Abstract
It has been 55 years since Nikolaas Tinbergen formalized the field of ethology by identifying four types of inquiry that address the “how” and “why” of animal behavior from both a contemporary and historical perspective. This framework has been particularly useful in exploring eusocial behavior among insects, due to integration across levels of analysis and timescales of influence. Although the former has proceeded quite deliberately, the latter has received less attention. Here, I synthesize recent findings regarding the mechanisms, ontogeny, evolution, and function of eusociality in ants, bees, and wasps. This synthesis reveals that there has been rapid gain of knowledge regarding the genetic underpinnings of eusocial behavior, but an understanding of the fitness consequences of these molecular mechanisms lags behind. Similarly, it has become clear that maternal or sibling effects on development are major drivers of caste-related behavior, but the mechanisms that produce these effects are largely unknown. Developmental caste determination and caste-biasing require sensitivities to social cues, but how this plasticity evolved from solitary ancestors is unknown. Understanding the origins of developmental plasticity is necessary to understand how plasticity shapes the evolutionary trajectory of social traits. Likewise, the influence of social function on molecular evolution has been studied within a robust theoretical framework; however, these studies will benefit from an understanding of how ancestral conditions promote the acquisition of social function in the first place. Future studies that span both levels of analysis and timescales of influence will further advance the integrative field of ethology that Tinbergen envisioned.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The modern era of the study of behavior is based on a framework established by Niko Tinbergen 55 years ago (Tinbergen 1963). In establishing the aims and methods of ethology, Tinbergen made the case that behavior can and should be studied like other traits. In doing so, he identified four categories of explanation that have become the pillars of biological investigation: causation, survival value, evolution, and ontogeny. This framework is largely responsible for the tremendous advances made within the field of animal behavior over the past several decades. One of the flagship areas of research that has been particularly fruitful in its application of Tinbergen’s approach is the study of social behavior in insects. The field of sociogenomics aims to integrate research focused on the “how” and the “why” of social behavior (Robinson et al. 2005). While this includes social behavior across the tree of life, research focused on sociality in insects has been particularly prolific in recent years. Here, I synthesize recent findings from social insect research with respect to Tinbergen’s framework, and I identify areas where further integration is needed.
Tinbergen’s framework for the study of behavior
Tinbergen was thorough and deliberate in his justification for each of his four lines of inquiry. In his discussion of causation, Tinbergen asked “How does it work?” Causal explanations focus on the mechanisms that generate behavioral phenotypes, and Tinbergen made the case that behaviors are influenced by both external and internal (i.e., neurophysiological) controls. In discussing survival value, Tinbergen asked the question “What is it for?” He cautioned against teleological thinking and advocated for experimental tests of the effects of behavior on fitness. He recognized the issues that come from performing such experiments in the lab, and urged that such experiments be replicated in ecological contexts. Bateson and Laland (2013) suggested the label “current utility” be used to describe this category of inquiry, to avoid presumptions about the adaptive value, and thus the historical processes that shaped the behavior. These historical processes are addressed under Tinbergen’s question about evolution. He asserted that behavior should be studied within a phylogenetic context, and recommended experimental evolution and population genetics be applied to understand how natural selection and other evolutionary forces have shaped the behavior. He intuitively predicted that antagonistic selection is likely to be particularly important in behavioral evolution, given the complexity and multifaceted nature of most behaviors. This is especially true of eusociality, which encompasses a complex suite of traits, and for which release from antagonistic pleiotropy has long been thought to be one of the driving forces in the origins of division of labor (Gadagkar 1997).
By appending ontogeny to Huxley’s original three questions (causation, survival value, evolution), Tinbergen called attention to the temporal nature of the external and internal controls shaping behavior. Interactions between individuals and the environment may have different effects on the phenotype, depending on the life stage at which they occur. Tinbergen recognized that the line between development and post-development may be blurred or overlapping in different components of behaviors. Nonetheless, he considered the temporal nature of mechanisms important enough to include as a separate category for ethological study. In this way, the study of ontogeny asks more than “how does it develop?”, but considers causation in a historical sense more generally.
Tinbergen’s four questions fit within the levels of analysis previously described by Mayr (1961). Mayr identified functional and evolutionary biology as overlapping, but differentiated, fields of biology. The former is concerned with proximate causes of traits, or those that answer the question “How?,” and the latter is primarily focused on ultimate causes of traits, or those that answer the question “Why?” Mayr suggested that proximate causes regulate neurophysiological responses to immediate factors from the environment, and ultimate causes involve molecular evolution over multiple generations. Mechanisms and ontogeny thus fit under the umbrella of proximate explanations, but with current and historical temporal influence, respectively. Current utility and evolution are then regarded as ultimate explanations, also with current and historical temporal influence, respectively (Fig. 1a) (Sherman 1988; MacDougall-Shackleton 2011).
Tinbergen’s four questions for the study of behavior. a The traditional view of Tinbergen’s four questions has partitioned research into proximate or ultimate levels of analysis and historical and contemporary timescales of influence. b A synthetic approach to Tinbergen’s four questions considers both levels of analysis and multiple timescales. This yields additional questions that are important in the study of animal behavior
Both Tinbergen and Mayr emphasized that biology could be best understood when explanations in one category informed the others through integrative approaches. For example, understanding the survival value of a trait can identify the important questions asked about causation. Tinbergen pointed out that Karl von Frisch studied the causative mechanisms of color vision in honey bees in order to test the survival value of flower colors. Tinbergen also pointed out that understanding the survival value of ontogenetic controls reveals the relative influence of the environment on behavior, similar to how evolutionary interactions with the environment refine the molecular instructions of genes. Tinbergen (1963) wrote, “It is useful both to distinguish between them and to insist that a comprehensive, coherent science of Ethology has to give equal attention to each of them and to their integration,” (p. 477). Yet even as recently as 2013, 50 years after the publication of this directive, integration across the four questions was recognized as one of the remaining challenges to the study of behavior (Bateson and Laland 2013).
This challenge has been most comprehensively met in research focused on social behavior in insects. Though some students of social behavior had adopted this approach early (Gamboa et al. 1986), the cementing of the relationship between proximate and ultimate explanations was greatly facilitated by the dawn of sociogenomics (i.e., the study of the genomic basis for social behavior; (Robinson 1999; Robinson et al. 2005)). This emerging field provided a conceptual framework to guide integration of explanations for social behavior that simultaneously answered the questions how and why (Robinson 1999; Robinson et al. 2005). The subsequent development of genomic resources for several ant and bee species served as a catalyst to enact this integrative approach within the field of sociobiology. In the years following, there has been a deliberate effort to simultaneously address both proximate and ultimate explanations for social behavior in insects, and this has provided a tremendous amount of insight into how and why insects cooperate (Miura 2004; Linksvayer and Wade 2005; Owens 2006; Monnin and Liebig 2008; Hofmann et al. 2014; Jeanson and Weidenmüller 2014).
Integration across contemporary and historical influences on social behavior has not been formalized to the same degree as has integration across proximate and ultimate. However, there are important hypotheses that invoke a temporal perspective on social behavior (e.g., the heterochrony model (Linksvayer and Wade 2005) and the evo-devo model of social evolution (Toth and Robinson 2007)), empirical tests of which have made important contributions to the study of social behavior (Toth et al. 2007). This suggests that we can gain fresh insight into the origins and elaborations of sociality via a more synthetic approach to integrating across Tinbergen’s four questions (Fig. 1b).
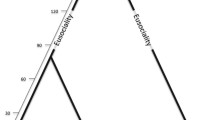
In this review, I synthesize the rapid progress that has been made in understanding the how and why of social behavior in insects, from both a historical and contemporary perspective. I focus on social complexity in the Hymenopteran insects for two reasons. First, eusociality is one of the most complex social behaviors known to animals, and the ants, bees, and wasps are exemplars of behavioral diversity in this respect. Although most bees and wasps are solitary, eusociality has been gained and lost multiple times within these groups and has evolved once in the common ancestor of all ants (Fig. 2). Eusociality, in its most basic form, is characterized by overlapping generations of queens and workers, reproductive division of labor, and cooperative brood care (Batra 1966) (Fig. 2a). By foregoing direct reproduction to help rear their siblings, workers display extreme altruism. Among the Hymenoptera, there have also been several independent elaborations of eusociality in species which form very large colonies with many thousands of individuals. Social behavior in these species is regulated by emergent properties, such as sophisticated modes of chemical communication and reproduction at the colony level (Wilson 1971; Anderson and McShea 2007) (Fig. 2a). Yet, the social biology of many ants, bees, and wasps does not fit squarely within these definitions. Many families and subfamilies include species with a broad spectrum of social features (Fig. 2b). Social complexity may be best thought of as a continuum of traits that promote increasing dependency among individuals (Fig. 2a).
Social diversity in Hymenopteran insects. a Social biology can be classified by a suite of traits associated with increasing interdependency between colony members linked to increasing social complexity. D.O.L. division of labor, Shaded boxes indicate that these traits are variable within this category. b Phylogenetic relationship among lineages within the aculeate Hymenoptera in which social behavior has evolved. Most lineages contain species with different types of social behavior, and sociality has been lost in some bee lineages. Adapted from Wenseleers and van Zweden (2017); Rehan and Toth (2015); phylogeny from Branstetter et al. (2017)
Second, the social insects are some of the most thoroughly studied with regard to each of Tinbergen’s four questions. Widespread global distribution and ecological abundance makes many species excellent subjects for field-based studies and comparative work, while their size and external brood rearing make them amenable to study in the lab for physiological and cross-generational experiments. Moreover, there are abundant genomic resources available for species within this group, which create the opportunity for a deeper understanding of the mechanisms underlying social behavior in relationship to other areas of behavior (Branstetter et al. 2018). Here, I explore social behavior in ants, bees, and wasps by synthesizing the results of recent studies across levels of analysis and timescales of influence. In doing so, I identify areas where integration is replete, in the context of priorities for future research.
Contemporary influence on proximate and ultimate causes of social behavior
Early calls for integration of proximate and ultimate studies of eusociality were primarily directed at behavioral ecologists, who for decades had pursued explanations for the origins of eusociality through the lens of inclusive fitness and kin selection, while remaining agnostic to the molecular mechanisms on which kin selection could potentially act (Owens 2006). Yet, the genetic architecture of a trait, especially a trait as complex as eusociality, can have significant impact on how it evolves (Lande 1982; Lande and Arnold 1983). In recent years, social insect research has provided a bridge between the proximate and the ultimate. The field of sociogenomics was born at a time when the only genetic knowledge of social insect behavior was in the form of quantitative trait loci (QTL) associated with pollen storage among honey bee colonies (Hunt et al. 1995). There are now myriad individual genes and molecular pathways known to regulate key aspects of social behavior. This provides partial answers to the question “how does it work?” Moreover, it is now recognized that understanding how it works provides the necessary information for a complete understanding of what it is for. With phenotype being the link between cause and consequence, we can ask the following two questions of the contemporary mechanisms and fitness consequences of social behavior (Fig. 1b).
How does genotype map to phenotype?
Social insect researchers asking how eusociality works have uncovered several genetic variants, epigenetic features, and even symbiotic relationships as potential regulators of social behavior. It has become clear that while there are several outstanding examples of genes and gene pathways involved in eusociality that are common to multiple lineages of ants, wasps, and bees, much of the genomic basis for eusociality is lineage specific (Gadau et al. 2012; Simola et al. 2013; Kapheim et al. 2015b). Many of the genomic signatures of eusociality have been recently reviewed elsewhere (Jandt and Toth 2015; Ronai et al. 2016; Shell and Rehan 2017; Toth and Rehan 2017; Weitekamp et al. 2017; Sumner et al. 2018), so here, I limit this discussion to only a few recent discoveries. I have chosen these examples to highlight the diversity of genetic elements that have been studied and the advanced set of tools employed to identify the causal mechanisms underpinning each of the traits that contribute to social complexity (Fig. 2a).
Caste differentiation (i.e., developmental canalization of queens and workers) is one of the marvels of eusociality, and thus, the genetic mechanisms associated with this phenomenon are highly sought after. New insight into the genomic processes by which caste determination occurs have come from a comparative study of a gene family called Osiris, which is restricted to insects and essential for chitin formation. Many of these genes are highly conserved throughout insects, both in expression patterns and synteny. They peak in expression during pupal development in both bumble bees (Bombus terrestris) and harvester ants (Pogonomyrmex barbatus), with consistently higher expression in workers than in queens, potentially related to variation among castes in wing formation (Smith et al. 2018). Further study of this gene family will be necessary to determine its social function in species for which caste is determined more by factors experienced in adulthood than in development.
Small, non-coding RNAs also function in developmental caste determination. Collins et al. (2017) recently identified two microRNAs that are more highly expressed in bumble bee (B. terrestris) larvae destined to become queens than larvae destined to become workers. These microRNAs are predicted to target mRNAs of genes involved in development and reproductive differentiation in honey bees. Yet, these microRNAs are distinct from those implicated in caste differentiation in honey bees (Guo et al. 2013; Shi et al. 2015; Ashby et al. 2016). This provides additional evidence that many of the genetic signatures of social behavior involve common molecular pathways, but the individual elements are lineage-specific. It is also noteworthy that the signal of differential expression of these bumble bee microRNAs was highest in late-instar cuticle, which could reflect their involvement in functional pathways that include the Osiris gene family.
A critical phenotype involved in eusociality is communication. Colony function relies on individuals responding to chemical and social cues from their nestmates and non-nestmates. For this reason, odorant-binding receptors have long been recognized as an important source of genetic variation associated with social function. Recently, this has been confirmed with the use of the genome engineering. Engineered mutations in the gene orco reduced responsiveness to conspecifics in workers of the jumping ant Harpegnathos saltator (Yan et al. 2017) and in clonal raider ants (Ooceraea biroi) (Trible et al. 2017).
Another recent study identified genes associated with abnormal social behavior among workers of the highly eusocial honey bee. In a laboratory assay, honey bee workers that did not show any aggression toward an unfamiliar conspecific or alloparental care toward a larva had a distinct neurogenomic profile in the mushroom bodies, which are the primary centers of sensory integration in insects (Shpigler et al. 2017). Genes that were differentially expressed between socially responsive and non-responsive workers were significantly enriched for genes that are associated with autism spectrum disorders in humans. The list of shared genes included several heatshock proteins, which function in protein regulation. Additional studies that include functional assays are necessary to determine the role these genes play in regulating social responsiveness.
From humble beginnings, sociogenomics researchers have generated a wealth of information regarding molecular mechanisms underpinning various aspects of social behavior in insects. The next step to integrating proximate and ultimate explanations for social behavior is to ask what these molecular signals are for. We must now use variation in these signals (either naturally-occurring or experimentally-induced) to ask how they impact individual and colony-level fitness associated with social behavior. From this, we will generate a more comprehensive understanding of cause and effect in eusociality.
How does phenotype map to fitness?
We can also approach this integration of contemporary influences on proximate and ultimate explanations from the inverse angle, by asking if what it is for helps us understand how it works. This requires understanding the fitness consequences of social behavior. Worker behavior has traditionally been modeled by the spread of an “allele for altruism” through kin selection, based on its effects on inclusive fitness (Hamilton 1964). However, it is also possible that worker behavior could evolve via parental manipulation, without inclusive fitness benefits to workers (Alexander 1974). Although these two models are both entirely consistent with kin selection, they provide different answers to the question “what is it for?”—namely, for increasing the inclusive fitness of workers or parents, respectively.
One way to test these alternative hypotheses is by comparing the inclusive fitness of helpers and non-helpers, using Hamilton’s rule. This is an impossible feat in species for which social behavior is obligate, because the alternative fitness outcomes cannot be accurately estimated. Moreover, the task of partitioning fitness benefits in social nests between queens and workers in social nests is non-trivial (Wolf and Wade 2001). Facultatively social species thus provide a unique opportunity to directly measure inclusive fitness of alternative social phenotypes.
Evidence from two such species suggests that Hamilton’s rule is not satisfied for workers. The benefits of helping relatives do not outweigh the costs of foregoing reproduction in a facultatively eusocial sweat bee (Megalopta genalis; Kapheim et al. 2015a) and a subsocial small carpenter bee (Ceratina calcarata; Shell et al. 2018). In both species, evidence strongly suggests that mothers who manipulate their daughters into becoming workers gain inclusive fitness benefits. Furthermore, in a model designed with parameters based on two years of field data for M. genalis, eusociality spread faster and under more realistic conditions if modeled as an allele for maternal manipulation, than it did when modeled as an allele for altruism (Kapheim et al. 2015a). Together, these studies support the emerging perspective that social behavior is a function of maternal effects on offspring behavior.
This is an important finding, because if the function of helping behavior is an increase in maternal fitness, then understanding its causation includes understanding the mechanisms of maternal manipulation. A search for genes involved in social behavior would therefore necessarily include a search for genes with maternal and other indirect genetic effects.
Proximate analysis of contemporary and historical influences on social behavior
By including ontogeny as one of his four questions, Tinbergen was attempting to reconcile an ongoing debate over the degree to which behavior is innate or acquired. He emphasized that while the former debate was largely semantic, it is important to ask how the machinery of behavior changes during development. That is, how do mechanisms operating during development shape adult behavior? He recognized that causation can be both contemporary and historical in nature, and that sometimes the distinction between these temporal influences is difficult to decipher. Tinbergen also submitted that once a developmental change in behavioral machinery was described, the next problem was to determine how such changes are controlled by external and internal regulators alike. He ascribed development a role in shaping behavior similar to that of evolution—as a form of trial-and-error-interaction with the environment. This is consistent with a more recent understanding for the role of development in both shaping and generating phenotypic variation (West-Eberhard 2003). With this in mind, we can investigate the temporal relationship between causative mechanisms of social behavior (Fig. 1b).
How does development influence the mechanisms of behavior?
With few exceptions, caste in social insects is environmentally determined, either during pre-imaginal development or in early adulthood. Understanding how ontogeny influences the mechanisms of behavior thus requires understanding the nature of these environmental interactions (Fig. 1b).
Environmental cues that influence caste determination during pre-imaginal stages of development are primarily in the form of larval diet. Honey bee larvae destined to become queens are fed a higher proportion of proteinaceous royal jelly (Haydak 1970), while workers receive a higher dose of the plant flavonoid p-coumaric acid (Mao et al. 2015). Proteins in the royal jelly fed to honey bee queens and dietary exposure to p-coumaric acid have downstream effects on nutrient signaling pathways, including insulin/insulin-like signaling (IIS) and target of rapamycin (TOR), which function in reproductive differentiation via their interaction with gonadotropic endocrine pathways (Patel et al. 2007; Wolschin et al. 2011; Wang et al. 2013; Mao et al. 2015). Trophallactic fluid transferred with larval food may also have a direct influence on caste development, via proteins, microRNAs, and gonadotropic hormones contained within the fluid (LeBoeuf et al. 2016). In this way, dietary nutrition influences the molecular pathways most notably involved in reproduction and division of labor.
There is accumulating evidence that larval nutrition may also influence the mechanisms of social behavior in species without derived specializations related to caste determination. For example, in the facultatively eusocial sweat bee, M. genalis, variation in the quality and quantity of larval provisions is linked to adult variation in the physiological correlates of social behavior (e.g., ovary maturation, body size) (Kapheim et al. 2011, 2012). Similarly, larval diet restriction in paper wasps (Polistes metricus) leads to worker-like physiology (Judd et al. 2015) and changes in the expression of genes related to worker behavior (Berens et al. 2015). In the small carpenter bee C. calcarata, larval food reduction leads to worker-like physiology and behavior, including reduced aggression (Lawson et al. 2016, 2017).
In the critical period of early adulthood, aggression is likely to be an important environmental control that regulates the mechanisms of behavior. In the facultatively eusocial sweat bee M. genalis, aggression from the queen to her worker-destined daughters begins upon eclosion and remains high even once the daughter begins working (Kapheim et al. 2016). This could indicate the effects of aggression span ontogenetic and contemporary influences on worker behavior. In small carpenter bees, being the recipient of aggression as an adult triggers expression changes in the brain of genes related to neurogenesis, olfactory-related behavior, and learning (Withee and Rehan 2017). Among newly eclosed Diacamma ants, being the recipient of aggression leads to immediate reproductive shutdown and gene expression shifts of nutrient-signaling genes, which are a major regulator of division of labor and other social behaviors among older adults (Okada et al. 2017).
Together, these results suggest that environmental exposure to nutritional and dominance cues can alter the mechanisms that regulate social behavior among adult insects (Kapheim 2017). What then are the mechanisms that influence these developmental cues?
What are the mechanisms that create different developmental environments?
As discussed in the sections above, nestmates are largely responsible for creating the developmental environments that shape adult social behavior. In highly eusocial species like ants and honey bees, larvae are reared as either queens or workers by nurses that specialize in brood feeding. In species closer to the solitary end of the social spectrum, developmental caste-biasing is influenced by maternal manipulation. Understanding the mechanisms that create different developmental environments thus requires understanding the mechanisms that cause variation in the way nurses or mothers rear larvae (Fig. 1b).
In highly eusocial species, nurses may specialize on rearing either queens or workers. In this case, understanding the mechanisms that create ontogenetic variation requires investigating neurogenomic differences between nurses with different specializations. Alternatively, individual nurses may rear both queens and workers, without specialization. This requires understanding the molecular shifts that occur as nurses switch from one type of brood care to another. Recent work with ants suggests that nurses do not specialize on feeding larvae of a particular caste, but are instead more likely to specialize on a particular developmental stage (Walsh et al. 2017). In honey bees, large differences in gene expression have been documented in nurses that rear queen-destined and worker-destined larvae (Vojvodic et al. 2015). These studies demonstrate that it is possible to identify the molecular mechanisms creating variation in ontogenetic environments, and suggest additional research is necessary to discern shared and species-specific mechanisms for social influences on behavioral development.
When maternal manipulation is the basis for developmental caste-biasing, as in facultatively social bees, specialization may occur in the sense that some females may only raise future queens (solitary females) and other females may raise both workers and queens (social colonies). In the facultatively eusocial sweat bee M. genalis, females that follow different social strategies also have significant differences in physiology. Females that pursue a solitary strategy are smaller, with lower titers of juvenile hormone, and reach reproductive maturity later than females pursuing a social strategy (Kapheim et al. 2013; Smith et al. 2013). However, there are relatively few differentially expressed genes in the abdomens and brains of solitary females and queens (Jones et al. 2017). Additional research is necessary to determine whether there are differences in maternal gene expression specifically associated with rearing future queens and future workers in facultatively social species. Comparisons of these maternal genes to those identified in specialized honey bee nurses may illuminate the degree to which the molecular basis for developmental variation is conserved across origins and elaborations of eusociality. Similarly, further investigation into the life history and physiological traits of honey bee nurses that specialize on queen or worker rearing could help to determine how sib-social care evolved from maternal care.
Historical influence on proximate and ultimate causes of social behavior
Historical perspectives on proximate and ultimate explanations for any phenotype require understanding the relationship between developmental and evolutionary processes. Although the parsing of proximate and ultimate levels of analysis has been embedded in behavioral biology for decades, some have recently argued that this distinction impedes, rather than facilitates, understanding (West-Eberhard 2003; Laland et al. 2011). These authors stress that developmental processes both shape and respond to natural selection, and thus span both proximate and ultimate causation. It is becoming clear that developmental plasticity can function as both a cause and a consequence of evolution, and this is likely to be particularly true for behavioral phenotypes, which tend to be sensitive to environmental cues (Renn and Schumer 2013). I propose that the conflict between proximate and ultimate levels of analysis, and what this means for the role of ontogeny, can be resolved when timescales of influence are also considered. From this perspective, ontogeny is a causal mechanism, which can respond to natural selection just like any other part of life history. However, the temporal distinction between causation and ontogeny also creates an avenue through which development can shape the evolutionary trajectory of traits, by influencing the mechanisms underlying phenotypes in adulthood. This is most evident among social insects, because of the important role the developmental environment has on shaping the mechanisms underlying social behavior (see above). By integrating across proximate and ultimate explanations at a historical timescale, we can ask how developmental plasticity evolves, and in turn, the role of developmental plasticity in evolution (Fig. 1b).
How does developmental plasticity evolve?
One of the fundamental features of the origins of eusociality from a solitary ancestor is the acquisition of developmental plasticity. Whether caste-determination is more influenced by environmental variation during larval stages or in early adulthood, the evolutionary origins of castes are tantamount to origins of novel responses to environmental cues. For example, the evidence reviewed above suggests that at least some caste-biasing, if not complete caste determination, is the result of variation in larval nutrition. However, this type of variation in the developmental environment is not unique to social insects. In fact, many insect species experience variation in larval nutrition under some conditions, and the molecular pathways that link nutrition to reproductive potential are highly conserved (Badisco et al. 2013). Larval nutrition is a principal factor in many insect polyphenisms that are unrelated to sociality (e.g., reproductive strategy and morphology in dung beetles (Reaney and Knell 2015), dispersal morphs in aphids (Müller et al. 2001)). Moreover, these polyphenisms are generated through highly conserved molecular pathways (Kijimoto et al. 2014; Kijimoto and Moczek 2016; Vellichirammal et al. 2016, 2017). Yet, caste differentiation as a response to larval diet restriction is unique to only a few lineages within the Hymenoptera. This highlights that the origins of social castes from common sources of environmental variation and highly conserved molecular pathways require a novel response to developmental cues.
One way to investigate the evolutionary origins of this novel plasticity is by comparing the response to environmental variation in closely related solitary and social species. There is very little known about how factors important in caste differentiation, such as nutritional, endocrine, and social cues, influence reproductive potential in solitary bees and wasps (Jandt and Toth 2015; Kapheim 2017). However, the following studies have begun to shed light on how developmental plasticity related to social behavior evolves.
Sensitivities to larval nutrition are also responsible for developmental plasticity associated with diapause in a broad range of species, including social insects (Hahn and Denlinger 2011). This observation has given rise to the diapause ground plan hypothesis for the origins of castes, which posits that the molecular pathways that regulate diapause in solitary insects have been co-opted for reproductive caste determination in social insects (Hunt and Amdam 2005). Evidence for this hypothesis comes from paper wasps (P. metricus), where future queens overwinter as adults prior to nest-founding and reproduction, and thus have larger fat stores, higher levels of storage proteins, longer development times, and inactivated ovaries at emergence (Hunt et al. 2007, 2010). Conversely, workers are smaller, with fewer nutrient stores, and are thus more similar to a non-diapausing phenotype. A recent study with the solitary alfalfa leafcutting bee (Megachile rotundata) provided additional evidence that facultative diapause is regulated by variation in larval diet (Fischman et al. 2017). This study is noteworthy, because the authors also showed that facultative diapause, as mediated by larval nutrition, has significant consequences on adult reproductive success. Thus, selection is free to act independently on diapausing and non-diapausing phenotypes, and this provides a potential pathway for the evolution of castes. Further investigation of the molecular mechanisms underlying this nutritionally induced plasticity in M. rotundata and other solitary bees and wasps is necessary to determine how developmental plasticity related to diapause may have facilitated developmental plasticity associated with reproductive castes.
Social cues are another important source of developmental caste-biasing, but how sensitivity to these cues evolved is also unknown. Experimental co-housing of some otherwise solitary bees can result in division of labor and reproductive suppression in some species (Sakagami and Maeta 1977, 1984, 1989, 1995). However, most of these species are secondarily solitary, and these results may reflect vestigial, rather than pre-existing plasticity. A more recent study with an ancestrally solitary halictid bee (Nomia melanderi), demonstrated that unlike social halictid bees (e.g., M. genalis), reproductive maturation is robust to cues from the social environment (Kapheim and Johnson 2017). This suggests that in the ~ 75 million years since social bees shared a common ancestor with N. melanderi, gene regulatory networks that function in development shifted such that they became sensitive to the social environment. Additional research in this area is critical to understand the molecular basis for the evolutionary acquisition of developmental plasticity related to social behavior.
What is the role of developmental plasticity in evolution?
Developmental plasticity is one of the hallmarks of social evolution, with multiple castes evolving from highly similar genomes. Studies of caste differentiation have focused primarily on the causes of plasticity. However, developmental plasticity is also likely to shape the trajectory of social evolution, and it is thus also important to understand the consequences of plasticity (Fig. 1b). Hymenopteran insects are characterized by holometabolous development and ploidy-based sex determination. Each of these traits is associated with extensive plasticity in gene expression through development. These ancient forms of developmental plasticity predate the origins of eusociality, and may have primed certain molecular pathways for co-option by social evolution. This priming may be the result of one or more corollaries of developmental plasticity.
First, phenotypes serve as the conduit between the genetic code and natural selection, and genes with reduced or conditional expression are thus less exposed to selection. Genes with reduced expression associated with developmental plasticity are therefore likely to harbor elevated amounts of genetic variation, because slightly deleterious mutations will be less effectively shed from the population via purifying selection. Relaxed selective constraint stemming from sex- or stage-specific developmental plasticity can lead to the accumulation of cryptic genetic variation that may become co-opted for social function and subsequent adaptive evolution. This hypothesis generates at least two testable predictions. First, expression plasticity during development should be positively correlated with genetic diversity in solitary species that represent the ancestors of eusocial species. Second, these genetic variants should show signatures of adaptive evolution associated with social behavior. To my knowledge, this hypothesis has not been tested directly in social insects, but indirect support comes from the finding that expression patterns of caste-biased genes have diverged more than sex- or stage-biased genes in eusocial vespid wasps (Hoffman and Goodisman 2007; Hunt and Goodisman 2010). One potential explanation for this finding is that genes involved in caste dimorphism are under positive selection in social wasps, as a result of genetic variants that accumulated under conditional expression associated with sexual dimorphism. More explicit tests of this hypothesis are required to understand the degree to which developmental plasticity produces genetic variants that are co-opted for social evolution.
The second way that developmental plasticity may influence social evolution is through the creation of gene networks that are primed for novel regulatory controls. Regulatory elements involved in sex differentiation pathways are sensitive to environmental cues, and are thus spring-loaded to develop sensitivities to novel cues, such as those associated with regulating caste differentiation (Klein et al. 2016). In support of this, sex-biased genes also tend to be differentially expressed among social castes of the ant Cardiocondyla obscurior (Klein et al. 2016). In C. obscurior, and perhaps others, high tier regulatory elements that function in endocrine response and nutrient-sensing (e.g., juvenile hormone pathways) can generate phenotypic novelty, such as that of social castes, with minor changes in spatio-temporal expression via downstream effects of transcriptional cascades (Klein et al. 2016). Additional tests of this hypothesis should include comparisons of gene regulatory networks associated with sex-, stage-, and caste-specific development to test the prediction that there are a significant portion of shared elements in these networks which function as master-regulators of environmentally sensitive pathways.
Finally, genes that function in developmental plasticity associated with stage or sex differentiation are likely to have high levels of inter-individual variation in expression. For inter-individual variation to be maintained in populations, they must be part of robust gene regulatory networks capable of absorbing fluctuation in response to environmental change. Gene networks associated with sex or stage related developmental plasticity are thus more likely to absorb variation associated with novel developmental phenotypes, such as castes, without significant fitness costs (Klein et al. 2016). In support of this, genes that are differentially expressed among social castes also tend to have high variability in expression within social castes in fire ants (Solenopsis invicta; (Hunt et al. 2013)) and tetraphenic ants (C. obscurior; (Schrader et al. 2017)).
Ultimate analysis of contemporary and historical influences on social behavior
The origins and elaborations of sociality involve evolutionary changes in the genome that include a combination of new genes and new patterns of gene regulation. Incipient forms of eusociality are characterized by the decoupling of a solitary life cycle among colony members engaged in division of labor, with queens specializing on reproduction and workers specializing on brood care. It has been hypothesized that novel regulatory patterns of existing gene networks are likely to be a primary mechanism of social origins (West Eberhard 1987; West Eberhard 1996; Johnson and Linksvayer 2010). The genetic toolkit required for all aspects of incipient social life is likely to be present in the ancestral genomes from which sociality arose, and can be thought of as the “social anatomy” (Johnson and Linksvayer 2010). The elaborations of eusociality from simple societies to superorganisms, however, are expected to require the evolution of new genes or novel gene networks that function in the emergent properties necessary for coordination of a “social physiology” at the group level (Johnson and Linksvayer 2010). Comparative genomics studies in bees and ants, as well as detailed studies of tissue-specific expression patterns, have provided an accumulating base of support for the mechanistic aspects of this hypothesis (reviewed in Kapheim 2016).
However, many of the ultimate aspects of this hypothesis remain unaddressed. That is, genes that make up the social anatomy and social physiology have been identified in several lineages with social species, but it is not clear why these genes or gene networks have been the targets of social evolution. To fill this gap, it is necessary to ask why certain gene networks, such as those involved in metabolism, endocrine function, or nutrient sensing are repeatedly the site of regulatory changes associated with social origins. We can also ask how novel social functions that arise from regulatory changes influence the evolution of emergent properties at the colony level. To address ultimate explanations for the genomic anatomy and physiology associated with social origins and elaborations, it is thus necessary to ask how historical patterns of selection prime the ancestral genome for current function in social behavior, and how social function in turn affects the trajectory of social evolution (Fig. 1b).
How does evolutionary history influence current utility?
A spate of comparative genomics and transcriptomics studies in the past several years have produced lists of genes with current social utility in a broad range of social insect species. These studies have revealed that key sets of genes or gene pathways have shared social function in multiple species. However, it is unclear why these particular genes have repeatedly evolved social function. In order to address this question, it is necessary to ask how ancestral conditions set the stage for current utility (Fig. 1b).
One way that evolutionary history may shape genes that function in social behavior is via processes that generate genetic diversity in solitary ancestors of social species. Selection on pre-existing genetic diversity is likely to be an important component of social evolution, because adaptive evolution is faster and more effective when acting on standing genetic variation than on new mutations (Barrett and Schluter 2008). Testing this hypothesis requires understanding patterns of genetic diversity in species that represent the solitary ancestors from which sociality evolved. If social function is derived from pre-existing genetic variation, then orthologs of genes involved in social behavior are expected to have elevated nucleotide diversity or fast evolutionary rates in solitary relatives of social insect species. A dearth of population genetic studies on solitary species has left this prediction largely untested, but other forms of evidence support this hypothesis more generally.
First, an early test of this hypothesis showed that orthologous genes which are differentially expressed between fire ant and honey bee castes have significantly higher rates of evolution in the parasitoid wasp, Nasonia vitripennis (Hunt et al. 2011). This result is consistent with the hypothesis that genes under relaxed selective constraint are more likely to be co-opted for social function. However, this result may alternatively stem from shared genetic mechanisms underlying a parasitoid and eusocial lifestyle. Female parasitoid wasps, like queens of eusocial colonies, outsource the care of their offspring. This reduction in maternal care in favor of increased fecundity is expected to lead to reduced constraint on genes associated with the non-reproductive aspects of offspring production in eusocial ants and bees (Gadagkar 1997), but a similar pattern may result from selection on parasitoid lifestyle. In this case, elevated rates of evolution in N. vitripennis orthologs of caste-biased genes in social insects may stem from shared expression-bias due to adaptive evolution, rather than standing genetic variation. Differentiating between these alternatives will require documenting the evolutionary rate and genetic diversity of orthologs of genes with social function in solitary species that engage in brood care and are more closely related to social bees and wasps.
A second line of evidence for this hypothesis comes from a recent study of gene duplication patterns. Among ten bee species that vary in social behavior, increasing rates of gene duplication were associated with increasing social complexity (Chau and Goodisman 2017). A closer examination of the consequences of gene duplication in honey bees showed that genes with biased expression between queens and workers were overrepresented among duplicated genes (Chau and Goodisman 2017). Together, these results could suggest that gene duplication precedes co-option for social function. Because duplication allows the accumulation of genetic variation in the duplicated copy of the gene, this could provide support for the hypothesis that social function arises from standing genetic variation in a solitary ancestor. However, this is likely to be filtered through adaptive evolution associated with sexual and cellular differentiation, as similar patterns of gene duplication were found for sex-biased and tissue-biased genes (Chau and Goodisman 2017).
Another potential source of standing genetic variation is recombination. Recombination is an important source of genetic diversity in the honey bee genome, as mismatches generated by crossing-over events are often imperfectly repaired, and this produces genetic variants (Yang et al. 2015). Evidence for the hypothesis that social function has been co-opted from genetic diversity produced by recombination stems from the finding that genes with higher expression in honey bee workers tend to be found in regions of the genome with high recombination (Kent et al. 2012; Liu et al. 2015). Understanding the role of recombination in priming the genome for co-option by social evolution requires comparisons of recombination rate across the genomes of closely related solitary and social species (Kapheim 2016). The finding that recombination generates genetic diversity in orthologs of worker-biased genes in solitary species would provide support for the hypothesis that elevated levels of genetic diversity preceded, and perhaps facilitated, adaptive evolution associated with social function.
How does current utility influence evolutionary trajectory?
Eusociality is expected to play a significant role in shaping the evolutionary trajectory of traits within populations. This role can best be understood by focusing on genes which function in social behavior, particularly genes with caste-biased patterns of expression. Genes with social function are expected to have increased rates of evolution and an accumulation of genetic variation, because they operate primarily through kin selection, and their effects on fitness are therefore indirect (Linksvayer and Wade 2009, 2016; Hall and Goodisman 2012). Due to these indirect fitness effects, the evolutionary trajectory of social genes is expected to differ from sex-limited or other conditionally-expressed genes with direct fitness effects (Linksvayer and Wade 2016). In accordance with these theoretical predictions, worker-biased genes have been shown to have reduced selection and higher rates of molecular evolution than queen-biased genes in bumble bees (Harpur et al. 2017) and ants (Monomorium pharaonis; (Warner et al. 2017)). In honey bees, however, genes with signatures of adaptive evolution are more enriched for worker-expressed genes than queen-expressed genes (Harpur et al. 2014). This finding is in contrast with the null expectations of the effects of kin selection on molecular evolution, and could suggest that worker traits have significantly larger effects on colony fitness than do queen traits.
It is notable that sociogenomic predictions of kin selection theory have been tested exclusively within obligately eusocial species, characterized by a moderate or high degree of social complexity. However, kin selection is not likely to be as strong at the origins of eusociality, where conflict between individual, kin, and group fitness is likely to be higher. The predictions for how kin selection should impact the molecular evolution of genes with social effects are based on the assumption that all else is equal (Linksvayer and Wade 2016). However, many genes with caste-biased expression are also conditionally expressed with regard to sex, tissue, or developmental stage (Klein et al. 2016). In highly derived, obligately eusocial species like ants and bumble bees, the strength of kin selection is apparently strong enough to supersede the potentially antagonistic direct effects of developmental plasticity. However, this may not be the case in species with incipient or facultative forms of sociality, such as M. genalis or C. calcarata. It will be necessary to disentangle the effects of developmental plasticity and social plasticity to fully evaluate how social function influences evolution at the origins of sociality. One potential outcome of this disentanglement could be the finding that the effects of other sources of plasticity have a larger role in shaping the earliest stages of social evolution than does kin selection (see above).
Eusociality may also influence the evolution of traits within populations via the effects of phenotypic plasticity. It has long been recognized that plasticity may precede and facilitate adaptive evolution (West-Eberhard 2003; Pfennig et al. 2010; Moczek et al. 2011; Gilbert et al. 2015), but there are few empirical tests of this “plasticity-first” hypothesis (Levis and Pfennig 2016). Evidence for effects of phenotypic plasticity on social evolution could include evidence of adaptive evolutionary change in the regulation of genes that function in social behavior (Aubin-Horth and Renn 2009; Levis and Pfennig 2016). Opportunities to look for such patterns can be found among families of bees and wasps which include closely related lineages or populations of both social and solitary species (Rehan and Toth 2015).
Comparative genomic studies in bees have provided the first evidence for a leading role of plasticity in social evolution. First, a comparative study of European honey bees and derived populations of highly aggressive Africanized bees evaluated the degree of overlap between plastic and evolved responses to alarm pheromone, which is an important signal for colony defense and social cohesion (Alaux et al. 2009). Remarkably, many of the genes for which changes in expression are induced by alarm pheromone in European honey bees have evolved differences in baseline expression between European and Africanized bees. This provides support for the idea that plastic responses to alarm pheromone preceded evolved differences in aggressive social behavior in honey bees.
A more recent study provides evidence of genetic accommodation that spans a broad taxonomic range. Species which exhibit facultative eusociality, such as the sweat bee M. genalis, can be studied as proxies for the ancestral state from which sociality evolved. Jones et al. (2017) used this approach to identify environmentally induced plasticity that may have preceded adaptive social evolution. They found that genes which are differentially expressed in one or more reproductive and social castes in M. genalis significantly overlap with caste-biased genes in obligately eusocial bees from different lineages, spanning multiple origins of sociality. Moreover, socially expressed genes in M. genalis were enriched for orthologs of genes undergoing positive selection in distantly related obligately eusocial species. This suggests that genetic accommodation of environmentally induced plasticity may be a mechanism of evolution that is common to multiple independent origins of eusociality.
Concluding remarks and the future of sociogenomics
In establishing the framework for the study of behavior, Tinbergen identified four questions that could be asked to explain behavioral phenotypes. These questions span multiple timescales and two levels of analysis. Tinbergen made it clear, however, that these questions should not be answered in isolation of one another, and that ethological research would be most valuable when approached from an integrative perspective. I propose that few areas of research have been as successful in achieving this level of integration as the study of the social lives of insects. We now have the capacity to answer reciprocal questions about how contemporary and historical processes influence mechanisms and evolutionary outcomes (Fig. 1).
Integrative research has provided the insight that the early stages of social evolution likely operate through maternal manipulation, which becomes transformed into sibling-directed caste determination with the evolution of increasing social complexity. This transformation is coupled with a temporal shift in the relative importance of nutrition such that nutritional effects on caste are perhaps more relevant during the larval stage in highly eusocial species than they are in species with incipient or facultative forms of eusociality. More research is needed to identify the mechanisms that cause mothers and sisters to direct the differential development of queens and workers. Comparative studies that include solitary insects will help fill gaps in our understanding of how developmental plasticity evolves in solitary ancestors, as well as how genes involved in other aspects of developmental plasticity may have been co-opted for social evolution. Additional research on solitary insects will also enable more complete tests of the hypothesis that social function emerged from standing genetic variation, and will help to identify the mechanisms that produce this variation. Comparative studies of selection patterns in closely related solitary and social species will also be necessary to evaluate the degree to which plasticity precedes social evolution. Finally, kin selection is expected to be weaker in species at the earliest stages of social evolution, and so molecular evolution studies in these species will improve our understanding of how social function influences evolution.
Filling some of these gaps in our understanding of eusociality will require new resources. This includes new genomic resources, particularly for solitary species from lineages with independent origins of sociality. However, natural history resources are also a critical component for the future of sociogenomics. This is particularly true for behaviors like social complexity that are best defined by a suite of traits, and are multidimensional in nature (Fig. 2). Tinbergen also stressed the need for observation in studies of ethology. In devoting an entire section of his treatise to the importance of observation, he delivered the following admonition, “However, if we overdo this in itself justifiable tendency of making description subject to our analytical aims, ...we might forget that naïve, unsophisticated, or intuitively guided observation may open our eyes to new problems. Contempt for simple observation is a lethal trait in any science, and certainly in a science as young as ours,” (p. 412). Our comparative genomic analyses are only as good as the natural history data that supports our comparisons. Complex phenotypes like eusociality encompass a suite of nuanced traits that cannot be easily compartmentalized into discreet categories. Detailed knowledge of life histories is thus exceedingly valuable. Yet, the amount of genomic knowledge we have about social insect species is rapidly verging upon that which we know about variation in behavior. The future of sociogenomics, and the insight promised by its integrative approach, thus depends in part on good observation.
Change history
31 January 2019
This article is a part of the topical collection “Social complexity: patterns, processes, and evolution”. For this reason, though it was originally inadvertently published in 2018, in Vol. 72, Issue 12, citation ID 186, it has now been reassigned and republished in 2019, in Vol. 73, Issue 1, citation ID 186.
References
Alaux C, Sinha S, Hasadri L et al (2009) Honey bee aggression supports a link between gene regulation and behavioral evolution. Proc Natl Acad Sci U S A 106:15400–15405. https://doi.org/10.1073/pnas.0907043106
Alexander R (1974) The evolution of social behavior. Annu Rev Ecol Syst 5:325–383
Anderson C, McShea DW (2007) Individual versus social complexity, with particular reference to ant colonies. Biol Rev 76:211–237. https://doi.org/10.1017/S1464793101005656
Ashby R, Forêt S, Searle I, Maleszka R (2016) MicroRNAs in honey bee caste determination. Sci Rep 6:18794. https://doi.org/10.1038/srep18794
Aubin-Horth N, Renn SCP (2009) Genomic reaction norms: using integrative biology to understand molecular mechanisms of phenotypic plasticity. Mol Ecol 18:3763–3780. https://doi.org/10.1111/j.1365-294X.2009.04313.x
Badisco L, Van Wielendaele P, Vanden Broeck J (2013) Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front Physiol 4:202. https://doi.org/10.3389/fphys.2013.00202
Barrett RDH, Schluter D (2008) Adaptation from standing genetic variation. Trends Ecol Evol 23:38–44. https://doi.org/10.1016/j.tree.2007.09.008
Bateson P, Laland KN (2013) Tinbergen’s four questions: an appreciation and an update. Trends Ecol Evol 28:712–718. https://doi.org/10.1016/j.tree.2013.09.013
Batra SWT (1966) Nests and social behavior of halictine bees of India (Hymenoptera: Halictidae). Indian J Entomol 28:375–393
Berens AJ, Hunt JH, Toth AL (2015) Nourishment level affects caste-related gene expression in Polistes wasps. BMC Genomics 16:235. https://doi.org/10.1186/s12864-015-1410-y
Branstetter MG, Danforth BN, Pitts JP, Faircloth BC, Ward PS, Buffington ML, Gates MW, Kula RR, Brady SG (2017) Phylogenomic insights into the evolution of stinging wasps and the origins of ants and bees. Curr Biol 27:1019–1025. https://doi.org/10.1016/j.cub.2017.03.027
Branstetter MG, Childers AK, Cox-Foster D, Hopper KR, Kapheim KM, Toth AL, Worley KC (2018) Genomes of the Hymenoptera. Curr Op Insect Sci 25:65–75. https://doi.org/10.1016/j.cois.2017.11.008
Chau LM, Goodisman MAD (2017) Gene duplication and the evolution of phenotypic diversity in insect societies. Evolution 71:2871–2884. https://doi.org/10.1111/evo.13356
Collins DH, Mohorianu I, Beckers M, Moulton V, Dalmay T, Bourke AFG (2017) MicroRNAs associated with caste determination and differentiation in a primitively eusocial insect. Sci Rep 7:45674. https://doi.org/10.1038/srep45674
Fischman BJ, Pitts-Singer TL, Robinson GE (2017) Nutritional regulation of phenotypic plasticity in a solitary bee (Hymenoptera: Megachilidae). Environ Entomol 46:1070–1079. https://doi.org/10.1093/ee/nvx119
Gadagkar R (1997) The evolution of caste polymorphism in social insects: genetic release followed by diversifying evolution. J Genet 76:167–179
Gadau J, Helmkampf M, Nygaard S, Roux J, Simola DF, Smith CR, Suen G, Wurm Y, Smith CD (2012) The genomic impact of 100 million years of social evolution in seven ant species. Trends Genet 28:14–21. https://doi.org/10.1016/j.tig.2011.08.005
Gamboa GJ, Reeve HK, Pfennig DW (1986) The evolution and ontogeny of nestmate recognition in social wasps. Annu Rev Entomol 31:431–454. https://doi.org/10.1146/annurev.en.31.010186.002243
Gilbert SF, Bosch TCG, Ledon-Rettig C (2015) Eco-Evo-Devo: developmental symbiosis and developmental plasticity as evolutionary agents. Nat Rev Genet 16:611–622. https://doi.org/10.1038/nrg3982
Guo X, Su S, Skogerboe G, Dai S, Li W, Li Z, Liu F, Ni R, Guo Y, Chen S, Zhang S, Chen R (2013) Recipe for a busy bee: microRNAs in honey bee caste determination. PLoS One 8:e81661. https://doi.org/10.1371/journal.pone.0081661
Hahn DA, Denlinger DL (2011) Energetics of insect diapause. Annu Rev Entomol 56:103–121. https://doi.org/10.1146/annurev-ento-112408-085436
Hall DW, Goodisman MA (2012) The effects of kin selection on rates of molecular evolution in social insects. Evolution 66:2080–2093. https://doi.org/10.1111/j.1558-5646.2012.01602.x
Hamilton WD (1964) The genetical evolution of social behaviour, I & II. J Theor Biol 7:1–52
Harpur BA, Kent CF, Molodtsova D, Lebon JM, Alqarni AS, Owayss AA, Zayed A (2014) Population genomics of the honey bee reveals strong signatures of positive selection on worker traits. Proc Natl Acad Sci U S A 111:2614–2619. https://doi.org/10.1073/pnas.1315506111
Harpur BA, Dey A, Albert J et al (2017) Queens and workers contribute differently to adaptive evolution in bumble bees and honey bees. Genome Biol Evol 9:2395–2402. https://doi.org/10.1093/gbe/evx182
Haydak M (1970) Honey bee nutrition. Annu Rev Entomol 15:143–156
Hoffman EA, Goodisman MA (2007) Gene expression and the evolution of phenotypic diversity in social wasps. BMC Biol 5:23. https://doi.org/10.1186/1741-7007-5-23
Hofmann HA, Beery A, Blumstein D et al (2014) An evolutionary framework for studying mechanisms of social behavior. Trends Ecol Evol 29:581–589. https://doi.org/10.1016/j.tree.2014.07.008
Hunt JH, Amdam GV (2005) Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 308:264–267. https://doi.org/10.1126/science.1109724
Hunt BG, Goodisman MA (2010) Evolutionary variation in gene expression is associated with dimorphism in eusocial vespid wasps. Insect Mol Biol 19:641–652. https://doi.org/10.1111/j.1365-2583.2010.01021.x
Hunt GJ, Page RE Jr, Fondrk MK, Dullum CJ (1995) Major quantitative trait loci affecting honey bee foraging behavior. Genetics 141:1537–1545
Hunt JH, Kensinger BJ, Kossuth JA, Henshaw MT, Norberg K, Wolschin F, Amdam GV (2007) A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc Natl Acad Sci U S A 104:14020–14025. https://doi.org/10.1073/pnas.0705660104
Hunt JH, Wolschin F, Henshaw MT, Newman TC, Toth AL, Amdam GV (2010) Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp. PLoS One 5:e10674. https://doi.org/10.1371/journal.pone.0010674
Hunt BG, Ometto L, Wurm Y, Shoemaker D, Yi SV, Keller L, Goodisman MAD (2011) Relaxed selection is a precursor to the evolution of phenotypic plasticity. Proc Natl Acad Sci U S A 108:15936–15941. https://doi.org/10.1073/pnas.1104825108
Hunt BG, Ometto L, Keller L, Goodisman MA (2013) Evolution at two levels in fire ants: the relationship between patterns of gene expression and protein sequence evolution. Mol Biol Evol 30:263–271. https://doi.org/10.1093/molbev/mss234
Jandt JM, Toth AL (2015) Physiological and genomic mechanisms of social organization in wasps (family: Vespidae). Adv Insect Physiol 48:95–130. https://doi.org/10.1016/bs.aiip.2015.01.003
Jeanson R, Weidenmüller A (2014) Interindividual variability in social insects – proximate causes and ultimate consequences. Biol Rev 89:671–687. https://doi.org/10.1111/brv.12074
Johnson BR, Linksvayer TA (2010) Deconstructing the superorganism: social physiology, groundplans, and sociogenomics. Q Rev Biol 85:57–79. https://doi.org/10.1086/650290
Jones BM, Kingwell CJ, Wcislo WT, Robinson GE (2017) Caste-biased gene expression in a facultatively eusocial bee suggests a role for genetic accommodation in the evolution of eusociality. Proc R Soc B 284:20162228. https://doi.org/10.1098/rspb.2016.2228
Judd TM, Teal PEA, Hernandez EJ, Choudhury T, Hunt JH (2015) Quantitative differences in nourishment affect caste-related physiology and development in the paper wasp Polistes metricus. PLoS One 10:e0116199. https://doi.org/10.1371/journal.pone.0116199
Kapheim KM (2016) Genomic sources of phenotypic novelty in the evolution of eusociality in insects. Curr Opin Insect Sci 13:24–32. https://doi.org/10.1016/j.cois.2015.10.009
Kapheim KM (2017) Nutritional, endocrine, and social influences on reproductive physiology at the origins of social behavior. Curr Opin Insect Sci 22:62–70. https://doi.org/10.1016/j.cois.2017.05.018
Kapheim KM, Johnson MM (2017) Juvenile hormone, but not nutrition or social cues, affects reproductive maturation in solitary alkali bees (Nomia melanderi). J Exp Biol 220:3794–3801. https://doi.org/10.1242/jeb.162255
Kapheim KM, Bernal SP, Smith AR, Nonacs P, Wcislo WT (2011) Support for maternal manipulation of developmental nutrition in a facultatively eusocial bee, Megalopta genalis (Halictidae). Behav Ecol Sociobiol 65:1179–1190. https://doi.org/10.1007/s00265-010-1131-9
Kapheim KM, Smith AR, Ihle KE, Amdam GV, Nonacs P, Wcislo WT (2012) Physiological variation as a mechanism for developmental caste-biasing in a facultatively eusocial sweat bee. Proc R Soc Lond B 279:1437–1446. https://doi.org/10.1098/rspb.2011.1652
Kapheim KM, Smith AR, Nonacs P, Wcislo WT, Wayne RK (2013) Foundress polyphenism and the origins of eusociality in a facultatively eusocial sweat bee, Megalopta genalis (Halictidae). Behav Ecol Sociobiol 67:331–340. https://doi.org/10.1007/s00265-012-1453-x
Kapheim KM, Nonacs P, Smith AR, Wayne RK, Wcislo WT (2015a) Kinship, parental manipulation and evolutionary origins of eusociality. Proc R Soc B 282:20142886. https://doi.org/10.1098/rspb.2014.2886
Kapheim KM, Pan H, Li C, Salzberg SL, Puiu D, Magoc T, Robertson HM, Hudson ME, Venkat A, Fischman BJ, Hernandez A, Yandell M, Ence D, Holt C, Yocum GD, Kemp WP, Bosch J, Waterhouse RM, Zdobnov EM, Stolle E, Kraus FB, Helbing S, Moritz RFA, Glastad KM, Hunt BG, Goodisman MAD, Hauser F, Grimmelikhuijzen CJP, Pinheiro DG, Nunes FMF, Soares MPM, Tanaka ED, Simoes ZLP, Hartfelder K, Evans JD, Barribeau SM, Johnson RM, Massey JH, Southey BR, Hasselmann M, Hamacher D, Biewer M, Kent CF, Zayed A, Blatti C, Sinha S, Johnston JS, Hanrahan SJ, Kocher SD, Wang J, Robinson GE, Zhang G (2015b) Social evolution. Genomic signatures of evolutionary transitions from solitary to group living. Science 348:1139–1143. https://doi.org/10.1126/science.aaa4788
Kapheim KM, Chan TY, Smith AR, Wcislo WT, Nonacs P (2016) Ontogeny of division of labor in a facultatively eusocial sweat bee Megalopta genalis. Insect Soc 63:185–191. https://doi.org/10.1007/s00040-015-0454-y
Kent CF, Minaei S, Harpur BA, Zayed A (2012) Recombination is associated with the evolution of genome structure and worker behavior in honey bees. Proc Natl Acad Sci U S A 109:18012–18017. https://doi.org/10.1073/pnas.1208094109
Kijimoto T, Moczek AP (2016) Hedgehog signaling enables nutrition-responsive inhibition of an alternative morph in a polyphenic beetle. Proc Natl Acad Sci U S A 113:5982–5987. https://doi.org/10.1073/pnas.1601505113
Kijimoto T, Snell-Rood EC, Pespeni MH, Rocha G, Kafadar K, Moczek AP (2014) The nutritionally responsive transcriptome of the polyphenic beetle Onthophagus taurus and the importance of sexual dimorphism and body region. Proc R Soc B 281:20142084. https://doi.org/10.1098/rspb.2014.2084
Klein A, Schultner E, Lowak H, Schrader L, Heinze J, Holman L, Oettler J (2016) Evolution of social insect polyphenism facilitated by the sex differentiation cascade. PLoS Genet 12:e1005952. https://doi.org/10.1371/journal.pgen.1005952
Laland KN, Sterelny K, Odling-Smee J, Hoppitt W, Uller T (2011) Cause and effect in biology revisited: is Mayr’s proximate-ultimate dichotomy still useful? Science 334:1512–1516. https://doi.org/10.1126/science.1210879
Lande R (1982) A quantitative genetic theory of life history evolution. Ecology 63:607–615. https://doi.org/10.2307/1936778
Lande R, Arnold SJ (1983) The measurement of selection on correlated characters. Evolution 37:1210–1226
Lawson SP, Ciaccio KN, Rehan SM (2016) Maternal manipulation of pollen provisions affects worker production in a small carpenter bee. Behav Ecol Sociobiol 70:1891–1900. https://doi.org/10.1007/s00265-016-2194-z
Lawson SP, Helmreich SL, Rehan SM (2017) Effects of nutritional deprivation on development and behavior in the subsocial bee Ceratina calcarata (Hymenoptera: Xylocopinae). J Exp Biol 220:4456–4462. https://doi.org/10.1242/jeb.160531
LeBoeuf AC, Waridel P, Brent C et al (2016) Oral transfer of chemical cues, growth proteins and hormones in social insects. eLife 5:e20375. https://doi.org/10.7554/eLife.20375
Levis NA, Pfennig DW (2016) Evaluating ‘plasticity-first’ evolution in nature: key criteria and empirical approaches. Trends Ecol Evol 31:563–574. https://doi.org/10.1016/j.tree.2016.03.012
Linksvayer TA, Wade MJ (2005) The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Q Rev Biol 80:317–336
Linksvayer TA, Wade MJ (2009) Genes with social effects are expected to harbor more sequence variation within and between species. Evolution 63:1685–1696. https://doi.org/10.1111/j.1558-5646.2009.00670.x
Linksvayer TA, Wade MJ (2016) Theoretical predictions for sociogenomic data: the effects of kin selection and sex-limited expression on the evolution of social insect genomes. Front Ecol Evol 4:65. https://doi.org/10.3389/fevo.2016.00065
Liu H, Zhang X, Huang J, Chen J-Q, Tian D, Hurst L, Yang S (2015) Causes and consequences of crossing-over evidenced via a high-resolution recombinational landscape of the honey bee. Genome Biol 16:15
MacDougall-Shackleton SA (2011) The levels of analysis revisited. Philos Trans R Soc B 366:2076–2085. https://doi.org/10.1098/rstb.2010.0363
Mao W, Schuler MA, Berenbaum MR (2015) A dietary phytochemical alters caste-associated gene expression in honey bees. Sci Adv 1:e1500795. https://doi.org/10.1126/sciadv.1500795
Mayr E (1961) Cause and effect in biology. Science 134:1501–1506. https://doi.org/10.1126/science.134.3489.1501
Miura T (2004) Proximate mechanisms and evolution of caste polyphenism in social insects: from sociality to genes. Ecol Res 19:141–148. https://doi.org/10.1111/j.1440-1703.2003.00618.x
Moczek AP, Sultan S, Foster S, Ledon-Rettig C, Dworkin I, Nijhout HF, Abouheif E, Pfennig DW (2011) The role of developmental plasticity in evolutionary innovation. Proc R Soc Lond B 278:2705–2713. https://doi.org/10.1098/rspb.2011.0971
Monnin T, Liebig J (2008) Understanding eusociality requires both proximate and ultimate thinking and due consideration of individual and colony-level interests. Oikos 117:1441–1443
Müller CB, Williams IS, Hardie J (2001) The role of nutrition, crowding and interspecific interactions in the development of winged aphids. Ecol Entomol 26:330–340. https://doi.org/10.1046/j.1365-2311.2001.00321.x
Okada Y, Watanabe Y, Tin MMY, Tsuji K, Mikheyev AS (2017) Social dominance alters nutrition-related gene expression immediately: transcriptomic evidence from a monomorphic queenless ant. Mol Ecol 26:2922–2938. https://doi.org/10.1111/mec.13989
Owens IPF (2006) Where is behavioural ecology going? Trends Ecol Evol 21:356–361
Patel A, Fondrk MK, Kaftanoglu O, Emore C, Hunt G, Frederick K, Amdam GV (2007) The making of a queen: TOR pathway is a key player in diphenic caste development. PLoS One 2:e509
Pfennig DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP (2010) Phenotypic plasticity’s impacts on diversification and speciation. Trends Ecol Evol 25:459–467. https://doi.org/10.1016/j.tree.2010.05.006
Reaney LT, Knell RJ (2015) Building a beetle: how larval environment leads to adult performance in a horned beetle. PLoS One 10:e0134399. https://doi.org/10.1371/journal.pone.0134399
Rehan SM, Toth AL (2015) Climbing the social ladder: the molecular evolution of sociality. Trends Ecol Evol 30:426–433. https://doi.org/10.1016/j.tree.2015.05.004
Renn SCP, Schumer ME (2013) Genetic accommodation and behavioural evolution: insights from genomic studies. Anim Behav 85:1012–1022. https://doi.org/10.1016/j.anbehav.2013.02.012
Robinson GE (1999) Integrative animal behaviour and sociogenomics. Trends Ecol Evol 14:202–205
Robinson GE, Grozinger CM, Whitfield CW (2005) Sociogenomics: social life in molecular terms. Nat Rev Genet 6:257–270
Ronai I, Vergoz V, Oldroyd BP (2016) The mechanistic, genetic, and evolutionary basis of worker sterility in the social Hymenoptera. Adv Study Behav 48:251–317. https://doi.org/10.1016/bs.asb.2016.03.002
Sakagami SF, Maeta Y (1977) Some presumably pre-social habits of Japanese Ceratina bees, with notes on various social types in Hymenoptera. Insect Soc 24:319–343
Sakagami SF, Maeta Y (1984) Multifemale nests and rudimentary castes in the normally solitary bee Ceratina-japonica (Hymenoptera, Xylocopinae). J Kansas Entomol Soc 57:639–656
Sakagami SF, Maeta Y (1989) Compatibility and incompatibility of solitary life with eusociality in two normally solitary bees Ceratina japonica and Ceratina okinawana (Hymenoptera, Apoidea), with notes on the incipient phase of eusociality. Jpn J Entomol 57:417–739
Sakagami SF, Maeta Y (1995) Task allocation in artificially induced colonies of a basically solitary bee Ceratina (Ceratinidia) okinawana, with a comparison of sociality between Ceratina and Xylocopa (Hymenoptera, Anthophoridae, Xylocopinae). Jpn J Ecol 63:115–150
Schrader L, Helanterä H, Oettler J (2017) Accelerated evolution of developmentally biased genes in the tetraphenic ant Cardiocondyla obscurior. Mol Biol Evol 34:535–544. https://doi.org/10.1093/molbev/msw240
Shell WA, Rehan SM (2017) Behavioral and genetic mechanisms of social evolution: insights from incipiently and facultatively social bees. Apidologie 49:13–30. https://doi.org/10.1007/s13592-017-0527-1
Shell WA, Rehan SM, Holman L (2018) The price of insurance: costs and benefits of worker production in a facultatively social bee. Behav Ecol 29:204–211. https://doi.org/10.1093/beheco/arx146
Sherman PW (1988) The levels of analysis. Anim Behav 36:616–619. https://doi.org/10.1016/S0003-3472(88)80039-3
Shi Y-Y, Zheng H-J, Pan Q-Z, Wang Z-L, Zeng Z-J (2015) Differentially expressed microRNAs between queen and worker larvae of the honey bee (Apis mellifera). Apidologie 46:35–45. https://doi.org/10.1007/s13592-014-0299-9
Shpigler HY, Saul MC, Corona F, Block L, Cash Ahmed A, Zhao SD, Robinson GE (2017) Deep evolutionary conservation of autism-related genes. Proc Natl Acad Sci U S A 114:9653–9658. https://doi.org/10.1073/pnas.1708127114
Simola DF, Wissler L, Donahue G, Waterhouse RM, Helmkampf M, Roux J, Nygaard S, Glastad KM, Hagen DE, Viljakainen L, Reese JT, Hunt BG, Graur D, Elhaik E, Kriventseva EV, Wen J, Parker BJ, Cash E, Privman E, Childers CP, Munoz-Torres MC, Boomsma JJ, Bornberg-Bauer E, Currie CR, Elsik CG, Suen G, Goodisman MAD, Keller L, Liebig J, Rawls A, Reinberg D, Smith CD, Smith CR, Tsutsui N, Wurm Y, Zdobnov EM, Berger SL, Gadau J (2013) Social insect genomes exhibit dramatic evolution in gene composition and regulation while preserving regulatory features linked to sociality. Genome Res 23:1235–1247. https://doi.org/10.1101/gr.155408.113
Smith AR, Kapheim KM, Perez-Ortega B, Brent CS, Wcislo WT (2013) Juvenile hormone levels reflect social opportunities in the facultatively eusocial sweat bee Megalopta genalis (Hymenoptera: Halictidae). Horm Behav 63:1–4. https://doi.org/10.1016/j.yhbeh.2012.08.012
Smith CR, Morandin C, Noureddine M, Pant S (2018) Conserved roles of Osiris genes in insect development, polymorphism and protection. J Evol Biol 31:516–529. https://doi.org/10.1111/jeb.13238
Sumner S, Bell E, Taylor D (2018) A molecular concept of caste in insect societies. Curr Opin Insect Sci 25:42–50. https://doi.org/10.1016/j.cois.2017.11.010
Tinbergen N (1963) On aims and methods in ethology. Z Tierpsychol 20:410–433
Toth AL, Rehan SM (2017) Molecular evolution of insect sociality: an eco-evo-devo perspective. Annu Rev Entomol 62:419–442. https://doi.org/10.1146/annurev-ento-031616-035601
Toth AL, Robinson GE (2007) Evo-devo and the evolution of social behavior. Trends Genet 23:334–341
Toth AL, Varala K, Newman TC, Miguez FE, Hutchison SK, Willoughby DA, Simons JF, Egholm M, Hunt JH, Hudson ME, Robinson GE (2007) Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 318:441–444. https://doi.org/10.1126/science.1146647
Trible W, Olivos-Cisneros L, McKenzie SK et al (2017) Orco mutagenesis causes loss of antennal lobe glomeruli and impaired social behavior in ants. Cell 170:727–735. e710. https://doi.org/10.1016/j.cell.2017.07.001
Vellichirammal NN, Madayiputhiya N, Brisson JA (2016) The genomewide transcriptional response underlying the pea aphid wing polyphenism. Mol Ecol 25:4146–4160. https://doi.org/10.1111/mec.13749
Vellichirammal NN, Gupta P, Hall TA, Brisson JA (2017) Ecdysone signaling underlies the pea aphid transgenerational wing polyphenism. Proc Natl Acad Sci U S A 114:1419–1423. https://doi.org/10.1073/pnas.1617640114
Vojvodic S, Johnson BR, Harpur BA, Kent CF, Zayed A, Anderson KE, Linksvayer TA (2015) The transcriptomic and evolutionary signature of social interactions regulating honey bee caste development. Ecol Evol 5:4795–4807. https://doi.org/10.1002/ece3.1720
Walsh JT, Warner MR, Kase A, Cushing BJ, Linksvayer TA (2017) Ant nurse workers exhibit behavioral and transcriptomic specialization on larval stage but not caste. bioRxiv:218834. https://doi.org/10.1101/218834
Wang Y, Azevedo SV, Hartfelder K, Amdam G (2013) Insulin-like peptides (AmILP1 and AmILP2) differentially affect female caste development in the honey bee (Apis mellifera). J Exp Biol 218:4347–4357. https://doi.org/10.1242/jeb.085779
Warner MR, Mikheyev AS, Linksvayer TA (2017) Genomic signature of kin selection in an ant with obligately sterile workers. Mol Biol Evol 34:1780–1787. https://doi.org/10.1093/molbev/msx123
Weitekamp CA, Libbrecht R, Keller L (2017) Genetics and evolution of social behavior in insects. Annu Rev Genet 51:219–239. https://doi.org/10.1146/annurev-genet-120116-024515
Wenseleers T, van Zweden JS (2017) Sensory and cognitive adaptations to social living in insect societies. Proc Natl Acad Sci U S A 114:6424–6426
West Eberhard MJ (1987) Flexible strategy and social evolution. In: Ito Y, Brown JL, Kikkawa J (eds) Animal societies: theories and facts. Japan Scientific Societies Press, Tokyo, pp 35–51
West Eberhard MJ (1996) Wasp societies as microcosms for the study of development and evolution. In: Turillazzi S, West Eberhard MJ (eds) Natural history and evolution of paper wasps. Oxford University Press, Oxford, pp 290–317
West-Eberhard MJ (2003) Developmental plasticity and evolution. Oxford University Press, Oxford
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge
Withee JR, Rehan SM (2017) Social aggression, experience, and brain gene expression in a subsocial bee. Integr Comp Biol 57:640–648. https://doi.org/10.1093/icb/icx005
Wolf JB, Wade MJ (2001) On the assignment of fitness to parents and offspring: whose fitness is it and when does it matter? J Evol Biol 14:347–356
Wolschin F, Mutti NS, Amdam GV (2011) Insulin receptor substrate influences female caste development in honeybees. Biol Lett 7:112–115. https://doi.org/10.1098/rsbl.2010.0463
Yan H, Opachaloemphan C, Mancini G, Yang H, Gallitto M, Mlejnek J, Leibholz A, Haight K, Ghaninia M, Huo L, Perry M, Slone J, Zhou X, Traficante M, Penick CA, Dolezal K, Gokhale K, Stevens K, Fetter-Pruneda I, Bonasio R, Zwiebel LJ, Berger SL, Liebig J, Reinberg D, Desplan C (2017) An engineered orco mutation produces aberrant social behavior and defective neural development in ants. Cell 170:736–747. e739. https://doi.org/10.1016/j.cell.2017.06.051
Yang S, Wang L, Huang J, Zhang X, Yuan Y, Chen JQ, Hurst LD, Tian D (2015) Parent-progeny sequencing indicates higher mutation rates in heterozygotes. Nature 523:463–467. https://doi.org/10.1038/nature14649
Acknowledgments
I am grateful to P. Kappeler, S. Schultz, D. Lukas, and T. Clutton-Brock for organizing this topical issue. This manuscript was improved by helpful discussion with lab members and by suggestions from P. Kappeler, D. Lukas, and two anonymous reviewers.
Funding
This study received financial support from Utah State University and the Utah Agricultural Experiment Station (project 1297).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that she has no conflict of interest.
Additional information
Communicated by P. M. Kappeler
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised as this was inadvertently published in 2018, in Vol. 72, Issue 12, citation ID 186, it has now been reassigned and republished in 2019, in Vol. 73, Issue 1, citation ID 186.
This article is a contribution to the Topical Collection Social complexity: patterns, processes, and evolution - Guest Editors: Peter Kappeler, Susanne Shultz, Tim Clutton-Brock, and Dieter Lukas
Rights and permissions
About this article
Cite this article
Kapheim, K.M. Synthesis of Tinbergen’s four questions and the future of sociogenomics. Behav Ecol Sociobiol 73, 186 (2019). https://doi.org/10.1007/s00265-018-2606-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2606-3