Abstract
Primate mothers have the potential to influence the development of species-typical aspects of their offspring’s behavior as well as their individual sociosexual and reproductive strategies. If mothers experience psychosocial stress during pregnancy or lactation, their own stress hormones can have a long-term impact on their offspring’s physiology. The mother’s own behavior, especially weaning-related rejection, can be stressful to infants and have long-lasting effects on their infants’ neuroendocrine reactivity to stress. Exposure to variable maternal style early in life has long-term effects on the development of offspring behavior, including exploration and play, affiliation and aggression, and parenting. Primate mothers influence the kinship- and rank-related social preferences of their offspring, typically by providing opportunities to interact with some individuals more than others. Although mothers can contribute to the development of sex-typical behavior in the offspring (along with other environmental and genetic factors), sex-typical behavior is generally not the product of maternal socialization. Mothers provide their offspring with opportunities for social learning but rarely teach their infants new skills. In primate species with despotic dominance hierarchies, maternal transmission of rank through agonistic aid to their offspring makes a crucial contribution to the offspring’s fitness (as the daughters of high-ranking mothers reproduce more successfully than the daughters of low-ranking mothers). Future studies of maternal influences on social development could benefit from a deeper theoretical and experimental investigation of the evolutionary significance of these effects as well as of their underlying proximate mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In nonhuman primates, mothers bear most (and in many cases, all) of the burden of childrearing across a wide range of parental activities (nutrition, transport, protection, social support, education) and for almost their entire lifespan, as maternal investment often continues into adulthood. Significant paternal investment is extremely rare and limited to highly specific socioecological and reproductive contexts: for example, in marmosets and tamarins, fathers “are forced” to carry their offspring because females give birth to twins and incur significant energetic constraints (Clutton-Brock 1991). Help in raising offspring from older siblings, other relatives, and unrelated adults is also rare (Maestripieri 1994).
Primate mothers establish intimate physiological connections with their offspring from the day of conception until their offspring are weaned from breast milk (a period of time that can last several years) as well as strong socio-emotional bonds from the birth of the offspring (or even earlier) through the last day of their own life. Primate mothers, therefore, have the potential to influence virtually any aspect of their offspring’s phenotype through a variety of different mechanisms: genetic, nutritional, hormonal, immunological, emotional, cognitive, social, and environmental (Maestripieri 2009). Mothers can even influence their offspring’s reproductive decisions and their investment in their own descendants for several subsequent generations (Maestripieri 2009).
Many maternal influences on offspring development concern basic aspects of their survival or their growth; this is the case for effects that occur through maternal nutrition of the offspring or through the transfer of immunological substances to them through the placenta or breast milk. However, there is a lot more to fitness in primates than nutrition and growth. For example, in most species of primates, and especially in humans, social success is a crucial component of reproductive success, as making friends and allies, achieving high status, and obtaining social support to buffer oneself from stress and improve health are important prerequisites for living a long life and raising offspring and grandoffspring (e.g., Silk et al. 2003). An important question, therefore, is whether and how primate mothers contribute to (in positive or in negative) the future social success of their offspring. Exploring maternal influences on offspring social development can also shed light on the development of primate sociality in general, which is still a poorly understood aspect of primate behavioral evolution.
In this article, I review our current knowledge of maternal influences on social development in nonhuman primates. This review focuses on the species of monkeys and apes for which most information is available and is biased towards studies conducted in naturalistic and semi-naturalistic conditions (e.g., in which individuals are free-ranging or are captive but live in naturalistic social groups) as opposed to those conducted in highly artificial laboratory conditions (e.g., those in which animals are housed individually or in pairs in small cages). Although a great deal is known about parental influences on social development in humans, human research is not systematically reviewed in this article, in part because in many cases we do not have nonhuman primate data that are directly comparable to human data to warrant a joint review and discussion. For example, much human research on maternal influences on social development has been conducted within the framework of attachment theory (Bowlby 1969), but little is known about attachment and social development in nonhuman primates (Maestripieri 2003).
The article begins with a review of the influence of maternal stress on the development of offspring physiological and behavioral reactivity to the environment, including reactivity to stress (section “Influence of of maternal stress on offspring neuroendocrine function”). This topic is relevant because overall reactivity to the environment can be an important component of social success. The rest of the article addresses social processes. Specifically, I review maternal influences on offspring’s behavior (section “Influence of maternal style on the offspring's behavior”) and social preferences (section “Maternal influences on the development of offspring’s social preferences”), the development of behavioral sex differences (section “Maternal influences on the development of sex differences in behavior”), the acquisition of dominance rank (section “Influence of maternal agonistic support on the offspring’s acquisition of dominance rank”), and last social learning (section “Maternal influences on offspring social learning”). The article ends with a brief summary and discussion of future research directions (section “Conclusions and future directions”).
Influence of maternal stress on offspring neuroendocrine function
Sex and individual differences in virtually all aspects of social behavior, including affiliative and agonistic behavior or sexual and parental behavior, originate, at the proximate level, from sex and individual differences in neuroendocrine function. In primates, variation in neuroendocrine function and its effects on behavior have been documented for hormones of the hypothalamic-pituitary-gonadal (HPG) axis (e.g., estrogen and testosterone), those of the hypothalamic-pituitary-adrenal (HPA) axis such as adrenocorticotropin hormone (ACTH) and cortisol, as well as for other hormones and brain neurotransmitters such as oxytocin, vasopressin, norepinephrine, dopamine, and serotonin (see Maestripieri 2010, for a review). Sex and individual differences in neuroendocrine function are in part genetically determined and in part influenced by the environment, especially early in life. For example, variation in the exposure to hormones or to environmental stress prenatally or postnatally has long-term effects on neuroendocrine function (Maestripieri and Wallen 2003; Del Giudice et al. 2011; Maestripieri and Klimczuk 2013). Early life effects on the development of neuroendocrine function are relevant to our understanding of the evolution of sociality in primates and other organisms because such effects play a key role in the development of life history strategies, including sociosexual strategies (Maestripieri and Mateo 2009; Del Giudice et al. 2011).
Primate mothers can have a significant influence on the development on their offspring’s sociosexual strategies (e.g., through their influence on their offspring’s personality, which in turn regulates many aspects of sociosexuality; Groothuis and Maestripieri 2013) and this influence may be mediated by long-term alterations in their offspring’s neuroendocrine reactivity to stress. One mechanism for these effects is the transfer of maternal hormones and other physiological substances (e.g., immunoglobulins and other antibodies) through the placenta during gestation and then through milk during the breastfeeding period (Groothuis and Maestripieri 2013). The transfer of physiological substances from mother to offspring during pregnancy or lactation can be influenced by the mother’s own genetic make-up as well as by her interaction with the environment, including the mother’s level of stress. The offspring therefore can experience prenatal stress through her mother’s body. In addition, the mother’s own behavior can be stressful to the infant and have both short-term and long-term effects on her offspring’s reactivity to stress (see below). Psychosocial stress induced in the offspring by the mother’s behavior will be referred to as “maternal stress.”
There is a great deal of evidence that prenatal and maternal psychosocial stress can influence the development of the offspring’s HPA axis in primates (Maestripieri and Klimczuk 2013). Long-term alterations in HPA axis activity or reactivity induced by prenatal or maternal psychosocial stress could underlie adaptive adjustments to environment; these may include, for example, long-term changes in emotional reactivity or metabolism (Flinn et al. 2011; Berghänel et al. 2016, 2017), or they could reflect chronic stress-related pathologies (Maestripieri and Wallen 2003; Parker and Maestripieri 2011). If mother and infant are exposed to the same environmental stress, one could expect similarities in the HPA axis activity of mother and infant (but these can also arise as the result of genetic similarities). The few primate (including human) studies that have investigated possible correlations between maternal and infant cortisol levels did not produce significant results. For example, Murray et al. (2018) did not report significant correlations between maternal fecal glucocorticoid concentrations during pregnancy and postnatal infant glucocorticoid concentrations in chimpanzees (but sons of low-ranking mothers appeared to have a downregulated glucocorticoid response). Similarly, Maestripieri et al. (2009) found no significant correlation between plasma cortisol concentrations of free-ranging rhesus macaque mothers and their infants’ postnatal plasma cortisol concentrations. Therefore, to this date, there is no evidence of correlations between maternal and infant stress hormones in primates. There is evidence, however, of significant associations between maternal stress and stress-related physiological parameters in the offspring (see below).
Primate studies exploring the effects of maternal gestational stress on the offspring have been conducted in highly artificial laboratory conditions and will not be discussed here (see Groothuis and Maestripieri 2013; Maestripieri and Klimczuk 2013, for reviews). Studies of maternal stress (including maternal abusive behavior) conducted in more naturalistic conditions have focused on one main aspect of stress-inducing maternal behavior: maternal rejection (see Bardi and Huffman 2006, for baboons; Maestripieri 2008 for macaques). Mothers reject their infants by holding them at a distance with an arm or by forcefully removing them from the nipple and pushing them away. Mothers also reject their infants by hitting or biting them. Maternal rejection typically elicits distress vocalizations by the infants as well as temper tantrums (for a review of behavioral conflict between mothers and infants, see Maestripieri 2002). Frequently rejected infants also show behavioral signs of depression such as reduced locomotor activity, lack of interest in play and other social interactions, and lethargy (Maestripieri 2002). Maternal rejection is clearly a stressful experience for primate infants (see Mandalaywala et al. 2014a).
In rhesus monkeys, infants are generally rejected in the third or fourth week of life at the average rate of one episode every 2 h (Maestripieri 1998). In rhesus monkeys, the rate of rejection gradually increases as infants grow older, peaking at 6 months of age when mothers resume their mating activities (for information on rejection in other primate species, see Maestripieri 2002). However, some infants do not experience rejection at all, while others are rejected at the rate of three to four or more episodes per hour as early as in their first week of life (Maestripieri 1998).
Positive correlations between high rates of maternal rejection and infant stress have been reported in both rhesus macaques and in baboons, using different measures of HPA axis activity such as fecal glucocorticoid concentrations, salivary cortisol concentrations, plasma cortisol levels in response to maternal separation, and plasma cortisol levels in response to a corticotropin hormone (CRH) challenge (Bardi et al. 2005; McCormack et al. 2009; Sanchez et al. 2010; Koch et al. 2014; Mandalaywala et al. 2014a; Petrullo et al. 2016). The general picture that emerges from primate studies of maternal stress, along with studies of other animals and humans, is that exposure to maternal stress early in life is associated with a hyperreactive HPA axis in the immediate aftermath of stress, while the HPA axis of chronically stressed individuals may become hypoactive in the long run (Parker and Maestripieri 2011; Petrullo et al. 2016).
In rhesus macaques, stressful maternal rejection is also associated with lower brain noradrenergic and serotonergic function in the infants (Maestripieri et al. 2006a, b, 2009; Sanchez et al. 2007). Specifically, Maestripieri et al. (2006a) reported that infants (both non-fostered and cross-fostered infants) reared by highly rejecting mothers exhibited lower cerebrospinal fluid (CSF) levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) and of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG) in the first 3 years of life when compared to infants reared by less rejecting mothers. In male rhesus macaques and vervet monkeys, low levels of CSF 5-HIAA and MHPG have been associated with high impulsivity, risk-taking behavior, and propensity to engage in severe forms of aggression (see Higley 2003, for a review). Furthermore, young vervet monkey males with low levels of CSF 5-HIAA showed greater propensities to become high ranking (Fairbanks et al. 2004). Young males with low CSF 5-HIAA also appear to be more likely to emigrate from their natal group at a young age (Kaplan et al. 1995; Mehlman et al. 1995; but see Howell et al. 2007, for opposite results). However, low CSF 5-HIAA seems to be correlated with reduced survival and lower mating success, at least in male rhesus macaques (Mehlman et al. 1997; Howell et al. 2007). Howell et al. (2007) argued that this trait has mostly deleterious consequences for male fitness but may be associated with phenotypic traits that are advantageous to females such as high levels of sociability and sexual receptivity.
Maternal rejection plays an important role in the process of offspring weaning, as high rates of rejection reduce the amount and frequency with which infants seek to be in contact and gain access to their mothers’ nipples for suckling, thereby encouraging their nutritional and social independence (Fairbanks 1996). Although it may be argued that the psychosocial stress generated in the offspring by maternal rejection is just an inevitable by-product of weaning, it may also be argued that this stress is not a by-product but a phenomenon that needs an explanation. And this explanation may be that when mothers reject their infants, they simultaneously accomplish different goals: they encourage their infants to be nutritionally and socially independent (so that mothers can invest in future offspring) and they also give them the opportunity to develop the appropriate tools to deal with psychosocial stress later in life (Parker et al. 2006; Parker and Maestripieri 2011).
It is possible that by exposing their fetuses, infants, and children to some psychosocial stress, mothers help them develop physiological, behavioral, and emotional/cognitive adaptations that allow them to cope with stress in an optimal way throughout their life (Parker et al. 2006; Del Giudice et al. 2011; Parker and Maestripieri 2011). This is similar to the process by which exposure to moderate amounts of pathogens early in life strengthens the immune system and inoculates the body against future exposure to the same or similar pathogens.
Influence of maternal style on the offspring’s behavior
Maternal behavior can influence not only the offspring’s physiology but also their behavior and through mechanisms independent from stress. Naturally occurring interindividual variation in maternal style can influence the development of a wide range of behaviors, such as exploration of the environment, reactivity to aversive stimuli, affiliative and agonistic behaviors, and also later sexual and parental behavior (Fairbanks 1996; Maestripieri 2009). Studies of intraspecific variation in maternal styles in cercopithecine monkeys have shown that most variability occurs along the two orthogonal dimensions of maternal protectiveness and rejection (Fairbanks 1996; Maestripieri 2001a; see De Lathouwers and van Elsacker 2004, for data on great apes). Although maternal protectiveness and rejection change in frequency as a function of infant age and the mother’s own age and experience, individual differences in maternal style are usually stable (Hinde and Spencer-Booth 1971; Fairbanks 1996).
There is evidence that young monkeys exposed to variable maternal style in infancy differ in their propensity to be independent from their mother and explore the environment later in life. In an early study of rhesus monkeys, infants reared by highly rejecting mothers were less likely to explore the environment at the end of the first year (Simpson 1985). In later studies of rhesus and other species of macaques, however, more rejecting (or more laissez-faire mothers) produced infants that were more independent and more likely to explore the environment on their own and play (Simpson and Simpson 1985; Simpson et al. 1989; Simpson and Datta 1990; Bardi and Huffman 2006). In vervet monkeys, less rejecting mothers produced more timid and less independent infants who are less likely to approach potentially dangerous individuals (Fairbanks and McGuire 1988, 1993). Finally, there is evidence that rhesus infants reared by more protective and higher ranking mothers look longer at threatening faces as opposed to faces with neutral facial expressions (indicating higher vigilance for threat; Mandalaywala et al. 2014b).
Since most studies of maternal style and infant development in primates are correlational, the possibility that associations between maternal and infant behaviors reflect inherited temperamental similarities between mothers and offspring cannot be ruled out. Evidence of cause-effect relationships, however, has been provided by experimental studies in which maternal style was altered through manipulations of the physical or the social environment (Fairbanks and McGuire 1987; Andrews and Rosenblum 1991; Vochteloo et al. 1993) or in which infants were cross-fostered between different mothers (Maestripieri 2005; Maestripieri et al. 2007). Genetic correlations between maternal and infant behaviors regulating the maintenance of contact, however, are likely to exist and be the result of co-adaptation or evolutionary mother-offspring conflict (Maestripieri 2004). For example, mothers who are genetically predisposed to break contact frequently are likely to have infants who are genetically predisposed to make contact frequently (Maestripieri 2004).
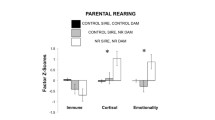
The effects of maternal style on offspring behavior can also be observed later in life. For example, there is evidence that maternal protectiveness has long-term effects on the tendency to enter a new enclosure and approach novel objects in juvenile vervet monkeys (Fairbanks and McGuire 1988, 1993, 1995), while greater maternal permissiveness early in life has been associated with greater tendency to approach and challenge an adult male in adolescent vervet monkeys (Fairbanks 1996). Long-term effects of maternal style have also been reported in Japanese macaques and in baboons. Macaque infants reared by more rejecting mothers were less likely to respond with submissive signals or with avoidance to an approach from another individual later in life (Schino et al. 2001); juvenile baboons whose mothers displayed a protective maternal style were characterized by greater vigilance when housed in a small cage, whereas juveniles of mothers that displayed high levels of stress-related behaviors had higher cortisol level and displayed greater locomotor activity in a small cage (Bardi et al. 2005). Finally, rhesus infants reared by more rejecting mothers spent more time away from their mother and engaged more in solitary play in the second year (Maestripieri et al. 2006b; this study involved infants cross-fostered at birth; therefore, the possibility that the effect of maternal style represented genetically inherited behavioral similarities between mothers and offspring could be excluded).
The best illustration of the effects of maternal style on the offspring’s adult behavior concerns the intergenerational transmission of maternal style from mothers to daughters. Multi-generational observational studies of maternal behavior in vervet monkeys and rhesus macaques reported similarities in behavior (especially with regard to rates of infant rejection) between mothers and daughters and suggested that these similarities may be the result of the daughters’ early experience (Fairbanks 1989; Berman 1990). This was confirmed by a study in which rhesus macaque females were cross-fostered at birth and reared by unrelated foster mothers (Maestripieri et al. 2007). This study reported cross-generational consistencies in the rejection dimension but not in the protectiveness dimension of maternal style. Significant similarities in maternal rejection between mothers and daughters were found for both non-fostered and cross-fostered rhesus females, suggesting that the daughters’ behavior was affected by exposure to their mothers’ rejection in their first 6 months of life. Since maternal styles may represent adaptations to particular maternal characteristics (e.g., dominance rank, body condition, or age) or demographic and ecological circumstances (e.g., availability of food or social support from relatives), the intergenerational transmission of maternal styles in primates may represent an example of non-genomic transmission of behavioral adaptations from mothers to daughters.
Maternal influences on the development of offspring’s social preferences
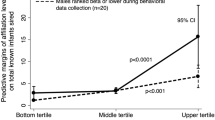
Social preferences for genetically related individuals, and especially for relatives of the same sex, play an important role in the origin and maintenance of primate philopatry and may significantly affect individual social strategies as well. Studies of the development of these preferences in rhesus macaques have shown that the social relationships infants develop with other individuals tend to mirror those of their mothers (see Roney and Maestripieri 2003; Berman 2004 for reviews). For example, rhesus mothers associate preferentially with close female relatives and have antagonistic relationships with unrelated females from other matrilines (even though rates of aggression between close maternal kin are high due to high proximity between them; see Widdig et al. 2002; Roney and Maestripieri 2003). Similarly, infants spend more time and affiliate with members of their own matriline and tend to ignore or avoid unrelated individuals (e.g., Kulik et al. 2015a, b). Furthermore, in macaque species in which the relationships between adult females and close female relatives are strong, so are the relationships between infants and these individuals, whereas in species in which the relationships between adult females and close female relatives are weak, infants show only a moderate or weak preference for these individuals (Berman 2004; see also Duboscq et al. 2017). Although interspecific differences in the strength of kin bias could be genetically based, studies have suggested that the development of kin-biased social behavior is influenced by the maternal environment (see below).
In rhesus macaques, young infants spend most of their time in contact or close proximity with their mothers. Since their mothers associate and affiliate with their close kin, infants begin interacting mostly with these individuals simply because these individuals happen to be nearby and are available for interaction. Although young infants initially do not seem to have an active preference for any kin other than their mothers (sex differences in infant behavior emerge gradually; Kulik et al. 2015a, b; see below for a discussion of sex differences), their social interactions are kin-biased by virtue of the fact that their immediate social environment is also kin-biased. Not only do mothers provide their offspring with opportunities to interact with particular individuals, they also serve as models for these interactions. In other words, by observing their mothers being amicable towards close relatives and avoidant or aggressive towards non-relatives, infants learn how to behave towards relatives and non-relatives accordingly. Thus, an important component of the formation of social relationships in young monkey infants involves being passively exposed to the mother’s environment and the mother’s behavior. There is little evidence to date that mothers actively encourage their infants to interact with particular individuals and form relationships with them, despite some suggestions to the contrary (de Waal 1990). However, mothers discourage interactions between their infants and certain other individuals (Maestripieri 1995c; Maestripieri et al. 2002). Furthermore, differences in maternal interactions with sons and daughters may influence the development of sons and daughters’ social relationships with other individuals (Kulik et al. 2015a, b, 2016; see below).
Through the joint action of multiple processes driven by mothers such as providing opportunities, modeling, and active shaping of behavior, monkey infants develop active social preferences for their close kin and seek out these individuals and initiate interactions with them. Since kin and non-kin, especially adults, respond differently to the infant’s behavior, learning through reinforcement and punishment in the course of social interactions with these individuals can contribute to the formation of infant social preferences. In rhesus and Japanese macaques, the development of active kin bias occurs some time in the first year of life and, for females, will continue to strengthen for the rest of their lives (Nakamichi 1989; de Waal 1996; Berman 2004). The social developmental trajectory of males, instead, will begin to diverge from that of females after 1–2 years (Hinde and Spencer-Booth 1967; Nakamichi 1989; Kulik et al. 2016). After this age, males will receive increasing amounts of aggression from their older female relatives and will become increasingly attracted to peers and adult males. Eventually, these processes will culminate in the peripheralization of the juvenile and subadult males in the group and their emigration (Roney and Maestripieri 2003; Kulik et al. 2015a, b, 2016).
It is important to emphasize that maternal influences on offspring social development probably act in conjunction with genetically inherited predispositions and that the relative contribution of maternal non-genetic versus genetic influences varies in relation to the type of relationships the offspring are forming. For example, whereas maternal influences on the development of offspring kin bias appear to be strong, at least in some primate species, the development of female attraction to infants, the development of play partner preferences, and the development of sex partner preferences seem to be relatively independent of maternal influences. Instead, they seem to be more dependent on a combination of inherited propensities and the species-typical social environment (i.e., young individuals must develop in social groups with species-typical size and demographic composition to be able to fully express these propensities; e.g., Maestripieri and Ross 2004). The relative importance of maternal influences versus inherited propensities for the development of social preferences also seems to vary greatly among primate species. For example, whereas maternal influences on the development of matrilineal kin bias appear to be strong in nepotistic primate species with female philopatry and male dispersal, different types of kin bias and of maternal influences occur in species in which males are philopatric and females disperse (e.g., chimpanzees: see Murray et al. 2014) or species in which both sexes disperse (e.g., gorillas; Berman 2004). Unfortunately, little is known about maternal influences on social development in these species. Little is also known about whether and how within-species variation in the maternal environment and in maternal behavior affects the offspring social and mating strategies in adulthood (but see Schino et al. 2004) and whether and how this behavioral variation translates into variation in survival or reproductive success.
Maternal influences on the development of sex differences in behavior
Developmental sex differences in social behavior are the result of genetic differences as well as of differences in maternal investment in sons and daughters (either as the result of direct influence of maternal investment on offspring behavior, or as a by-product of the effect of maternal investment on offspring growth and onset of reproduction; Hinde 2009; Maestripieri 2009). Such differences in maternal investment are expected to occur whenever either the costs or the benefits of raising sons or daughters are significantly different to the mothers (Maestripieri 2009). The costs and benefits of raising sons and daughters, in turn, may vary in relation to maternal characteristics such as body condition or dominance rank (e.g., Trivers and Willard 1973).
Many primate studies investigating differential maternal investment in sons and daughters have used birth sex ratios as their dependent variable (e.g., Hiraiwa-Hasegawa 1993); therefore, the results of these studies are not relevant to the issues of maternal influences on offspring behavior. Studies that have looked at reproductive intervals following the births of sons of daughters provide important information, as longer interbirth intervals indicate greater maternal postnatal investment. In a study with one of the largest sample sizes to date (N > 3000 births), Maestripieri (2001b) reported that rhesus macaques mothers had significantly longer interbirth intervals following the births of daughters than following the births of sons, indicating greater maternal investment in daughters (see Hoffman et al. 2010; Hoffman and Maestripieri 2011, for further data on interbirth intervals in a different population of rhesus macaques). In rhesus macaques, greater maternal investment in daughters may result from greater costs of raising daughters (as young daughters receive more aggression from unrelated adult females than sons do), or greater benefits (as adult daughters are able to cooperate more with their mothers than sons do), or both (Maestripieri 2007).
Longer reproductive intervals following the births of daughters should be associated with daughter-biased maternal behavioral investment but there is little evidence of significant differences in maternal behavior towards sons and daughters at any age (see Roney and Maestripieri 2003; Lonsdorf 2017 for reviews; for sex-biased milk-related investment, see Hinde 2009). However, the results of the few studies that do report sex-biased maternal behavior tend to be in the same direction. In cercopithecine monkeys, there is some evidence that mothers spend more time in contact and proximity to their daughters, physically restrain their daughters more, groom their daughters more, support their daughters in fights more, and reject their daughters less when compared to sons (see Kulik et al. 2016; Lonsdorf 2017). This evidence suggests that in female philopatric species mothers form stronger social bonds with their daughters than with their sons (Kulik et al. 2016). Since in these species, males typically disperse from their natal group at puberty, their weaker bonds with their mothers (which also include receiving aggression from the mother) in infancy may be a proximate factor contributing to their emigration from the group (Kulik et al. 2016). In chimpanzees, a male philopatric species, Murray et al. (2014) found that mothers of sons spent more time in large groups and in groups containing adult males, especially in the first 6 months of infant life, than mothers of daughters. Later in life, sons seemed to socialize with more individuals overall and with more adult males than daughters (see also Lonsdorf et al. 2014a, b). Lonsdorf (2017) therefore has raised the possibility that chimpanzee mothers actively socialize their infants according to their future sex-specific social roles (see also Maestripieri and Ross 2004, for gorillas).
Developmental sex differences in grooming behavior in cercopithecine monkeys may be the result of differential maternal grooming given to daughters and sons. In macaques, daughters groom their mothers and other females earlier in life and more frequently than sons do, perhaps to reciprocate the high rates of grooming young females receive from their mothers and other adult females (see Roney and Maestripieri 2003). In addition to sex differences in grooming, there are also sex differences in rough and tumble play (with males doing more than females), in infant-related interactions (with females doing more than males), and possibly in aggression around puberty (with males performing and receiving more aggression than females) (e.g., Symons 1978; Nakamichi 1989; Maestripieri 2005). Roney and Maestripieri (2003), however, have argued that sex differences in social play and play parenting are unlikely to be the product of maternal socialization given that mothers do not interact differently with sons and daughters in these domains.
Early sex differences in social behavior can be interpreted in light of the different life histories and reproductive strategies of males and females. For example, the intense female-oriented grooming behavior exhibited by young females clearly foreshadows their integration and acceptance into the female core of the social group whereas the grooming patterns of young males can be viewed as a prelude to emigration from the natal group (e.g., Kulik et al. 2015a, b, 2016). Early differences in play behavior are also likely adaptations to the different life histories of males and females, with a primary emphasis on the development of fighting skills for males and parenting skills for females (Maestripieri and Ross 2004). Some of these sex differences in early social behavior can be viewed as species-typical adaptations whereas others can be regarded as a more general mammalian or primate pattern. For example, sex differences in grooming behavior are expected to vary as a function of the patterns of philopatry and dispersal of the species. Sex differences in play fighting and play parenting, however, are more likely to reflect patterns of mating competition and parental investment that are shared by most mammalian species. Although mothers are likely to bias their investment towards sons or daughters depending on variations in the cost/benefits ratios of raising sons and daughters, evidence that such sex-biased investment contributes to sex-typical social development is still limited.
Influence of maternal agonistic support on the offspring’s acquisition of dominance rank
In cercopithecine monkeys and other primates in which females are philopatric and establish clear dominance hierarchies within their group (e.g., most baboons and macaques), sons and daughters acquire their mothers’ dominance rank early in life through the agonistic support they receive from mothers and through observations of their mother’s behavior towards other individuals (see Holekamp and Smale 1991; Chapais 1992; Pereira 1995 for reviews). In chimpanzees, in which males are philopatric, maternal rank influences the offspring’s acquisition of rank but direct maternal interventions in offspring’s conflicts are rare (Markham et al. 2015). In rhesus macaques, immature offspring are aided by their mothers during conflicts with other individuals (Maestripieri 2007). When mothers are higher ranking than the offspring’s opponents and the opponents’ mothers, the mother’s intervention results in a positive outcome of the conflict. Therefore, offspring learn that they can consistently win fights with certain opponents, and these opponents begin to show submissive behavior to the offspring the way they do it to their mothers. In contrast, when mothers are lower ranking than their offspring’s opponents and the opponents’ mothers, both offspring and their mothers are defeated. Therefore, offspring learn that they can be consistently defeated by certain opponents and, as a result, begin to show submissive behavior to them. This learning process is reinforced by the offspring’s observations of interactions between their mothers and other individuals. Offspring observe that their mothers consistently attack and defeat some individuals but are consistently attacked and defeated by others. Through a combination of observational learning and shaping through punishment and reward, offspring eventually match their mothers’ behavior towards other individuals. As a result, offspring acquire a dominance rank adjacent to that of their mothers and in particular immediately below that of their mothers because offspring remain subordinate to their mothers (rhesus macaques: Maestripieri 2007; baboons: Cheney 1977; vervet monkeys: Horrocks and Hunte 1983). The non-genetic nature of rank transmission in cercopithecine monkeys has been confirmed by observations that immatures that are adopted at birth by unrelated females acquire the dominance rank of their adoptive mothers and not that of their biological mothers (Chapais 1992). Furthermore, the social mechanisms of rank acquisition and transmission have been elucidated with experimental manipulations of group composition and alteration of conflict outcomes (Chapais 1992). In cercopithecine monkeys with female philopatry, female ranks can be stable throughout a female’s lifetime. As long as males remain in their natal group, they acquire dominance ranks within their matrilines, but when they join a new group, their new rank is established through seniority or through fighting and is generally unrelated to their rank in their group of origin (e.g., van Noordwijk and van Schaik 1985, 1988; Higham and Maestripieri 2010; Georgiev et al. 2016). Therefore, while rank-related maternal influences on the fitness of the philopatric offspring may last a lifetime, such influences generally end at puberty for the dispersing offspring.
The direct effects of maternal dominance rank on offspring fitness are expected to be stronger in some primate species and environments than in others (Majolo et al. 2012). Socioecological theory predicts that rank-related maternal effects on offspring fitness should be strongest in female-bonded primate species such as rhesus macaques and savannah baboons in which female social relationships are classified as despotic and nepotistic (i.e., dominance relationships are strongly unidirectional and asymmetrical and strongly affected by kinship; van Schaik 1989). Despotic and nepotistic female social relationships, in turn, should be associated with ecological conditions in which resources are highly clumped leading to high within-group contest competition (van Schaik 1989; Sterck et al. 1997). Such within-group contest competition may be exacerbated in environments with high predation risk resulting in large foraging groups (Sterck et al. 1997). The direct effects of maternal dominance rank on offspring fitness are also expected to be stronger in wild than in captive or free-ranging but food-provisioned primates (Majolo et al. 2012; but see Blomquist et al. 2011, for evidence for the food-provisioned rhesus macaques on Cayo Santiago). This is because one of the most important functions of female rank is to regulate priority of access to food resources when such resources are limited.
Maternal influences on offspring social learning
Dealing effectively with the complexity of primate societies presumably requires significant social learning in early life. A full understanding of the development of primate sociality, therefore, requires knowledge of the occurrence and characteristics of early social learning. Primate mothers could contribute to their offspring’s acquisition of knowledge or skills pertinent to their physical or social environment if they behaved in ways that provided opportunities for offspring social learning. For example, mothers might bring their infants in the proximity of unfamiliar individuals or expose them to unfamiliar social circumstances so that their infants could learn about these individuals or circumstances through observation or direct interaction. Similarly, mothers in chimpanzee populations that are known to use tools for fishing termites or cracking nuts might leave appropriate tools in the vicinity of their infants so that infants have opportunities to play with them and learn their use in a foraging context (see below). These maternal activities would be functionally related to offspring social learning and be best classified as “social facilitation,” “local enhancement,” or “scaffolding” (Maestripieri 1995a). A separate question, at least from a cognitive point of view, is whether primate mothers facilitate their offspring’s social learning not only functionally but also intentionally. This would require that mothers engage in behavior that has no goal other than promote offspring social learning and that they have some understanding of the consequences of their behavior for the offspring’s skills or even knowledge (the latter would require that mothers attribute mental states such as knowledge and ignorance to their infants). In other words, the question would be whether primate mothers are capable of intentional teaching (Caro and Hauser 1992; Maestripieri 1995a; Hoppitt et al. 2008; Thornton and Raihani 2008).
The evidence from both observational and experimental primate studies is stronger for “functional” (and more for great apes than for monkeys) than for “intentional” teaching. Overall, however, and contrary to expectations, maternal involvement in offspring social learning is very rare and highly domain-specific. Mother-infant interactions suggestive of teaching have been observed in several domains such as tool-use, alarm calls, food choice and processing, gestural communication, and infant independent locomotion (reviewed by Maestripieri 1995a). For example, rhesus and pigtail macaque mothers exhibit highly flexible and sophisticated behavior when they encourage their young infants to walk independently, which includes making eye-to-eye contact and displaying the bared-teeth display, lip-smacking, or the pucker face to their infants while walking backwards, and repeating (Maestripieri 1995b, 1996a, b). Older and more experienced mothers encourage their infants more than younger and inexperienced mothers, and infants who are encouraged more walk on their own earlier in life than those who are not encouraged (Maestripieri 1995b, 1996a). Macaque mothers typically use facial expressions such as bared-teeth display, lip-smacking, or the pucker face to regulate proximity to their infants and to encourage their infants to come closer to them, follow, or make contact (Maestripieri and Wallen 1997; Maestripieri 1996b; for other primate species including great apes, see Cartmill and Maestripieri 2012). Ferrari and collaborators have shown that rhesus infants whose mothers lipsmack to them more often early in life exhibit not only earlier locomotor independence but also greater sociability and social competence later in life, suggesting that early maternal behavior can influence their infants’ social and cognitive development (Ferrari et al. 2009; Dettmer et al. 2016). Maternal encouragement of infant independent locomotion has also been reported in chimpanzees (van de Rijt-Plooy and Plooy 1987) and in gorillas (Whiten 1999; Maestripieri et al. 2002), but the developmental consequences of these early mother-infant interactions have not been investigated in these species.
Social learning is known to play an important role in complex foraging techniques, which involve the use of tools, or coordination of behavior between two or more individuals, or food sharing. The importance of social learning for tool using has been best documented in capuchin monkeys, chimpanzees, and orangutans (see Lonsdorf and Ross 2012 for a review). The question of whether mothers in these species contribute in some way to their offspring’s learning of skills in foraging or feeding contexts has been addressed by several studies. A study of captive lowland gorillas reported that food sharing between mother and infant was rare and generally initiated by infants, while mothers simply showed passive tolerance (Maestripieri et al. 2002). In this study, gorilla mothers did not encourage their infants in any way but discouraged their infants from interacting with particular individuals, especially the male silverback. In contrast, the infants showed interest in their mothers’ activities, encouraged their mothers to share food, play, or follow them, and also repeated their behavior. Thus, gorilla infants took the initiative in creating situations that were potentially conducive to the acquisition of knowledge or skills.
In chimpanzees, social learning plays an important role in the ontogeny of tool-use skills such as nut-cracking and termite-fishing. In a population of chimpanzees in which nut-cracking is common, Boesch (1991) suggested that chimpanzee mothers sometimes leave in the vicinity of their infants tools for nut-cracking such as rocks or sticks that can be used as the “anvil” or the “hammer,” thereby engaging in social facilitation (local enhancement) of their skill acquisition. Boesch (1991) also reported a few anecdotal observations of mothers who appeared to be tutoring their infants by positioning the nut correctly on the anvil or demonstrating how to hit the nut most effectively with the hammer.
Potential maternal influences on the offspring’s acquisition of skills in the context of termite-fishing have been investigated both in wild and captive chimpanzees. In the Gombe population of chimpanzees, Lonsdorf and collaborators have reported significant sex differences in the development of termite-fishing skills (Lonsdorf et al. 2004). Female infants observed their mothers’ termite-fishing behavior more often than male infants and subsequently showed shorter acquisition times and greater proficiency at this task. Female infants were also better at matching their mothers’ precise technique (Lonsdorf 2005; Lonsdorf and Ross 2012). There were, however, no significant differences in the way in which mothers behaved towards male or female offspring during termite-fishing activities (Lonsdorf 2006, 2017). Lonsdorf (2006) did not observe any instances of active instruction on the part of the mother (or any other individuals). However, since individual mothers differed significantly from one another in the frequency with which they engaged in termite-fishing near their infants, as well as in their proficiency, it is likely that significant variation exists in the extent to which mothers provide opportunities for social learning to their infants (Lonsdorf 2006). Another field study of chimpanzees has shown that mothers provide their infants with sticks that can serve as appropriate tools for termite-fishing (Musgrave et al. 2016); the authors of this study have argued that such tool transfer meets the functional criteria for teaching proposed by Caro and Hauser (1992), although no active tutoring was observed. The conclusions of these field studies are generally consistent with those of a captive study, in which chimpanzee infants learned tool-use by observing their mothers and other adults but without any active tutoring by their mothers (Hirata and Celli 2003).
Conclusions and future directions
Given the prominence of maternal care for offspring development and the close associations that primate mothers maintain with their offspring, maternal influences on social development are ubiquitous and highly significant across primate species. Maternal influences on offspring development are a crucial component of the development of primate sociality and of its maintenance across generations. In this article, I focused on maternal neuroendocrine and behavioral influences on offspring’s physiology and social behavior and did not address research on maternal genetic or nutritional effects on their offspring’s growth, health, or reproduction (see Maestripieri 2009, for a review of some of this research). Mothers can have long-term effects on stress-sensitive physiological systems in the offspring, such as the HPA axis. With the exception of extreme circumstances, these effects are likely adaptive and prepare the offspring to deal with future environmental challenges (Maestripieri and Mateo 2009). Long-term effects of early maternal stress, such as that induced by rejection, on offspring neuroendocrine reactivity to the environment can also be the source of adaptive variation in sociosexual and reproductive strategies when offspring reach adulthood (Del Giudice et al. 2011). Through their own variable maternal style, mothers can also shape many aspects of their offspring’s behavior, including exploration, affiliation, and aggression, and also future parental behavior. Transgenerational effects of maternal style can be a mechanism that promotes or maintains behavioral adaptations to particular social characteristics or micro-environments (Maestripieri et al. 2007).
A crucial component of primate social systems is kinship- and rank-related social preferences; such preferences are often different in males and females. Although mothers do influence the sex-typical social preferences of their offspring and other sex-typical behavior such as grooming and emigration from the group, they do so mainly indirectly by providing opportunities to engage in particular activities more than in others and thus reinforcing biological predispositions for sexually differentiated behavior that are the result of genetic factors or prenatal hormonal exposure. Maternal influences on their offspring’s social learning too are with some exceptions mainly indirect and consist of providing opportunities for learning, rather than intentionally tutoring or teaching offspring directly. One domain in which primate mothers in some species play a direct and significant influence on their offspring’s social development is the transmission of dominance rank across generations. Since rank is a crucial determinant of survival and reproductive success in primate species with despotic hierarchies, maternal transmission of rank through agonistic aid to their offspring makes a crucial contribution to the offspring’s fitness (Blomquist et al. 2011) (and at the same time to the maintenance of the special-typical social system).
Overall, primate mothers have the potential to influence both “normative” (i.e., species-typical) aspects of social development and interindividual variation in behavior, physiology, and reproduction (i.e., sociosexual and reproductive strategies). Much of the research describing these maternal effects has been purely descriptive; therefore, more research is needed both on the functional significance of these effects for fitness and on the proximate mechanisms underlying these effects. The extent to which the offspring themselves contribute to driving their mothers’ behavior (e.g., by “extracting” maternal investment or soliciting affiliative and agonistic support) also needs to be further investigated. Finally, the perspective taken in this article emphasizes that the evolutionary interests of mothers and offspring are often aligned and overlapping, but this is not necessarily always the case. Thus, future studies of primate maternal influences on offspring social development should explicitly consider evolutionary conflicts of interests between mothers and infants, and the consequent possibility that the long-term outcomes of mother-infant interactions may be more advantageous to one party and less to the other, depending on how the conflict is resolved.
References
Andrews MW, Rosenblum LA (1991) Attachment in monkey infants raised in variable and low-demand environments. Child Dev 62:686–693
Bardi M, Bode AE, Ramirez SM (2005) Maternal care and development of stress responses in baboons. Am J Primatol 66:263–278
Bardi M, Huffman MA (2006) Maternal behavior and maternal stress are associated with infant behavioral development. Dev Psychobiol 48:1–9
Berghänel A, Heistermann M, Schülke O, Ostner J (2016) Prenatal stress effects in a wild, long-lived primate: predictive adaptive responses in an unpredictable environment. Proc R Soc B 283:20161304
Berghänel A, Heistermann M, Schülke O, Ostner J (2017) Prenatal stress accelerates offspring growth to compensate for reduced maternal investment across mammals. Proc Natl Acad USA 114:E10658–E10666
Berman CM (1990) Intergenerational transmission of maternal rejection rates among free-ranging rhesus monkeys. Anim Behav 39:329–337
Berman CM (2004) Developmental aspects of kin bias in behavior. In: Chapais B, Berman CM (eds) Kinship and behavior in primates. Oxford University Press, Oxford, pp 317–346
Blomquist GE, Sade DS, Berard JD (2011) Rank-related fitness differences and their demographic pathways in semi-free-ranging rhesus macaques (Macaca mulatta). Int J Primatol 32:193–208
Boesch C (1991) Teaching among wild chimpanzees. Anim Behav 41:530–532
Bowlby J (1969) Attachment and loss. Attachment, vol I. Basic Books, New York
Caro TM, Hauser MD (1992) Is there teaching in nonhuman animals? Q Rev Biol 67:151–174
Cartmill E, Maestripieri D (2012) Socio-cognitive specializations of nonhuman primates: evidence from gestural communication. In: Vonk J, Shackelford T (eds) The Oxford handbook of comparative evolutionary psychology. Oxford University Press, Oxford, pp 166–193
Chapais B (1992) The role of alliances in social inheritance of rank among female primates. In: Harcourt A, de Waal FBM (eds) Coalitions and alliances in humans and other animals. Oxford University Press, New York, pp 29–59
Cheney DL (1977) The acquisition of rank and the development of reciprocal alliances among free-ranging immature baboons. Behav Ecol Sociobiol 2:303–318
Clutton-Brock TH (1991) The evolution of parental care. Princeton University Press, Princeton
De Lathouwers M, van Elsacker L (2004) Comparing maternal styles in bonobos (Pan paniscus) and chimpanzees (Pan troglodytes). Am J Primatol 64:411–423
de Waal FBM (1990) Do rhesus mothers suggest friends to their offspring? Primates 31:597–600
de Waal FBM (1996) Macaque social culture: development and perpetuation of affiliative networks. J Comp Psychol 110:147–154
Del Giudice M, Ellis BJ, Shirtcliff EA (2011) The adaptive calibration model of stress responsivity. Neurosci Biobehav Rev 35:1562–1592
Dettmer AM, Kaburu SSK, Simpson EA et al (2016) Neonatal face-to-face interactions promote later social behavior in infant rhesus monkeys. Nat Commun 7:11940
Duboscq J, Neumann C, Agil M, Perwitasari-Farajallah D, Thierry B, Engelhardt A (2017) Degrees of freedom in social bonds of crested macaque females. Anim Behav 123:411–426
Fairbanks LA (1989) Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Dev Psychobiol 22:669–681
Fairbanks LA (1996) Individual differences in maternal styles: causes and consequences for mothers and offspring. Adv Study Behav 25:579–611
Fairbanks LA, Jorgensen MJ, Huff A, Blau K, Hung Y, Mann JJ (2004) Adolescent impulsivity predicts adult dominance attainment in male vervet monkeys. Am J Primatol 64:1–17
Fairbanks LA, McGuire MT (1987) Mother-infant relationships in vervet monkeys: response to new adult males. Int J Primatol 8:351–366
Fairbanks LA, McGuire MT (1988) Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Dev Psychobiol 21:711–724
Fairbanks LA, McGuire MT (1993) Maternal protectiveness and response to the unfamiliar in vervet monkeys. Am J Primatol 30:119–129
Fairbanks LA, McGuire MT (1995) Maternal condition and the quality of maternal care in vervet monkeys. Behaviour 132:733–754
Ferrari PF, Paukner A, Ionica C, Suomi SJ (2009) Reciprocal face-to-face communication between rhesus macaque mothers and their newborn infants. Curr Biol 19:1768–1772
Flinn MV, Nepomnaschy PA, Muehlenbein MP, Ponzi D (2011) Evolutionary functions of early social modulation of hypothalamic-pituitary-adrenal axis development in humans. Neurosci Biobehav Rev 35:1611–1629
Georgiev AV, Christie D, Rosenfield KA, Ruiz-Lambides A, Maldonado E, Emery Thompson M, Maestripieri D (2016) Breaking the succession rule: the costs and benefits of an alpha-status take-over by an immigrant rhesus macaque on Cayo Santiago. Behaviour 153:325–351
Groothuis TTG, Maestripieri D (2013) Parental influences on offspring personality traits in oviparous and placental vertebrates. In: Carere C, Maestripieri D (eds) Animal personalities: behavior, physiology, and evolution. The University of Chicago Press, Chicago, pp 317–352
Higham JP, Maestripieri D (2010) Revolutionary coalitions in male rhesus macaques. Behaviour 147:1889–1908
Higley JD (2003) Aggression. In: Maestripieri D (ed) Primate psychology. Harvard University Press, Cambridge, pp 17–40
Hinde K (2009) Richer milk for sons but more milk for daughters: sex-biased investment during lactation varies with maternal life history in rhesus macaques. Am J Hum Biol 21:512–519
Hinde RA, Spencer-Booth Y (1971) Towards understanding individual differences in rhesus mother-infant interaction. Anim Behav 19:165–173
Hinde RA, Spencer-Booth Y (1967) The behaviour of socially living rhesus monkeys in their first two and a half years. Anim Behav 15:169–196
Hirata S, Celli ML (2003) Role of mothers in the acquisition of tool-use behaviors by captive infant chimpanzees. Anim Cogn 6:235–244
Hiraiwa-Hasegawa M (1993) Skewed birth sex ratios in primates: should high ranking mothers have daughters or sons? Trends Ecol Evol 8:395–400
Hoffman CL, Higham JP, Mas-Rivera A, Ayala JE, Maestripieri D (2010) Terminal investment and senescence in rhesus macaques (Macaca mulatta) on Cayo Santiago. Behav Ecol 21:972–978
Hoffman CL, Maestripieri D (2011) Costs of reproduction among rhesus macaque females on Cayo Santiago. In: Wang Q (ed) Bones, genetics, and behavior of rhesus macaques. Springer, Berlin, pp 209–226
Holekamp KE, Smale L (1991) Dominance acquisition during mammalian social development: the "inheritance" of maternal rank. Am Zool 31:306–317
Hoppitt WJE, Brown GR, Kendal R, Rendell L, Thornton A, Webster MM, Laland KN (2008) Lessons from animal teaching. Trends Ecol Evol 23:486–493
Horrocks J, Hunte W (1983) Maternal rank and offspring rank in vervet monkeys: an appraisal of the mechanisms of rank acquisition. Anim Behav 31:772–782
Howell S, Westergaard G, Hoos B, Chavanne TJ, Shoaf SE, Cleveland A, Snoy PJ, Suomi SJ, Higley JD (2007) Serotonergic influences on life-history outcomes in free-ranging male rhesus macaques. Am J Primatol 69:851–865
Kaplan JR, Fontenot MB, Berard J, Manuck SB, Mann JJ (1995) Delayed dispersal and elevated monoamine activity in free-ranging rhesus monkeys. Am J Primatol 35:229–234
Koch H, McCormack KM, Sanchez MM, Maestripieri D (2014) The development of the hypothalamic-pituitary-adrenal axis function in rhesus monkeys: effects of age, sex, and early experience. Dev Psychobiol 56:86–95
Kulik L, Amici F, Langos D, Widdig A (2015a) Sex differences in the development of social relationships in rhesus macaques (Macaca mulatta). Int J Primatol 36:353–376
Kulik L, Amici F, Langos D, Widdig A (2015b) Sex differences in the development of aggressive behavior in rhesus macaques (Macaca mulatta). Int J Primatol 36:764–789
Kulik L, Langos D, Widdig A (2016) Mothers make a difference: mothers develop weaker bonds with immature sons than daughters. PLoS One 11:e0154845
Lonsdorf EV (2005) Sex differences in the development of termite-fishing skills in wild chimpanzees (Pan troglodytes schweinfurthii) of Gombe National Park, Tanzania. Anim Behav 70:673–683
Lonsdorf EV (2006) What is the role of the mother in the acquisition of tool-use skills in wild chimpanzees? Anim Cogn 9:36–46
Lonsdorf EV (2017) Sex differences in nonhuman primate behavioral development. J Neurosci Res 95:213–221
Lonsdorf EV, Anderson KE, Stanton MA, Shender M, Heintz MR, Murray CM (2014a) Boys will be boys: sex differences in wild infant chimpanzee social interactions. Anim Behav 88:79–83
Lonsdorf EV, Markham AC, Heintz MR, Anderson KE, Ciuk DJ, Goodall J, Murray CM (2014b) Sex differences in wild chimpanzee behavior emerge during infancy. PLoS One 9:e99099
Lonsdorf EV, Pusey AE, Eberly L (2004) Sex differences in learning in chimpanzees. Nature 428:715–716
Lonsdorf EV, Ross SR (2012) Socialization and development of behavior. In: Mitani JC, Call J, Kappeler PM, Palombit RA, Silk JB (eds) The evolution of primate societies. The University of Chicago Press, Chicago, pp 245–268
Maestripieri D (1994) Social structure, infant handling, and mothering styles in group-living Old World monkeys. Int J Primatol 15:531–553
Maestripieri D (1995a) Maternal encouragement in nonhuman primates and the question of animal teaching. Hum Nat 6:361–378
Maestripieri D (1995b) First steps in the macaque world: do rhesus mothers encourage their infants' independent locomotion? Anim Behav 49:1541–1549
Maestripieri D (1995c) Assessment of danger to themselves and their infants by rhesus macaque (Macaca mulatta) mothers. J Comp Psychol 109:416–420
Maestripieri D (1996a) Maternal encouragement of infant locomotion in pigtail macaques, Macaca nemestrina. Anim Behav 51:603–610
Maestripieri D (1996b) Gestural communication and its cognitive implications in pigtail macaques (Macaca nemestrina). Behaviour 133:997–1022
Maestripieri D (1998) Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav 55:1–11
Maestripieri D (2001a) Intraspecific variability in parenting styles of rhesus macaques: the role of the social environment. Ethology 107:237–248
Maestripieri D (2001b) Female-biased maternal investment in rhesus macaques. Folia Primatol 72:44–47
Maestripieri D (2002) Parent-offspring conflict in primates. Int J Primatol 23:923–951
Maestripieri D (2003) Attachment. In: Maestripieri D (ed) Primate psychology. Harvard University Press, Cambridge, pp 108–143
Maestripieri D (2004) Genetic aspects of mother-offspring conflict in rhesus macaques. Behav Ecol Sociobiol 55:381–387
Maestripieri D (2005) Effects of early experience on female behavioral and reproductive development in rhesus monkeys. Proc R Soc Lond B 272:1243–1248
Maestripieri D (2007) Macachiavellian intelligence: how rhesus macaques and humans have conquered the world. The University of Chicago Press, Chicago
Maestripieri (2008) Neuroendocrine mechanisms underlying the intergenerational transmission of maternal behavior and infant abuse in rhesus monkeys. In: Pfaff D, Kordon C, Chanson P, Christen Y (eds) Hormones and social behavior. Springer, Berlin, pp 121–130
Maestripieri D (2009) Maternal influences on offspring growth, reproduction, and behavior in primates. In: Maestripieri D, Mateo JM (eds) Maternal effects in mammals. The University of Chicago Press, Chicago, pp 256–291
Maestripieri D (2010) Neurobiology of social behavior. In: Platt M, Ghazanfar A (eds) Primate Neuroethology. Oxford University Press, Oxford, pp 359–384
Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM (2006a) Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques. Behav Neurosci 120:1017–1024
Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD (2009) Mother-infant interactions in free-ranging rhesus macaques: relationships between physiological and behavioral variables. Physiol Behav 96:613–619
Maestripieri D, Klimczuk ACE (2013) Prenatal and maternal psychosocial stress in primates: adaptive plasticity or vulnerability to pathology? In: Laviola G, Macrí S (eds) Adaptive and maladaptive aspects of developmental stress. Springer, Berlin, pp 45–64
Maestripieri D, Lindell SG, Higley JD (2007) Intergenerational transmission of maternal behavior in rhesus monkeys and its underlying mechanisms. Dev Psychobiol 49:165–171
Maestripieri D, Mateo JM (eds) (2009) Maternal effects in mammals. The University of Chicago Press, Chicago
Maestripieri D, McCormack KM, Lindell SG, Higley JD, Sanchez MM (2006b) Influence of parenting style on the offspring's behavior and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res 175:90–95
Maestripieri D, Ross SR (2004) Sex differences in play among western lowland gorilla (Gorilla gorilla gorilla) infants: implications for adult behavior and social structure. Am J Phys Anthropol 123:52–61
Maestripieri D, Ross SR, Megna NL (2002) Mother-infant interactions in western lowland gorillas (Gorilla gorilla gorilla): spatial relationships, communication, and opportunities for social learning. J Comp Psychol 116:219–227
Maestripieri D, Wallen K (1997) Affiliative and submissive communication in rhesus macaques. Primates 38:127–138
Maestripieri D, Wallen K (2003) Nonhuman primate models of developmental psychopathology: problems and prospects. In: Cicchetti D, Walker E (eds) Neurodevelopmental mechanisms in psychopathology. Cambridge University Press, Cambridge, pp 187–214
Majolo B, Lehmann J, de Bortoli Vizioli A, Schino G (2012) Fitness-related benefits of dominance in primates. Am J Primatol 147:652–660
Mandalaywala TM, Higham JP, Heistermann M, Parker KJ, Maestripieri D (2014a) Physiological and behavioral responses to weaning conflict in free-ranging primate infants. Anim Behav 97:241–247
Mandalaywala TM, Parker KJ, Maestripieri D (2014b) Early experience affects the strength of vigilance for threat in rhesus monkey infants. Psychol Sci 25:1893–1902
Markham AC, Lonsdorf EV, Pusey AE, Murray CM (2015) Maternal rank influences the outcome of aggressive interactions between immature chimpanzees. Anim Behav 100:192–198
McCormack KM, Newman TK, Higley JD, Maestripieri D, Sanchez MM (2009) Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav 55:538–547
Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers JH, Suomi SJ, Linnoila M (1995) Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am J Psychiatry 152:907–913
Mehlman PT, Higley JD, Fernald BJ, Sallee FR, Suomi SJ, Linnoila M (1997) CSF 5-HIAA, testosterone, and sociosexual behaviors in free-ranging male rhesus macaques in the mating season. Psychiatry Res 72:89–10
Murray CM, Lonsdorf EV, Stanton MA, Wellens KR, Miller JA, Goodall J, Pusey AE (2014) Early social exposure in wild chimpanzees: mothers with sons are more gregarious than mothers with daughters. Proc Natl Acad Sci USA 111:18189–18194
Murray CM, Stanton MA, Wellens KR, Santymire RM, Heintz MR, Lonsdorf EV (2018) Maternal effects on offspring stress physiology in wild chimpanzees. Am J Primatol 80:e22525
Musgrave S, Morgan D, Lonsdorf EV, Mundry R, Sanz C (2016) Tool transfers are a form of teaching among chimpanzees. Sci Rep 6:34783
Nakamichi M (1989) Sex differences in social development during the first 4 years in a free-ranging group of Japanese monkeys, Macaca fuscata. Anim Behav 38:737–748
Parker KJ, Buckmaster CL, Schatzberg AF, Lyons DM (2006) Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA 30:924–929
Parker KJ, Maestripieri D (2011) Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev 35:1466–1483
Pereira ME (1995) Development and social dominance among group living primates. Am J Primatol 37:143–175
Petrullo LA, Mandalaywala TM, Parker KJ, Maestripieri D, Higham JP (2016) Effects of early life adversity on cortisol/salivary alpha-amylase symmetry in free-ranging juvenile rhesus macaques. Horm Behav 86:78–84
Roney JR, Maestripieri D (2003) Social development and affiliation. In: Maestripieri D (ed) Primate psychology. Harvard University Press, Cambridge, pp 171–204
Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH (2007) Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry 12:895–897
Sanchez MM, McCormack KM, Grand AP, Fulks R, Graff A, Maestripieri D (2010) Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev Psychopathol 22:45–53
Schino G, Aureli F, Ventura R, Troisi A (2004) A test of the cross-generational transmission of grooming preferences in macaques. Ethology 110:137–146
Schino G, Speranza L, Troisi A (2001) Early maternal rejection and later social anxiety in juvenile and adult Japanese macaques. Dev Psychobiol 38:186–190
Silk JB, Alberts SC, Altmann J (2003) Social bonds of female baboons enhance infant survival. Science 302:1231–1234
Simpson MJA (1985) Effects of early experience on the behavior of yearling rhesus monkeys (Macaca mulatta) in the presence of a strange object: classification and correlation approaches. Primates 26:57–72
Simpson MJA, Datta SB (1990) Predicting infant enterprise from early relationships in rhesus macaques. Behaviour 116:42–63
Simpson MJA, Gore MA, Janus M, Rayment FDG (1989) Prior experience of risk and individual differences in enterprise shown by rhesus monkey infants in the second half of their first year. Primates 30:493–509
Simpson AE, Simpson MJA (1985) Short-term consequences of different breeding histories for captive rhesus macaque mothers and their young. Behav Ecol Sociobiol 18:83–89
Sterck EHM, Watts DP, van Schaik CP (1997) The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol 41:291–309
Symons D (1978) Play and aggression. In: A study of rhesus monkeys. Columbia University Press, New York
Thornton A, Raihani NJ (2008) The evolution of animal teaching. Anim Behav 75:1823–1836
Trivers RL, Willard D (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
van de Rijt-Plooy HHC, Plooy FX (1987) Growing independence, conflict and learning in mother-infant relations in free-ranging chimpanzees. Behaviour 101:1–86
van Noordwijk MA, van Schaik CP (1985) Male migration and rank acquisition in wild long-tailed macaques (Macaca fascicularis). Anim Behav 33:849–861
van Noordwijk MA, van Schaik CP (1988) Male careers in Sumatran long-tailed macaques (Macaca fascicularis). Behaviour 107:24–43
van Schaik CP (1989) The ecology of social relationships amongst female primates. In: Standen V, Foley R (eds) Comparative socioecology: the behavioral ecology of humans and other mammals. Blackwell, Boston, pp 195–218
Vochteloo JD, Timmermans PJA, Duijghuisen JAH, Vossen JMH (1993) Effects of reducing the mother's radius of action on the development of mother-infant relationships in longtailed macaques. Anim Behav 45:603–612
Whiten A (1999) Parental encouragement in gorilla in comparative perspective: implications for social cognition and the evolution of teaching. In: Parker ST, Mitchell RW, Miles HL (eds) The mentalities of gorillas and orangutans: comparative perspectives. Cambridge University Press, Cambridge, pp 342–366
Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch F (2002) Affiliation and aggression among adult female rhesus macaques: a genetic analysis of paternal cohorts. Behaviour 139:371–391
Acknowledgments
I am grateful to Federica Amici and Anja Widdig for their invitation to contribute to the Behavioral Ecology and Sociobiology topical collection “An evolutionary perspective on the development of primate sociality.” I also thank Anja Widdig and two anonymous reviewers for helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
This article is a contribution to the Topical Collection An evolutionary perspective on the development of primate sociality – Guest Editors: Federica Amici and Anja Widdig
Rights and permissions
About this article
Cite this article
Maestripieri, D. Maternal influences on primate social development. Behav Ecol Sociobiol 72, 130 (2018). https://doi.org/10.1007/s00265-018-2547-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2547-x