Abstract
Universal predictions on the occurrence of cooperative breeding are still elusive. This breeding strategy is strongly linked to phylogeny; therefore, studies on species within groups where cooperative breeding is more prevalent could improve our understanding. Many diurnal raptors exhibit cooperative breeding, although occurrence rates are mostly based on anecdotal observations at nests. Here, we present a detailed study of the reproductive output and social organisation of the African pygmy falcon. Using data from six breeding seasons, we found helpers at 19% of nests. Helper presence had a positive effect on the body condition of the chicks as brood size increased, likely due to their contribution to feeding the chicks. Cooperative breeding groups were more likely to occur following years of higher reproductive output. Indeed, most of the helpers (77%) were non-dispersed offspring from the previous year, whereas the other helpers were immigrant adults (23%). We identified groups that included retained offspring (46%), immigrant adults (27%) or both types of helpers (27%). Breeding groups were also described as multi-male (65%), multi-female (26%) and multi-male-female (9%). Pygmy falcon group composition proved to be highly variable and diverse compared to other raptor species, in which helpers are generally unrelated adult males, showing that selection pressures leading to group formation in diurnal raptors may be more diverse than previously thought.
Significance statement
Cooperative breeding occurs when more than two individuals contribute to raising a brood of young. It occurs in approximately 9% of bird species and it is particularly frequent in diurnal raptors. We studied the breeding performance and social organisation of the African pygmy falcon and found that they form breeding groups at 19% of the nests. These breeding groups included the breeding pair plus helpers that are either males or females. Helpers were also either adult immigrants or retained offspring. We also found that groups produced healthier fledglings than pairs and that groups are more likely to occur following years with high fledgling success. With this work, we expand the number of studies on raptor cooperative breeding and explore the fitness advantages of group breeding in this species as well as the mechanisms behind group formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cooperative breeding occurs when more than two individuals contribute to raising a brood of young (Koenig and Dickinson 2004). Despite occurring in just 9% of bird, 3% of mammal and < 0.1% of fish species (Emlen 1991; Taborsky 1994; Cockburn 2006), this breeding system is widely studied in behavioural ecology. Both indirect and direct fitness benefits of cooperative breeding likely help to explain the occurrence of kin and non-kin cooperative breeding, e.g. increased survival, offspring production and load-lightening (Hamilton 1964; Koenig and Dickinson 2004; Riehl 2013). However, no universal selection pressures for the evolution of cooperative breeding across taxa or geographic regions have been identified (Ford et al. 1988; Du Plessis et al. 1995; Arnold and Owens 1998; Arnold 1999; Jetz and Rubenstein 2011; Shen et al. 2017). Similarly, there is no general consensus of the conditions that underlie individuals’ decisions to help or what the fitness consequences for kin and non-kin helpers are (Bergmüller et al. 2007; Brosnan and Bshary 2010; Kingma et al. 2014). Further studies on cooperative breeding, ideally in a wide variety of taxa, are needed to improve current knowledge.
Cooperative breeding is strongly linked to phylogeny (Edwards and Naeem 1993). In birds, it occurs in more than 50 families, with some families showing cooperative breeding in most species. Diurnal raptors show cooperative breeding in 14% of extant species (Kimball et al. 2003), although occurrence is likely underestimated due to the difficulties and scarcity of studying this functional group (Kimball et al. 2003; Koenig and Dickinson 2004). Anecdotal accounts are mainly used to confirm cooperative breeding, but for species with adequate data, Kimball et al. (2003) found that auxiliary individuals in cooperative groups were generally unrelated adult males (social polyandry). Kimball et al. (2003) concluded that in contrast to many cooperatively breeding passerine species, cooperative breeding in diurnal raptors is independent of delayed dispersal. They listed several other factors that may promote cooperative breeding through direct and indirect benefits to unrelated helpers, such as spatial and temporal variability of resources (Parker and Ports 1982; Faaborg 1986), limited territories due to habitat saturation (Faaborg 1986; Heredia and Donázar 1990; Tella 1993), lack of mates (Garcelon et al. 1995), increased survivorship in groups (Faaborg et al. 1980; Faaborg and Bednarz 1990) and hunting larger prey (Bednarz 1988; Malan 1998). Unfortunately, the limited studies and data do not allow for discrimination among these benefits.
Breeding groups have been linked to an increase in the number and quality of offspring produced per breeding event (Emlen and Wrege 1991; Heinsohn and Cockburn 1994; Hatchwell et al. 2004; Valencia et al. 2006; Canestrari et al. 2008; Covas et al. 2008). Helpers in cooperatively breeding birds may benefit breeding by feeding chicks, building the nest and defending it against predators (reviewed by Koenig and Dickinson 2004). Reproduction-related duties by helpers may increase offspring condition, which is often a good proxy for higher survival of the young and increased probability of recruitment into the breeding population (Hatchwell et al. 2004; Moreno et al. 2005; Rodríguez et al. 2016). Group breeding may also influence laying behaviour by increasing the number of clutches produced per season (Brown and Brown 1981; Russell and Rowley 1988; Canestrari et al. 2008) or by advancing the time of laying (Dias et al. 2015).
In raptors, only three cooperative species have been studied such that comparisons can be made between the reproductive success of breeding groups compared with that of breeding pairs: pale chanting goshawk Melierax canorus trios suffered lower nest predation (Malan and Jenkins 1996) and successfully fledged second broods more often than pairs (Malan et al. 1997); Harris’ hawk Parabuteo unicinctus groups produced larger offspring and were more likely to double brood in a season (Bednarz 1987); and Galapagos hawks Buteo galapagoensis produce more fledglings when breeding in groups (Faaborg et al. 1980).
Here, we present the first detailed account of cooperative breeding in the African pygmy falcon Polihierax semitorquatus. In one of the few published studies of this species, Maclean (1970) described aspects of the breeding biology of this tiny falcon (50–60 g), but never mentioned the occurrence of breeding groups. Thomsett (1991) initially reported a single observation of two males copulating in turns with the same female, describing the species as potentially polyandrous. Several years later, Spottiswoode et al. (2004) described four cases of a third group member helping on the feeding of the chicks, confirming the facultative cooperative behaviour. In this study, we describe the dynamics of group composition in the pygmy falcon and examine three questions: (i) How frequent are cooperatively breeding groups in pygmy falcons? (ii) Who are the auxiliary individuals in terms of relatedness and sex? (iii) Do helpers influence the reproductive success of the breeding pair?
Methods
Study site
The study was done in Tswalu Kalahari, a reserve in the Northern Cape Province of South Africa (27.225° S 22.478° E). The study area comprised an ~ 130 km2 area of the reserve. The study site is a semi-arid area at the southern border of the Kalahari Desert. It is characterised by savanna vegetation in the plains and dunes that comprises scattered trees (camelthorn Vachellia erioloba and shepherd’s trees Boscia albitrunca).
Data collection
Pygmy falcons do not build their own nests but use those built by other species; in their southern African range, they exclusively use chambers within the massive colonial nests built by sociable weavers (Philetairus socius; Maclean 1970; Maclean 1973). Pygmy falcons are sexually dimorphic, non-migratory resident and breed from August to March (Maclean 1970). At the beginning of each breeding season (2011 to 2016), we surveyed all sociable weaver colonies within the study area (Table 1) to find signs of recent chamber use by falcons or falcon activity around colonies. Falcon chambers are usually easily identifiable due to the conspicuous white faecal mat visible at the entrance (Maclean 1970; Krochuk et al. 2018). We checked falcon chambers using a telescopic mirror fitted with a LED light every 7 to 10 days until breeding was confirmed. Sometimes falcons use more than one chamber within the weaver colony; therefore, when we refer to a falcon nest, it means the one chamber where breeding was detected. Falcon nests were then visited weekly to follow the breeding attempts. The annual period of fieldwork increased during the study: 2 months (Oct–Nov) in 2011 and 2012, 3 months (Oct–Dec) in 2013, 4 months (Oct–Jan) in 2014 and 6 months (Sep–Feb) in 2015 and 2016.
Falcons were captured at their roosting/breeding chambers in the weaver colonies and ringed. Between 2011 and 2014, all individuals were ringed with numbered metal rings. From 2015, all adult birds were fitted with a unique combination of one numbered metal ring and three coloured plastic rings. Nestlings and recent fledglings were only fitted with numbered metal rings, but if captured in subsequent years as adults they received colour rings. If any adult was not colour ringed at a colony, we performed a capture to identify individuals with metal rings (and add coloured ones) or to add full metal/colour ring combinations to unringed birds. All captures and ringing were done when chicks were 1–2 weeks from fledging or older. We performed captures just before sunrise using a fabric bag sewn around a metal ring that was placed flush against the chamber. We then softly pushed a blunt stick into the grass of the colony to coax the bird(s) out of the chamber. Our method allowed targeted falcon captures. Standard measurements, wing, tail, maximum tarsus and mass were taken from all captured adults and chicks. We used maximum tarsus and mass measurements to calculate a body condition index (BCI) to assess chick quality. The BCI was calculated as the residuals of the regression of body mass on tarsus length of the chicks. We used this method because chicks were not always measured at the same age, and this type of index is widely used as the results are independent from body size (here measured as tarsus length), which allows to analyse data from chicks of different age (Labocha and Hayes 2012).
All falcons using a given weaver colony were usually captured during a trapping event. If we noticed that one or more birds escaped capture, we attempted another capture after about 2 weeks. It was easy to determine if any birds were missed or escaped capture, because they would stay near the colony after sunrise calling to contact the rest of the group. The number and identity of individuals at a colony was confirmed during capture events. After colour ringing individuals, we revised the number and identity of birds at the colonies through direct observations or video cameras (Canon Legria camcorders) mounted on a tripod below the nests, focusing on the entrance to the breeding chamber. When we captured more than two adult falcons within the same colony, found either in one or more chambers, we classified the breeding unit type as a breeding group, as opposed to a breeding pair. We never detected more than one breeding pair or group of falcons using the same weaver colony at the same time.
With an individually marked population, we were able to classify helpers at a nest as (1) retained offspring or as (2) immigrants. Firstly, if we caught multiple birds of a gender at a nest, we classified the bird that was found at the same nest in previous years as the breeder. The rest of the individuals were classified as helpers. When two or more birds were originated from the same year at the nest, we classified the individual that was earlier in the study site as the breeder. Retained offspring were adult individuals captured known to be previous offspring of any other adult captured in the same colony. Immigrants were any other adult that was ringed in previous years at a different colony, or was captured unringed and was therefore new to the study population.
For each falcon nest, we aimed to record the breeding unit type (pair/group), laying date, clutch size, fledgling production (number of offspring that successfully fledged from the nest) and the cause of failure when not successful. During 2011, we detected some cases of likely nest abandonment at the egg stage after the incubating individual was flushed during nest inspection. We therefore minimised the number of visits to the nests once we found a clutch. As a result, we could not accurately determine laying date for most nests, and clutch size also remained unknown in some cases. Resolution for laying date was limited to laying month, which was back calculated using 27- to 31-day incubation and 30-day nestling periods (Maclean 1970). It was not possible to record data blind because our study involved focal animals in the field.
In this study, we monitored 25–39 falcon nests per season and followed a total of 189 falcon breeding attempts (Table 1). We ringed a total of 130 adults (67 males and 63 females) and 258 fledglings and individually identified all falcons in 155 nests.
Statistical analyses
Data from the pilot season (2011) were removed from analyses because field monitoring was less accurate than in subsequent years. We carried out all analysis using R statistical package version 3.4.2 (R Development Core Team 2017). Data were analysed using generalised linear mixed models (GLMM) with season and colony ID fitted as random factors in all analyses to control for repeated measures across seasons and colonies. Using colony ID as a random factor in the analyses, also partially controlled for territory/pair quality, which are potential confounding factors when assessing the effect of group size on breeding success and were not independently evaluated in this study. We did not include Breeding pair ID as random factor because Colony ID explained variance better, and both random factors appeared confounded when included together. We used R package lme4 (Bates et al. 2015) and fit the models with Poisson distribution, unless the model residuals were overdispersed, in which case we set binomial distribution using R package MASS (Venables and Ripley 2002).
We tested six response variables linked to breeding and reproductive output: laying month, probability of breeding attempt, clutch size, nest success, fledgling production and chick body condition. Breeding unit type (pair/group) was the explanatory variable of interest and it was included in all analyses. We also added laying month as explanatory variable when analysing clutch size, nest success, nest fledgling production and chick body condition. In addition, in the analysis of chick body condition, we included brood size and its interaction with the breeding unit type as explanatory variables. We included interactions when relevant and removed non-significant interactions from the final models.
We tested whether the probability of a breeding unit being a group in a particular season depended on the nest fledgling production of that same breeding unit the previous year. We fitted a GLMM with breeding unit type (group/pair) as the binomial response variable and number of fledglings produced in the previous year as an explanatory variable. We used binomial distribution and logit link function with the glmer function in lme4 package (Bates et al. 2015).
Data availability
The datasets analysed during the current study are available from the corresponding author on request.
Results
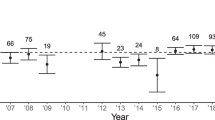
Of the 155 nests monitored, 81% contained a pair of adults (one female and one male), while the remaining 19% (n = 30) contained three or more adult individuals (hereafter groups) using the same weaver colony. The average number of group members was 3.32, ranging from three to five individuals. The occurrence of groups was variable between years (Table 2). Other than pairs, which consisted of one male and one female, we identified three types of cooperative groups: multi-male, when the group included only one female but more than one male; multi-female, for groups with only one male but more than one female and multi-male-female, if there were simultaneously more than one male and one female. The occurrence of these categories was 66.7%, 23.3% and 10% respectively (Table 2). Average sex ratio per group was 1.40 skewed toward males (Table 2). A total of 31 of the auxiliary individuals were males and 10 were females.
We identified 27 auxiliary individuals in 22 groups as immigrant adults (n = 10) or retained offspring (now adults) from previous years (n = 17). We found that groups included a pair and only immigrant adults in 27.3% of cases, only retained offspring in 45.5% of cases and both types of individuals in the remaining 27.3% of cases (Table 2). The retained offspring was a female in four groups; whereas, one or more males were found in the rest of the groups that included any retained offspring (n = 16).
In most cases, a single offspring delayed dispersal for a single year. We found one retained individual from the previous year in 11 occasions (68.8%). More rarely, two retained siblings were found in three occasions (18.8%), one individual that delayed dispersal for 2 years in one occasion (6.3%) and one individual that dispersed after fledging and came back as a helper to the natal nest after 1 year (6.3%). Retained offspring helped during 1 year and then disappeared/died in 13 occasions (4 females and 9 males). Offspring that delayed dispersal in different breeding seasons never co-occurred in the same group.
Helpers that were delayed offspring also had success in gaining breeding status in the future. On one occasion, a male helped during 1 year and became the main breeder the following year. In further two occasions, a retained male offspring helped during 1 or 2 years and then became main breeder at a different nest.
Immigrant helpers showed a male bias (males n = 9, females n = 3). In six cases, the immigrant helped for 1 year and then disappeared/died (one female and five males). In one case each, a male immigrant helped for 3 years and a female helped for 1 year and then became the main breeders at a different nest. On two occasions, the immigrant helper became the main breeder of the nest after helping during 1 (a female) and 2 (a male) years.
Reproductive output explained the occurrence of nests with cooperatively breeding adults (Table 3, Fig. 1). The probability of a breeding unit, being a group in a given year, was significantly explained by the number of fledglings produced in the previous year. The probability of helpers at a nest increased by about 10% per fledgling produced in the previous breeding season.
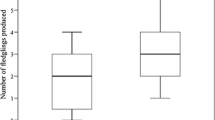
Chick body condition index variation according to brood size for pygmy falcon breeding groups and pygmy falcon pairs (model predicted values). Sample sizes were 13, 64 and 68 for pairs and 3, 12 and 12 for groups for one-, two- and three-chick broods, respectively. Vertical bars show 95% confidence intervals
Breeding unit type (group/pair) did not explain the response of several reproduction-related measures. Laying month of the breeding attempts (n = 124) did not differ between groups and pairs (Table 3). The type of breeding unit did not explain the probability of attempting to breed (Table 3); overall percentage of breeding attempts was 83.9%. Breeding unit type also did not explain the variation in clutch size (Table 3) which was on average 2.53 (range 1–3 eggs). Nest success was 70.3%, and mean fledgling production per nest was 1.43 (range 0–3), but neither were explained by breeding unit type (Table 3). Laying month explained marginal variation in both nest success and number of fledglings, with a negative effect on both probability of success and number of fledglings as the season advanced (Table 3).
Chick body condition was influenced by a significant interaction between breeding unit type and brood size (Table 3, Fig. 2). We used the function lstrends from lsmeans package (Lenth 2016) in R (R Development Core Team 2010) to calculate the trend of nestling body condition in relation to the increase on brood size. This post-hoc analysis revealed that there was a significant negative trend for pairs (trend ± CI = − 1.77 ± 1.21; Fig. 1), whereas no significant tendency was found for groups (trend ± CI = 1.00 ± 2.92; Fig. 1). Nestling body condition significantly decreased as brood size increased in pairs, but not in groups where it remained stable despite the graphic tendency (Fig. 1).
Discussion
We found that pygmy falcons regularly breed in groups, and that these groups are diverse in terms of the sex and origin of the auxiliary individuals. The type of breeding unit (pair vs. group) did not impact most breeding variables examined, except for the relative increase in offspring body condition as brood size increases. The occurrence of breeding groups each season is linked to the fledgling production of the nest in the previous year.
Pygmy falcon breeding groups
Pygmy falcon breeding groups had similar features to those described in other cooperative raptors, but also displayed some unique aspects. First, group size in pygmy falcons ranged from three to five individuals. Groups of three individuals were the most common, similar to other cooperatively breeding raptors (Bednarz 1987; Malan et al. 1997; Watson et al. 1999; Bertran and Margalida 2002). Only the Galapagos hawk exhibits larger group sizes (up to 9 individuals), possibly because of habitat saturation in the Galapagos (Faaborg et al. 1980). Second, we found that the sex ratio of helpers is skewed toward males in pygmy falcons. However, we found that 25% of helpers were female, which contrasts other cooperative raptor species where female helpers were relatively rare (Kimball et al. 2003). Only in two other species, Harris’ hawk and Madagascar fish eagle Haliaeetus vociferoides (Faaborg et al. 1995; Watson et al. 1999), were both females and males reported as helpers. In most cooperative raptors (n = 20), the helpers are only males; in seven species, only females and the sex is unknown for 12 species (Kimball et al. 2003). Lastly, we found that most of the helpers were retained offspring, showing that pygmy falcons are one of only two raptor species currently identified that commonly form groups through delayed dispersal; Harris’ hawk, the other (Bednarz 1987; Kimball et al. 2003).
Group formation and mating system
Our results suggest that delayed dispersal is an important driver of group formation in pygmy falcons. We found that the occurrence of breeding groups was predicted by the fledgling production of each group during the previous season. This finding contrasts with earlier suggestions that delayed dispersal may have evolved secondarily in social raptors, and that other benefits of group living may have primarily driven group formation in raptors (Kimball et al. 2003). Increased philopatry after a productive breeding season may be due to an increase in competition for breeding territories, making it more beneficial for offspring to delay dispersal (habitat saturation hypothesis, Emlen 1982). Low occupation rates of weaver-colonies by falcons (11.7 to 16.9%) suggest limiting factors restricting colony use, such as strong territoriality, environmental conditions or unsuitability of the colonies. Unfortunately, most of the retained offspring disappeared from the study site after 1 year of helping, and we do not know whether they dispersed successfully outside of the study area or died. Only four of the retained offspring became breeders after 1 or 2 years of helping, making it difficult to elucidate whether they acquired skills during their helping period that improved future breeding success.
Group formation in pygmy falcons does not depend only on offspring production, but also on migration of individuals from other territories. Approximately, one third of the helpers were immigrant adults. The causes that may lead an adult individual to join an established unrelated breeding pair, instead of settling in a new territory, are likely the same as for offspring delaying dispersal. In our study area, harsh environmental conditions are likely to be an important limiting factor to access breeding opportunities. Kimball et al. (2003) pointed out that high temporal and spatial variability of resources may promote cooperative breeding in raptors, independently of retained offspring dispersal. Savannah habitats are characterised by unpredictable and highly variable environmental conditions where the presence of helpers can buffer reproductive failure in harsh years (Du Plessis et al. 1995; Rubenstein and Lovette 2007; Jetz and Rubenstein 2011; Cornwallis et al. 2017). Indeed, Parker and Ports (1982) argued that Mississipi kites Ictinia mississippiensis form breeding trios to overcome unpredictable environmental factors such as nest predation, storms and food availability. However, the pale chanting goshawk, which also inhabits semi-arid areas of southern Africa, has been reported to form groups only in prey rich areas (Malan 2004).
We propose two main factors that provide direct fitness benefits for pygmy falcon helpers. First, adult immigrants may have access to extra-pair copulations. We have observed a case where two immigrant males of the same group copulated in turns with the female (RLT unpublished data), as previously reported by Thomsett (1991) in a different population. This suggests that breeding groups (perhaps only those with immigrant males) may be polyandrous, and paternity may be shared among males to incentivise helping. Similarly, all the males of the socially polyandrous groups in pale chanting goshawks, bearded vultures Gypaetus barbatus and Galapagos hawks have been observed to copulate with the breeding female (Faaborg et al. 1980; Malan et al. 1997; Bertran and Margalida 2002). Secondly, both adult immigrants and retained offspring helpers may inherit the territory. After helping in the same group during one or more years, both retained offspring and adult immigrants may become the main breeder. Both cases, once and twice respectively, were detected in our study population. Nevertheless, limiting breeding opportunities may also lead to immigrant males to take over a territory forcefully, without joining as a group helper (Lowney et al. 2017). Only one case of non-aggressive territory inheritance is reported in other raptors; in Galapagos hawks, breeding group size decreases until the last male dies and a new group forms; therefore, all males share the inheritance of the territory (Faaborg et al. 1980).
The diverse nature of the group members in the pygmy falcon makes more difficult the categorisation of their complex mating system without DNA genotyping. Since most of the auxiliary individuals are retained offspring, a monogamous pair of main breeders plus helpers seems a plausible system, as it avoids inbreeding. However, observations of two males mating with the same female point to cooperative polyandry as a likely scenario too. In Harris’ hawks, retained male offspring do not actively help during the first year (Bednarz 1987), which may reduce attempts of incestuous copulations. Observations on helping behaviours and genetic analyses of parentage are clearly the next step to disentangle the breeding dynamics of the pygmy falcon groups.
Group breeding performance
Breeding groups produced offspring of higher condition than pairs. Specifically, body condition of the nestlings raised by groups remained similar even when brood size increased; in contrast, nestling body condition decreased with brood size when raised by breeding pairs. This suggests that helpers appeared to counteract the negative effect of brood size increase on the body condition of the chicks. Helpers likely participated in chick feeding, as often recorded in cooperative breeding studies (Koenig and Dickinson 2004). Indeed, we observed helpers, both male and female, bringing food to the nest. Our results are similar to previous studies on cooperative breeding raptors, which showed that groups produced increased number or size of the fledglings (Faaborg et al. 1980; Bednarz 1987; Malan et al. 1997). Other avian cooperative breeders also showed that the presence of helpers might only have a significant effect on reproduction under adverse circumstances. For example, in situations when brood reduction through nestling starvation is frequent, the presence of helpers had a positive effect on the number of fledglings (Hatchwell 1999). Similarly, breeding groups of sociable weavers are better able to respond to the needs of experimentally enlarged broods than pairs alone (Covas and du Plessis 2005). However, the survival and fitness consequences of body condition on pygmy falcons remain to be tested as the long-term data accumulates in our population.
There are several aspects of the pygmy falcon breeding system that deserve further research. The genetic relatedness of the adult falcons to the chicks is a crucial aspect, as are the levels of help provided by helpers in feeding the chicks or defending the nest. Also, due to shortage of data, we could not evaluate the effect of age on breeding performance of the individuals, which has shown to be important in other raptor species (Clum 1995; Murgatroyd et al. 2016), nor on the probability of being in a group. Interestingly, the complexity and variability of the breeding ecology of the pygmy falcon offers a good opportunity to study cooperative breeding occurrence in arid, unpredictable environments, as well as its prevalence in diurnal raptors.
References
Arnold KE (1999) Cooperative breeding in birds: the role of ecology. Behav Ecol 10:465–471
Arnold KE, Owens IPF (1998) Cooperative breeding in birds: a comparative test of the life history hypothesis. Proc R Soc Lond B 265:739–745
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bednarz JC (1987) Pair and group reproductive success, polyandry, and cooperative breeding in Harris’ hawks. Auk 104:393–404
Bednarz JC (1988) Cooperative hunting Harris’ hawks (Parabuteo unicinctus). Science 239:1525–1527
Bergmüller R, Johnstone RA, Russell AF, Bshary R (2007) Integrating cooperative breeding into theoretical concepts of cooperation. Behav Process 76:61–72
Bertran J, Margalida A (2002) Social organization of a trio of bearded vultures (Gypaetus barbatus): sexual and parental roles. J Raptor Res 36:66–70
Brosnan SF, Bshary R (2010) Cooperation and deception: from evolution to mechanisms. Phil Trans R Soc B 365:2593–2598. https://doi.org/10.1098/rstb.2010.0155
Brown JL, Brown ER (1981) Kin selection and individual fitness in babblers. In: Alexander RD, Tinkle DW (eds) Natural selection and social behavior. Chiron, New York, pp 244–256
Canestrari D, Marcos JM, Baglione V (2008) Reproductive success increases with group size in cooperative carrion crows, Corvus corone corone. Anim Behav 75:403–416
Clum NJ (1995) Effects of aging and mate retention on reproductive success of captive female peregrine falcons. Integr Comp Biol 35:329–339. https://doi.org/10.1093/icb/35.4.329
Cockburn A (2006) Prevalence of different modes of parental care in birds. Proc R Soc Lond B 273:1375–1383
Cornwallis CK, Botero CA, Rubenstein DR, Downing PA, West SA, Griffin AS (2017) Cooperation facilitates the colonization of harsh environments. Nat Ecol Evol 1:0057. https://doi.org/10.1038/s41559-016-0057
Covas R and du Plessis MA (2005) The effect of helpers on artificially increased brood size in sociable weavers (Philetairus socius). Behav Ecol Sociobiol 57:631–636
Covas R, Du Plessis MA and Doutrelant C (2008) Helpers in colonial cooperatively breeding sociable weavers Philetairus socius contribute to buffer the effects of adverse breeding conditions. Behav Ecol Sociobiol 63:103–112
Dias RI, Webster MS, Macedo RH (2015) Helping enhances productivity in campo flicker (Colaptes campestris) cooperative groups. Sci Nat 102:31
Du Plessis MA, Siegfried WR, Armstrong AJ (1995) Ecological and life history correlates of cooperative breeding in South African birds. Oecologia 102:180–188
Edwards SV, Naeem S (1993) The phylogenetic component of cooperative breeding in perching birds. Am Nat 141:754–789
Emlen ST (1982) The evolution of helping. I An ecological constraints model. Am Nat 119:29–39
Emlen ST (1991) Evolution of cooperative breeding in birds and mammals. In: Krebs R, Davies NB (eds) Behavioural ecology: an evolutionary approach. Blackwell Scientific, Oxford, pp 301–335
Emlen ST and Wrege PH (1991) Breeding biology of White-fronted Bee-eaters at Nakuru: The influence of helpers on breeder fitness. J Anim Ecol 60:309–326
Faaborg J (1986) Reproductive success and survivorship of the Galapagos hawk Buteo galapagoensis: potential costs and benefits of cooperative polyandry. Ibis 128:337–347. https://doi.org/10.1111/j.1474-919X.1986.tb02684.x
Faaborg J, Bednarz J (1990) Galapagos and Harris’ hawks: divergent causes of sociality in two raptors. In: Stacey PB, Koenig WD (eds) Cooperative breeding in birds: long term studies of ecology and behaviour. Cambridge University Press, Cambridge, pp 357–384
Faaborg J, Vries T, Patterson CB, Griffin CR (1980) Preliminary observations on the occurrence and evolution of polyandry in the Galapagos hawk (Buteo galapagoensis). Auk 97:581–590
Faaborg J, Parker PG, DeLay L, de Vries T, Bednarz JC, Maria Paz S, Naranjo J, Waite TA (1995) Confirmation of cooperative polyandry in the Galapagos hawk (Buteo galapagoensis). Behav Ecol Sociobiol 36:83–90
Ford HA, Bell H, Nias R, Noske R (1988) The relationship between ecology and the incidence of cooperative breeding in Australian birds. Behav Ecol Sociobiol 22:239–249
Garcelon DK, Slater GL, Danilson CD, Helm RC (1995) Cooperative nesting by a trio of bald eagles. J Raptor Res 29:210–213
Hamilton W (1964) The genetical evolution of social behaviour. J Theor Biol 7:1–52
Hatchwell BJ (1999) Investment strategies of breeders in avian cooperative breeding systems. Am Nat 154:205–219
Hatchwell BJ, Russell AF, MacColl ADC, Ross DJ, Fowlie MK, McGowan A (2004) Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav Ecol 15:1–10
Heinsohn R and Cockburn A (1994) Helping is costly to young birds in cooperatively breeding White-winged Choughs. Proc R Soc B 256:293–298
Heredia R, Donázar J (1990) High frequency of polyandrous trios in an endangered population of lammergeiers Gypaetus barbatus in Northern Spain. Biol Conserv 53:163–171. https://doi.org/10.1016/0006-3207(90)90083-2
Jetz W, Rubenstein DR (2011) Environmental uncertainty and the global biogeography of cooperative breeding in birds. Curr Biol 21:72–78. https://doi.org/10.1016/j.cub.2010.11.075
Kimball RT, Parker PG, Bednarz JC (2003) Occurrence and evolution of cooperative breeding among the diurnal raptors (Accipitridae and Falconidae). Auk 120:717–729
Kingma SA, Santema P, Taborsky M, Komdeur J (2014) Group augmentation and the evolution of cooperation. Trends Ecol Evol 29:476–484
Koenig WD, Dickinson J (2004) Ecology and evolution of cooperative breeding in birds. Cambridge University Press, Cambridge
Krochuk BA, Bolopo D, Lowney A, Meyers PR, Spottiswoode CN, Raman RMG, Thomson RL (2018) Why defaecate on your doorstep? Investigating an unusual behaviour in Africa’s smallest falcon. Ostrich (published online. https://doi.org/10.2989/00306525.2018.1529001)
Labocha MK, Hayes JP (2012) Morphometric indices of body condition in birds: a review. J Ornithol 153:1–22
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. https://doi.org/10.18637/jss.v069.i01
Lowney A, Green K, Ngomane BP, Thomson RL (2017) Mortal combat: intraspecific killing by an African pygmy-falcon ( Polihierax semitorquatus ) to acquire new mate and territory. J Raptor Res 51:89–91. https://doi.org/10.3356/JRR-16-64.1
Maclean GL (1970) The pygmy falcon Polihierax semitorquatus. Koedoe 13:1–22
Maclean GL (1973) The sociable weaver, part 4: predators, parasites and symbionts. Ostrich 44:241–253
Malan G (1998) Solitary and social hunting in pale chanting goshawk (Melierax canorus) families: why use both strategies? J Raptor Res 32:195–201
Malan G (2004) The relative influence of prey abundance and co-breeders on the reproductive performance of polyandrous pale chanting-goshawks. Ostrich 75:44–51. https://doi.org/10.2989/00306520409485411
Malan G, Jenkins AR (1996) Territory and nest defence in polyandrous pale chanting goshawks: do co-breeders help ? S Afr J Zool 31:170–176
Malan G, Crowe TM, Biggs R, Herholdt JJ (1997) The social system of the pale chanting goshawk Melierax canorus; monogamy v polyandry and delayed dispersal. Ibis 139:313–321. https://doi.org/10.1111/j.1474-919X.1997.tb04630.x
Moreno J, Merino S, Sanz JJ, Arriero E, Morales J, Tomás G (2005) Nestling cell-mediated immune response, body mass and hatching date as predictors of local recruitment in the pied flycatcher Ficedula hypoleuca. J Avian Biol 36:251–260. https://doi.org/10.1111/j.0908-8857.2005.03413.x
Murgatroyd M, Underhill LG, Rodrigues L, Amar A (2016) The influence of agricultural transformation on the breeding performance of a top predator: Verreaux’s eagles in contrasting land use areas. Condor 118:238–252. https://doi.org/10.1650/CONDOR-15-142.1
Parker JW, Ports M (1982) Helping at the nest by yearling Mississippi kites. J Raptor Res 16:14–17
R Development Core Team (2010) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
R Development Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna http://www.R-project.org
Riehl C (2013) Evolutionary routes to non-kin cooperative breeding in birds. Phil Trans R Soc B 280:20132245
Rodríguez S, van Noordwijk AJ, Alvarez E, Barba E (2016) A recipe for postfledging survival in great tits Parus major: be large and be early (but not too much). Ecol Evol 6:4458–4467. https://doi.org/10.1002/ece3.2192
Rubenstein DR, Lovette IJ (2007) Temporal environmental variability drives the evolution of cooperative breeding in birds. Curr Biol 17:1414–1419
Russell E, Rowley I (1988) Helper contributions to reproductive success in the splendid fairy-wren (Malurus splendens). Behav Ecol Sociobiol 22:131–140
Shen S-F, Emlen ST, Koenig WD, Rubenstein DR (2017) The ecology of cooperative breeding behaviour. Ecol Lett 20:708–720
Spottiswoode C, Herrmann E, Rasa OAE, Sapsford CW (2004) Co-operative breeding in the pygmy falcon Polihierax semitorquatus. Ostrich 75:322–324
Taborsky M (1994) Sneakers, satellites, and helpers: parasitic and cooperative behavior in fish reproduction. Adv Stud Behav 23:1–100
Tella JL (1993) Polyandrous trios in a population of Egyptian vultures. J Raptor Res 27:119–120
Thomsett S (1991) Polyandrous pygmy falcons? Gabar 6:73
Valencia J, Solís E, Sorci G, de la Cruz C (2006) Positive correlation between helpers at nest and nestling immune response in a cooperative breeding bird. Behav Ecol Sociobiol 60:399–404
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Watson RT, Razafindramanana S, Thorstrom R, Rafanomezantsoa S (1999) Breeding biology, extra-pair birds, productivity, siblicide and conservation of the Madagascar fish eagle. Ostrich 70:105–111. https://doi.org/10.1080/00306525.1999.9634522
Acknowledgments
We thank the Oppenheimer family, Tswalu Foundation and Tswalu Kalahari for permitting us to conduct research on their property. We thank also Anna Gamero for comments on previous versions of this manuscript and Arjun Amar for help with statistical analyses. Thanks to Stephen Pruett-Jones, Christie Riehl and Jim Bednarz for their review and helpful suggestions to improve the manuscript.
Funding
The field work was funded by the NRF-DST Centre of Excellence of the FitzPatrick Institute of African Ornithology and by a Tswalu Foundation grant. RLT was initially supported by the Academy of Finland (grant no. 138049) and the University of Turku Collegium for Science and Technology grant for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The project conformed to the legal requirements of South Africa and has received research FAUNA permits from the Northern Cape Province’s Department of Environment and Nature Conservation, and an ethics approval from the Science Animal Ethics Committee (SFAEC) of the University of Cape Town, South Africa.
Additional information
Communicated by S. Pruett-Jones
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bolopo, D., Lowney, A.M. & Thomson, R.L. Helpers improve fledgling body condition in bigger broods of cooperatively breeding African pygmy falcon. Behav Ecol Sociobiol 73, 16 (2019). https://doi.org/10.1007/s00265-018-2630-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2630-3