Social evolution in primates: the role of ecological factors and male behaviour. Proc Brit Acad 88:9–31
van Schaik CP, Dunbar R (1990) The evolution of monogamy in large primates: a new hypothesis and some crucial tests. Behaviour 115:30–61
van Schaik CP, Kappeler PM (1997) Infanticide risk and the evolution of male–female association in primates. Proc R Soc Lond B 264:1687–1694
Vehrencamp SL (2000) Handicap, index, and conventional signal elements of bird song. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir Academic Press, Trondheim, pp 277–300
Wich SA, Assink PR, Becher F, Sterck EHM (2002) Playbacks of loud calls to wild Thomas langurs (Primates; Presbytis thomasi): the effect of location. Behaviour 139:65–78
Wilson ML, Kahlenberg SM, Wells M, Wrangham RW (2012) Ecological and social factors affect the occurrence and outcomes of intergroup encounters in chimpanzees. Anim Behav 83:277–291
Yi Y (2020). Intra-group and inter-group social interactions in a pair-living primate, the Javan gibbon (Hylobates moloch). PhD thesis, Ewha Womans University, Seoul
Yi Y, Fichtel C, Kim E, Choe JC (2020a) Impacts of intergroup interactions on intragroup behavioral changes in Javan Gibbons (Hylobates moloch). Int J Primatol 41:363–381
Yi Y, Kim Y, Hikmat A, Choe JC (2020b) Information transfer through food from parents to offspring in wild Javan gibbons. Sci Rep 10:714
Acknowledgments
This project was conducted in collaboration with the Department of Natural Resource Conservation and Ecotourism at the Institut Pertanian Bogor (IPB). We thank the Indonesian Ministry of Research and Technology (RISTEK), the Indonesian Ministry of Forestry’s Department for the Protection and Conservation of Nature (PHKA), and the Gunung Halimun-Salak National Park (GHSNP) for the research permissions. We thank Agus Hikmat, Rinekso Soekmadi, Ani Mardiastuti, Mirza Kusrini, and GHSNP staff for their assistance and cooperation. We also thank Sanha Kim for contributions to the establishment of the field site and also for helpful comments, Sunyoung Ahn for administrative support and coordination in Korea, and especially Rahayu Oktaviani for administrative support and coordination in Indonesia. We thank Yena Kim for helpful comments and Kyungmin Kim for helping us with the graphical output. We are grateful to Roger Mundry for his help with statistical analysis. We thank our field assistants, Muhamad Nur, Ri Rudini, Isra Kurnia, and Iyan Sopian, for their hard work in the field. Finally, we are grateful to the editor and two anonymous reviewers for their comments and edits to this manuscript.
Funding
This work was supported by the Amore Pacific Academic and Cultural Foundation (AACF), Ewha Womans University, and German Academic Exchange Service (DAAD).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This study involved observation of animals in their natural habitat and was not disruptive to the subjects and the ecosystem. Our research protocol was approved by the Indonesian Ministry of Research and Technology (RISTEK; SIP: 375/SIP/FRP/SM/X/2014, 91/P/TNGHS/2015, 652/FRP/SM/VI/2015), the Indonesian Ministry of Forestry’s Department for the Protection and Conservation of Nature (PHKA), and the Gunung Halimun-Salak National Park.
Additional information
Communicated by E. Huchard
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1627 kb)
Rights and permissions
About this article
Cite this article
Yi, Y., Fichtel, C., Ham, S. et al. Fighting for what it’s worth: participation and outcome of inter-group encounters in a pair-living primate, the Javan gibbon (Hylobates moloch). Behav Ecol Sociobiol 74, 96 (2020). https://doi.org/10.1007/s00265-020-02879-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-020-02879-0