Abstract
Fish rival birds and mammals in many of their cognitive skills, and have been shown to successfully discriminate between a range of stationary and moving objects. The present study tested the ability of Pseudotropheus zebra to recognize unique movement patterns shown in the form of a single moving dot, point displays (PDs), point-light displays (PLDs), and videos of moving organisms in two alternative forced-choice experiments. Cichlids successfully distinguished between (1) different directions of movement, (2) a biological vs. a random movement, (3) a biological (human walking) vs. a scrambled motion pattern, and (4) two biological motion patterns (human walking to the left vs. to the right). Following training in (3), it was tested if the walking human was also correctly identified when either the positive, the alternative, or both stimuli were altered or presented inverted; following training in (4), stimuli were presented inverted or moving backwards. With the exception of the presentation of inverted and backwards-moving stimuli, fish excelled at these tasks. Furthermore, cichlids successfully discriminated between videos of different organisms such as eel vs. trout, human vs. dog, eagle vs. bat, and dolphin vs. shark. Following each training, a series of transfer tests elucidated whether P. zebra could also recognize these organisms when shown in transfer test trials (a) from a different perspective (front or sideways), (b) enlarged or downsized, or (c) as PDs. With few exceptions, all individuals learned all tasks and significantly often chose the previously reinforced (but altered) training stimulus over the alternative one during transfer tests. This indicates that cichlids have the ability to recognize a familiar organism under new conditions, for example, based on its movement alone, which may be helpful in recognizing approaching predators early on.
Significance Statement
Fish can solve a variety of learning and memory tasks including visual discrimination of objects. Here it was tested if cichlids (Pseudotropheus zebra) can distinguish between pairs of videos featuring individually moving dots, dot patterns and moving organisms. Overall, fish were very successful and solved most of the presented tasks. They differentiated between movement directions and movement types, recognized stimuli in transfer tests under altered conditions, and identified familiar organisms when shown resized, from new perspectives or in form of point displays. To be able to recognize movement and to identify organisms based on their specific movement patterns (alone) in the wild is essential to individual survival, as predators, conspecifics and/or prey are usually mobile. Additionally, predators are often encountered at night or when light levels are low, making it harder to successfully distinguish general body features such as coloration and adding significance to being able to quickly recognize motion cues. It should be equally advantageous to be able to recognize organisms such as predators from unfamiliar perspectives. Stimuli used in this study were not of any ecological relevance; they were chosen purely for their characteristic type of movement and just served as vectors to determine transfer abilities of gained knowledge onto new situations. Nonetheless, having excelled at tasks using these stimuli, it seems quite likely that cichlids would perform even better under ecologically significant conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behavioral experiments assessing visual abilities of fish have come a long way in the last century. After initial experiments revealed that teleosts can see color (e.g., Schiemenz 1924; Schaller 1926) and can distinguish between geometric figures, sizes, surface areas, contrasts, and numerous other variables (reviewed in Herter 1953), fish have been the subject of a wide range of diverse discrimination experiments. From size and form constancy (Douglas et al. 1988; Schuster et al. 2004; Frech et al. 2012; Schluessel et al. 2014a, b) to object recognition and categorization (e.g., Neri 2012; Schluessel et al. 2012), to recognition of symmetrical symbols (Schluessel et al. 2015), optical illusions (e.g., Wyzisk and Neumeyer 2007), numerical competency (e.g., Agrillo et al. 2014; Mehlis et al. 2015), and the ability for amodal completion (e.g., Sovrano and Bisazza 2008; Darmaillacq et al. 2011), fish have excelled in many cognitive tasks, both in the laboratory and in the wild, in training tasks as well as innate preference tests (reviewed in Herter et al. 1953; Brown et al. 2011; Schluessel 2015). Still, surprisingly few studies have used moving stimuli to assess movement perception and discrimination abilities on a cognitive level (e.g., Sashar et al. 2005; Baldauf et al. 2009; Gori et al. 2014; Nakayasu and Watanabe 2014; Schluessel et al. 2015; Fuss et al. 2017) or in studies unrelated to cognitive investigations (e.g., Clark 1981; Orger et al. 2000; Turnell et al. 2003; Gerlai et al. 2009; Abaid et al. 2012a, b).
Biological motions describe characteristic movement patterns of living organisms (Johansson 1973). These are usually visualized in the form of white dots on a black background (point-light display, PLD), where the dots are situated in strategically important places such as joints, the spine, and the head. The more dots, the greater the information content; however, in most cases, five to ten dots are sufficient for humans to recognize human movement (Johansson 1973). Apart from just movement, humans can also recognize many additional features from human PLDs, such as emotion, gender, friends, and even themselves (Cutting and Kozlowski 1977; Kozlowski and Cutting 1977; Beardsworth and Buckner 1981; Mather and Murdoch 1994). Humans use biological motion information furthermore to identify activities, gestures, and other motions. This can be specifically useful in social or cultural situations, and may also be important to other (social) species, including fish. Biological motion perception has been studied in several non-human species so far, e.g., primates (e.g., Siegel and Andersen 1988; Tomonaga 2001; Parron et al. 2007; Brown et al. 2010; Vangeneugden et al. 2010), rats (e.g., MacKinnon et al. 2010; Foley et al. 2012), cats (Blake 1993), dolphins (Hermann et al. 1990), birds (e.g., Dittrich and Lea 1993; Dittrich et al. 1998; Regolin et al. 2000; Vallortigara et al. 2005; Vallortigara and Regolin 2006; Ortega et al. 2009; Troje and Aust 2013), and fish (Orger et al. 2000; Nakayasu and Watanabe 2014; Schluessel et al. 2015; Fuss et al. 2017). All of the assessed species were successful in either discriminating between biological and scrambled motions or in more advanced tasks involving complex manipulations. However, not all animals were able to generalize learned knowledge (e.g., MacKinnon et al. 2010).
Studies using PLDs or point displays (PDs) in fish are rare (Orger et al. 2000; Nakayasu and Watanabe 2014; Schluessel et al. 2015; Fuss et al. 2017). Schluessel et al. (2015) studied two teleost species in regard to the discrimination of simple and complex movement patterns of dots and objects, including biological motion patterns using PDs and PLDs. Cichlids successfully differentiated between most stimuli presented but, surprisingly, failed to recognize familiar organisms when shown as PDs, i.e., when the information content of the stimulus was reduced to the movement of the organism. A similar study on juvenile bamboo sharks gave comparable results; sharks successfully discriminated between videos of dots moving in different directions and videos of moving organisms (Fuss et al. 2017) but in most cases failed to transfer the learned information to new forms of stimulus presentations, i.e., when these were shown from new perspectives, enlarged or size reduced or in the form of PDs. However, as opposed to the cichlids in the study by Schluessel et al. (2015), sharks recognized two of their training stimuli, i.e. “eel” and “trout” when presented as PDs.
As the results of the first study on cichlids (Schluessel et al. 2015) were generally promising but could not provide direct support for the fishes’ ability to transfer learned information to PD presentations, the present study was designed to explore this topic in greater depth. Two aims were formulated; first, it was assessed whether cichlids can distinguish between different combinations of biological and scrambled biological motion (presented as dot patterns) and whether the learned information can be applied to new or altered stimulus presentations (e.g., new proportions, inverted, walking backwards). Previous studies have shown that once a PLD is presented upside down, humans’ ability to recognize the stimulus is strongly impaired (e.g., Sumi 1984; Pavlova et al. 2002; Chang and Troje 2008). Similar inversion effects were also observed in several other species, such as cats (Blake 1993) and chickens (Vallortigara and Regolin 2006), and at least to some degree in pigeons (Troje and Aust 2013).
An interesting aspect about the observed inversion effects in terrestrial animals is the role of gravity. In humans, it was shown that the direction of gravitational acceleration experienced by upper and lower limbs, and particularly by the feet of a walking individual, provides a strong cue that validates the visual stimulus as being animate and potentially interesting (Troje and Westhoff 2006; Hirai et al. 2011a, b; Troje and Chang 2013). Gravitational acceleration forms a visual invariant that constitutes a general detection system that draws attention to other animals even if they appear in the very periphery of the visual field (Thompson et al. 2007). It triggers orientation responses (Hirai et al. 2011a, b) and seems to be present already in newborn babies (Simion et al. 2008) as well as in newly hatched chickens (Vallortigara and Regolin 2006).
In aquatic environments, gravity plays only a minor role in locomotion and the terrestrial “life detector” would be useless. In that context, we are interested in exploring if fish are sensitive to inversion effects in biological motion at all. If they are, then it is likely not a response to inverted gravity, but rather demonstrating sensitivity to configural aspects of perceived body shape.
The second part of the study aimed at examining whether cichlids can recognize videos of different organisms and altered variations thereof (i.e., shown from new perspectives, enlarged or size reduced or in the form of PDs). Models were chosen based on motion similarity between pairs (e.g., bats and birds generally feature a similar type of movement yet different enough to be told apart) and dissimilarity among different pairs (terrestrial, aquatic, aerial) as to assess different types of movement. Variation in the type of movement was considered to be more important than ecological relevance of individual stimuli, as the overall aim was to test if organisms, even naturally unfamiliar ones, can be identified (after a training exposure) based on biological motion itself. Previous studies have shown that cichlids have profound categorization abilities (Schluessel et al. 2012, 2014a, b) indicating that they can solve tasks without paying sole attention to selected stimulus features, such as edges or lines, but to the stimulus as a whole. This ability should theoretically aid or enable cichlids also to recognize familiar objects when presented under altered conditions. Furthermore, considering that fish constantly move in a three-dimensional world, the ability to recognize organisms from new, previously not encountered perspectives and based on characteristic movements alone would be highly advantageous and is therefore expected.
Material and methods
Animals
The subjects were eight Malawi cichlids (Pseudotropheus zebra) obtained from a commercial aquarist shop. All but one individual had already participated in other behavioral experiments. All individuals were bred in captivity and ranged between 6.0 and 7.0 cm in total length. Sex could not be determined phenotypically. The number of animals used varied between the individual experiments and is provided at the beginning of each respective section.
Setup
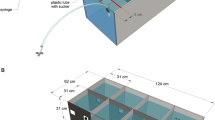
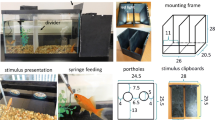
The cichlids were individually housed in 50-L aquaria (62 cm × 31 cm × 31 cm), filled with aerated and filtered water and kept at a temperature of 25–26 °C. The aquarium served both as a holding as well as an experimental tank (see Fig. 1). The walls and floor of each tank consisted of light gray polyvinylchloride, while the front was made of white frosted plastic. A partition was inserted into the middle of the tank, separating a back from a front compartment. The back compartment contained a pump and filter system (Duetto Cobra DJC 50) as well as a heating element. In the center, the gray partitioning was fitted with a small transparent guillotine door, controlled remotely by a hand-operated device. In the absence of testing, the door was kept open and the fish could move freely between compartments. Prior to training, the door was closed, restricting the fish to the back compartment. On the inside of each aquarium front, two feeders were installed (Fig. 1), consisting of two small pieces of plastic pipe that were attached to the wall with suckers. Into each pipe, a hose filled with food (food pellet, ©Sera, Germany) was inserted. On the other end, the hose was connected to a syringe through which the food delivery was controlled remotely. The front compartment was further divided into a right and a left compartment; parallel to the front wall, a line indicated a 5.0-cm-wide area, which together with the right and left divisions created two areas into which the fish had to swim in order for its stimulus choice to be valid. Stimuli (black shapes on a light gray background) were projected onto the acrylic glass front of the tank just below the feeders, using an LCD projector (ES 521 Optoma, DLP®, China), which was located in front of the tank and connected to a laptop computer. Markings on the acrylic glass guaranteed a projection onto the same fixed area in each session. The stimuli were projected at the same height as the guillotine door to ensure that individuals could immediately see them when exiting the door.
Experimental procedure
Experiments were conducted during daylight hours; there was a natural light:dark cycle. All experiments were conducted as two alternative forced-choice experiments. Individuals had to choose between two videos, forming a stimulus pair. One video presented the “positive” stimulus and choosing it was rewarded while choosing the alternative, “negative” stimulus, was unrewarded. Sessions were conducted daily and approximately at the same time of day with each animal. Each session consisted of ten trials (these were termed “regular” trials to distinguish them from “transfer test” trials, which are described in the “Transfer tests” section). Both feeders were baited in each trial to prevent olfactory cues guiding the fish. Stimuli were projected onto the milk-colored front. Locations of the two stimuli were counterbalanced. The order was randomized; however, the same configuration was not shown more than twice in a row.
At the beginning of each trial, the fish was in the back compartment. The guillotine door was opened and the fish was allowed to enter the front compartment. Once the door was opened, the fish had to make a choice within 2 min; otherwise, the trial was terminated and no response was registered. A response was recorded as soon as the fish crossed over the decision line as indicated by markers. A correct choice was rewarded with a food pellet. Immediately following an incorrect choice or after consuming the reward, the fish was ushered back into the back compartment with the help of a small net. Intertrial time was 30 s. Training ended when the learning criterion was reached which was established at performing successfully in at least seven out of ten trials (≥ 70% correct choices; one exception: nine out of 12, i.e., ≥ 75% in E6) in three consecutive sessions (χ2 (1) test, p ≤ 0.05). If the learning criterion was not reached within 30 sessions, the task was classified as not learned. For each session, the average trial time and the percentage of correct choices were recorded. Trial time was defined as the time the fish needed to swim through the guillotine door and cross the line in front of the food holders. Time was recorded using a stopwatch.
Pretraining
Prior to training, the fish had to get used to the apparatus and to feeding from the feeders. The guillotine door was open, the feeders were in place, and the fish were only fed at the feeders. In a second step, the door was closed and only opened when the fish pressed against it or waited in front of it. After the fish had learned to swim through the open door and approach the feeders while the projector was on, training started.
Training
With the exception of experiment 1 (in which group 1 (N = 4) was trained to prefer the horizontally moving stimulus and group 2 (N = 4) the vertically moving stimulus), the designated positive stimulus was always the same for all individuals (N = 8) in each experiment. In total, there were eight experiments, each featuring a different stimulus pair. Please refer to the following section on “stimuli” for construction of these; screenshots of the stimuli (videos) are provided in Figs. 2, 3, 4, 5, 6, 7, 8, and 9, and experimental conditions are summarized in Table 1. Please see supplementary videos E1–E8 for complete stimulus presentations (videos are named after the experiments; there is only one video per experiment; however, during the sessions, two identical versions were used in which only the position of the positive stimulus varied (either on the right or on the left).
Stimulus pairs (videos) for E5. a Training stimuli, eel* vs. trout. b–e Transfer stimuli. b Enlarged positive stimulus or size-reduced alternative stimulus, c size-reduced positive stimulus or enlarged alternative stimulus, d same color scheme, e PD. Scale bars in b and c indicate that stimuli were enlarged or size reduced to a ratio of 3:1 (not visible in presentations)
Stimulus pairs (videos) for E6. a, b Training stimuli, human* vs. dog (shown interchangeably and equally often). c–g Transfer stimuli. c Enlarged positive stimulus or size-reduced alternative stimulus, d size-reduced positive stimulus or enlarged alternative stimulus, e, f new perspective (front), g PD. Scale bars in c and d give the original size of training stimuli (not visible in presentations)
Stimulus pairs (videos) for E7. a Training stimuli, eagle* vs. bat. b–e Transfer stimuli. b Enlarged positive stimulus/size-reduced alternative stimulus, c size-reduced positive stimulus/enlarged alternative stimulus, d PD, e new perspective (sideways). Scale bars in b and c indicate that stimuli were enlarged or size reduced to a ratio of 3:1 (not visible in presentations)
Stimulus pairs (videos) for E8. a Training stimuli, dolphin* vs. shark. b–e Transfer stimuli. b Enlarged positive stimulus/size-reduced alternative stimulus, c size-reduced positive stimulus/enlarged alternative stimulus, d new perspective (sideways), e PD. Scale bars in b and c indicate that stimuli were enlarged or size reduced to a ratio of 3:1 (not visible in presentations)
Experiments E1–E4 assessed the ability of cichlids to discriminate dots moving in different directions (E1a, b; Fig. 2), biological* (the asterisks denotes the positive stimulus from here onwards) vs. random motion (E2; Fig. 3; video ESM_1), biological* (human walking) vs. scrambled motion (E3; Fig. 4a–d; videos ESM_2, ESM_3), and right-facing point-light walkers* from left-facing point-light walkers (E4; Fig. 5a–c; video ESM_4), (Table 1). In E3, additional transfer tests (see below) were performed, in which stimulus pairs were shown featuring (a) an altered positive stimulus (“big” human instead of “normal” sized), (b) an altered alternative stimulus (different dot pattern), (c) both stimuli altered as in a and b (video ESM_3), and (d) both stimuli inverted (Fig. 4). Training in E4 consisted of three parts, i.e., as the fish had difficulty distinguishing the stimuli, the speed of movement was consecutively reduced from normal, veridical speed (1.0) to speeds that were either 0.7 or 0.4 times the veridical speed. Transfer tests in E4 tested whether point-light walkers (speed 0.4) could still be identified when they were (a) inverted or (b) moving backwards (Fig. 5).
In the second set of experiments, the fish had to discriminate between videos showing the silhouettes of moving organisms, i.e., eel* vs. trout (E5, Fig. 6a–e; videos ESM_5, ESM_6), human* vs. dog (E6, Fig. 7; videos ESM_7, ESM_8), eagle* vs. bat (E7, Fig. 8; videos ESM_9, ESM_10), and dolphin* vs. shark (E8, Fig. 9; videos ESM_11, ESM_12; Table 1). Following each training, four sets of transfer tests T1–T4 (see below) were conducted for each experiment (E5–E8) in which the organisms were shown (a) enlarged, (b) size reduced, (c) in the same color (only E5), (d) from a new perspective (front or sideways, E6–E8), and (e) in the form of a PD (see respective Fig. 7b–e; videos ESM_6, ESM_8, ESM_10, ESM_12). In E6, training stimuli consisted of two different sets of videos, one in which the human and dog were of “normal” size and one in which the human was reduced to the height of the dog (Fig. 7). As the two stimuli varied considerably in height, this attempted to take the focus away from height as a deciding factor. Both versions were presented within each session in a random order but equally often (n = 5; n = 6 in E6).
Transfer tests
Transfer test trials elucidated whether the fish could apply previously gained knowledge during training to a new situation. Transfer tests were only conducted if the learning criterion was reached. As the transfer tests were unrewarded, they also served as a control to determine if baiting had any unwanted effect on the stimulus selection process. After the fish reached the learning criterion in the regular trials, an 80% rewarding scheme was introduced. From then on, food was only provided in a maximum of eight (out of ten) correct trials; prior to each session, it was randomly determined which two trials would remain unrewarded (regardless of choice). This served to prepare the fish for the subsequent introduction of the unrewarded transfer test trials, i.e., by keeping the fish from realizing that only these trials were unrewarded and therefore not worth participating in. If performance during the 80% rewarding scheme remained unaffected, transfer trials started [n = 10 (E3–E4) and n = 20 (E5–E8) per individual]. Two transfer trials were randomly interspersed with the ten “regular” trials within a session.
Stimuli
Dot pattern (experiment E1)
To generate the artificial dot pattern, a sinusoidal frequency was applied to the motion of single dots/points using MATLAB (R2011a). The amplitude of the point motion was determined in proportion to the image size and frequency given in hertz (see also Schluessel et al. 2015).
Biological vs. random movement (experiment E2)
Both animations were created using MATLAB (R2011a). Seven moving dots were arranged to mimic the motion of a stationary human runner presented from a sagittal viewpoint. The dots forming the “legs” were joint at a central dot point, which was moved up and down in a sinusoidal motion. Each “leg” consisted of two dots, indicating knee and foot. The rotational velocity of the knee joints around the central point was sinusoidal, with the knee joints swinging backwards and forwards. The rotational velocity of the feet around the knees was also sinusoidal, with the feet swinging backwards and forwards. In the case of the random motion, the animation also consisted of seven moving dots, with a central point providing the attachment site of the “knees” and the “feet.” However, as opposed to the biological motion depicting a “runner,” the rotational velocity of knees and feet was not sinusoidal but random.
Motion PLDs (experiments E3 and E4)
Biological motion point-light displays were based on generic walking obtained by averaging 50 male and 50 female walking sequences that were recorded using optical motion capture (Troje 2002, 2008). For stimuli design, the program “Biomotionlab” (NF Troje) was used. The veridical gait frequency of the walker was 1.05 Hz. In E4, speed was slowed down by factors 0.7 and 0.4. All walkers in E3 were shown in front view and in E4 in profile view (either left facing or right facing), walking stationary as if on a treadmill. Walkers consisted of 11 dots representing major joints of the body (two ankles, two knees, one hip, two wrists, two elbows, one shoulder) as well as the center of the head. The dots were white on a black background and had a diameter of 1 mm on the computer screen, but were larger (2.5 mm) once presented to the fish via the projector (from now on, only the size of the dots as seen by the fish is provided). Each walker display measured 9 cm × 4 cm. Videos were generated at 25 fps. They were encoded with the MPEG4 codec and were played back with Windows Media Player embedded into MS PowerPoint. The scrambled biological motions in E3 were based on the point-light walkers described above. They were created by randomly displacing the individual trajectories of the walkers within the area covered by the walker, then randomizing the phase relations between the dots. For the transfer tests in both experiments, the individual trajectories of the dots were changed according to the specific task.
Eel vs. trout (experiment E5)
A real trout and eel were filmed from below in a flow channel (working section: width 28 cm, height 40 cm, length 100 cm, water level 28 cm, flow 0–8 cm/s). Video data were processed with MATLAB (R2011a). First, a background image was subtracted and a threshold used to convert the video to a black and white format. A series of equidistant points was created through the centerline of the real fish with a straight posture. Points were plotted—without the image of the fish—for each frame to produce the PD. The dots measured 5 mm in diameter.
Other pairs of organisms (experiments E6–E8)
Prefabricated 3D models of each organism were downloaded from the website www.cadnav.com and imported into the program “Autodesk Maya 2015” (Autodesk Inc., San Rafael, CA). Imported organisms first appeared onscreen as black templates into which seven or more joints in strategically important body positions were added. Joints and skin were connected in preparation to create movement. Frames were constructed and standard settings used. Each video consisted of 200 frames and was integrated into PowerPoint as an infinite loop. Prior to constructing a video, a virtual camera was added to the processing area, which was positioned as desired (here from the front and from the side). Organisms were presented in a dark gray color against a light gray background. All videos were exported as .avi formats and then changed into MP4s, for integration in PowerPoint. Point displays featured dots ranging from 3 mm (bird, bat, shark, dolphin) to 4 mm (human and dog) in diameter.
Data analysis
Sessions were recorded by a webcam (Logitech Webcam C210, Logitech, Apples). The learning criterion was established to be ≥ 70% correct choices in three consecutive sessions. The probability of achieving this by chance is less than 5% (χ2 test, p < 0.05). A sign and binomial test was performed to test whether the individual fish chose the positive stimulus significantly more often than the alternative one during the transfer test trials. For all tests, a p ≤ 0.05 (two-tailed test) was considered significant. For group results, generalized linear mixed models (GLMM) were run in R [maximum likelihood (Laplace Approximation) using individual as random factor]. It was not possible to record data blind because our study involved close observation of the reaction of animals in the laboratory.
Results
E1a (horizontally vs. vertically moving dot, Fig. 2a)
Five out of eight fish reached the learning criterion within Ø 9.8 ± 7.5 (SD) sessions. The average trial time (±SD) was 1.53 ± 0.8 s. In group 1 (horizontally moving stimulus rewarded), only one fish mastered the task (5 sessions), while all four fish in group 2 (vertically moving stimulus rewarded) were successful (4–22 sessions).
E1b (diagonally moving dots, moving in different directions; Fig. 2b)
Six out of eight fish reached the learning criterion within Ø 14.33 ± 10.58 sessions. The average trial time was 1.22 ± 0.49.
E2 (biological* vs. random movement, Fig. 3; video ESM_1)
All fish (N = 8) reached the learning criterion within Ø 23.38 ± 8.77 sessions. The average trial time was 1.22 ± 0.62 s.
E3 (walking human* vs. scrambled movement, Fig. 4; videos ESM_2, ESM_3)
All fish (N = 8) reached the learning criterion within Ø 13.13 ± 5.39 sessions. The average trial time was 1.09 ± 0.48 s. In the trials of transfer test T1 (n = 80), the fish selected the “big” human more often (68 times; 85%) over the alternative stimulus (X2 = − 4.65, df = 1, p < 0.0001). The average trial time was 1.09 ± 0.76 s. In the T2 trials (N = 80), the fish chose the human significantly more often (64 times; 80%) than the alternative stimulus (X2 = − 3.38, df = 1, p < 0.0001). The average trial time was 0.87 ± 0.5 s. In the T3 trials (N = 80), the fish chose the “big” human significantly more often (59 times; 74%) than the altered alternative stimulus (X2 = − 3.33, df = 1, p < 0.0001). The average trial time was 1.03 ± 0.76 s. In the T4 trials (N = 80), the fish chose the inverted human only 29 times (37%) and the inverted alternative stimulus 51 times; this result was significant (X2 = 2.85, df = 1, p = 0.004). The average trial time was 1.14 ± 0.85 s.
E4 (right walker vs. left walker, Fig. 5; video ESM_4)
Two out of eight fish reached the learning criterion within Ø 21.0 ± 4.0 sessions when speed 1 was used. The average trial time was 1.6 ± 0.6 s. Due to the poor performance, the speed of walking was reduced to 0.7 in the following training. Only three fish participated; two reached the learning criterion in 10.5 ± 0.71 sessions. The average trial time was 1.17 ± 0.42 s. When the speed was further reduced to 0.4, five out of eight fish reached the learning criterion in 17.2 ± 8.20 sessions. The average trial time was 1.22 ± 0.49 s. Transfer tests (n = 50) showed that none of the fish could identify the right walker correctly when both stimuli were shown inverted (group result 25:15; X2 = 0.839, df = 1, p = 0.402) or walking backwards (group result 23:17; X2 = − 0.945, df = 1, p = 0.345).
E5 (eel* vs. trout, Fig. 6; videos ESM_5, ESM_6)
All fish (N = 7) reached the learning criterion within Ø 13.0 ± 8.12 sessions. The average trial time was 1.49 ± 0.73 s. In the T1 trials (n = 140), the fish (as a group) selected the enlarged eel* significantly often (126 times; 90%) over the size-reduced trout (X2 = − 3.73, df = 1, p < 0.001). Only one individual did not choose the stimulus significantly more often (14*:6, p = 0.115), but still more often than the alternative. In the T2 trials (n = 140), the fish (as a group) selected the size-reduced eel* significantly more often (101 times; 72%) than the trout (X2 = − 2.853, df = 1, p = 0.004). However, four individuals chose according to chance (14*:6, p = 0.115; 13*:7, p = 0.263). In the T3 trials (n = 140), the fish (as a group) selected the black eel* significantly more often (125 times; 89%) over the black trout (X2 = − 4.948, df = 1, p < 0.0001). Only one individual did not choose the eel* significantly more often (14*:6, p = 0.115). In the T4 trials (n = 140), the fish (as a group) selected the eel* shown as a PD significantly more often (105 times; 75%) over the trout shown as a PD (X2 = − 4.57, df = 1, p < 0.0001). However, three individuals chose according to chance (13*:7, p = 0.263; 12*:8, p = 0.503).
E6 (human* vs. dog, Fig. 7; videos ESM_7, ESM_8)
All fish (N = 7) reached the learning criterion within Ø 7.0 ± 3.79 sessions. The average trial time was 1.20 ± 0.64 s. In the T1 trials (n = 140), the fish (as a group) selected the enlarged human* significantly often (129 times; 92%) over the size-reduced dog (X2 = − 5.871, df = 1, p < 0.0001). In the T2 trials (n = 140), the fish (as a group) selected the size-reduced human* significantly more often (94 times; 67%) over the dog (X2 = − 3.67, df = 1, p = 0.0002). However, six out of seven individuals chose according to chance (14*:6, p = 0.115; 12*:8, p = 0.503; 11*:9, p = 0.824). In the T3a trials (n = 140), the fish as a group selected the human shown from the front significantly more often (122 times; 87%) than the dog shown from the front (X2 = − 7.58, df = 1, p < 0.0001). In the T3b trials (n = 140), in which the human and dog were of equal height, the fish (as a group) selected the human shown from the front also significantly more often (94 times; 67%) than the dog shown from the front (X2 = − 2.481, df = 1, p = 0.013), but again, four individuals did not choose the positive stimulus significantly more often than the alternative (14*:6, p = 0.115; 10*:10, p = 1.176; 7*:13, p = 0.263). In the T4 trials (n = 140), the fish (as a group) selected the human* shown as a PD significantly more often (118 times; 84%) than the dog shown as PD (X2 = − 4.86, df = 1, p < 0.0001). Only one individual chose according to chance (12*:8, p = 0.503).
E7 (eagle* vs. bat, Fig. 8; videos ESM_9, ESM_10)
All fish (N = 7) reached the learning criterion within Ø 6.0 ± 1.91 sessions. The average trial time was 1.48 ± 0.54 s. In the T1 trials (n = 140), the fish (as a group) tended to select the enlarged eagle* more often (87 times; 62%) than the size-reduced bat (X2 = − 1.878, df = 1, p = 0.0604). The results for only two of seven individuals were significant (17*:3, p = 0.003; 16*:4, p = 0.011). In the T2 trials (n = 140), the fish (as a group) selected the size-reduced eagle* significantly more often (127 times; 91%) than the enlarged bat (X2 = − 3.63, df = 1, p < 0.001). One individual chose according to chance (13*:7, p = 0.263). In the T3 trials (n = 140), the fish (as a group) selected the eagle shown sideways* significantly more often (108 times; 77%) than the bat shown sideways (X2 = −6.044, df = 1, p < 0.0001). One individual chose according to chance (12*:8, p = 0.503). In the T4 trials (n = 140), the fish (as a group) selected the eagle* shown as a PD significantly more often (92 times; 66%) than the bat shown as PD (X2 = −3.654, df = 1, p < 0.001). However, five individuals chose according to chance (14*:6, p = 0.115; 13*:7, p = 0.263; 12*:8, p = 0.503; 10*:10, p = 0.176).
E8 (dolphin* vs. shark, Fig. 9; videos ESM_11, ESM_12)
All fish (N = 7) reached the learning criterion within Ø 3.29 ± 0.49 sessions. The average trial time was 0.87 ± 0.36 s. In the T1 trials (n = 134), the fish (as a group) selected the enlarged dolphin* significantly more often (113 times; 84%) than the size-reduced shark (X2 = − 7.08, df = 1, p < 0.0001). In the T2 trials (n = 136), the fish (as a group) selected the size-reduced dolphin* significantly more often (116 times; 85%) than the enlarged shark (X2 = − 6.605, df = 1, p < 0.0001). One individual chose according to chance (13*:7, p = 0.263). In the T3 trials (n = 132), the fish (as a group) selected the dolphin shown sideways* significantly more often (105 times; 77%) than the shark shown sideways (X2 = − 6.042, df = 1, p < 0.0001). One individual chose according to chance (13*:7, p = 0.263). In the T4 trials (n = 120), the five participating fish (as a group) selected the dolphin* shown as a PD significantly more often (84 times; 70%) than the shark shown as PD (X2 = − 5.768, df = 1, p < 0.0001). Only one individual chose according to chance (13*:7, p = 0.263).
A representative learning curve (E5) for fish 1 is shown in Fig. 10. Table 2 gives an overview of what tasks were accomplished by the group and by individuals.
Representative learning curve for fish 1 in E5 (eel* vs. trout). Shown are the correct choices (%) per session (black circles) and average trial time (s, ±SD) per session (white circles). The line depicts the 70% threshold. The learning criterion was reached in session 11; transfer tests were conducted starting in session 15
Discussion
Cichlids in the present study successfully learned to discriminate between videos of moving dots, PLDs, and moving organisms in a series of experiments. Performance was high, with six out of eight experiments (E2, E3, E5–E8) being successfully accomplished by all individuals. In contrast to previous results, cichlids also successfully generalized learned information and reliably identified altered training stimuli in a range of transfer tests, with the exception of those in which stimuli were presented inverted or moving backwards. Here, performance dropped to chance level.
Discrimination of motion in PDs and PLDs
All fish learned to distinguish between a random vs. a biological (E2) and a biological vs. a scrambled motion (E3). Surprisingly, the fish needed quite a long time (Ø 23 sessions) to distinguish between a biological and a random movement. The literature suggests that biological motions are generally and even innately preferred by most species, such as human infants (Fox and McDaniel 1982; Kuhlmeier et al. 2010), medaka fish (Nakayasu and Watanabe 2014), common marmoset (Brown et al. 2010), and chicks (Regolin et al. 2000; Vallortigara et al. 2005). It had therefore been expected that such a preference would also exist in Pseudotropheus and aid the decision-making process. As no spontaneous preference tests were performed, it cannot be clarified whether Pseudotropheus agrees with other species in this respect but current data as seen from E2 do not immediately support such an assumption. In the subsequent task (E3), the average session number to reach the learning criterion decreased by almost 45% (Ø 13 sessions). However, this was probably caused by previous training in E2 familiarizing the fish with the type of experiment, rather than to a spontaneous preference for the biological stimulus.
When either the positive or the alternative stimulus, or when both stimuli, were exchanged for alternatives in selected transfer tests (E3, T1–T3), the fish were still able to choose the altered training stimulus reliably often. Results thereby confirm previous ones (Newport et al. 2013; Schluessel et al. 2012, 2015), in that fish learned and remembered not just the meaning of the positive but also of the alternative stimulus. Additionally, small alterations in both stimuli as seen in T3 did not inhibit effective decision-making, suggesting that small figural elements were not used (exclusively) for differentiation, something also observed previously (Schluessel et al. 2012, 2014a, b).
Transfer test 4 (E3) showed that training stimuli were not recognized anymore once they were presented inverted. This result agrees with studies on other species such as humans (Chang and Troje 2008) and cats (Blake 1993). There are several theories as to why species across all assessed taxa have difficulties recognizing upside down presentations of biological motions. As stated previously, a “life detector” that depends on gravity would be rather useless in aquatic environments, as gravity plays only a minor role in locomotion (Chang and Troje 2008). Nonetheless, fish also encounter objects and other animals in characteristic positions or orientations, i.e., fish rarely swim “upside down” and bottom-dwelling animals such as crustaceans maintain their position in general. As in terrestrial animals, characteristic elements of movements that are expected to occur in certain places (such as the feet being close to the ground) may have not been found anymore by the fish once the stimuli were inverted, thereby providing confusing or conflicting information.
In a previous study (Gierszewski et al. 2013), cichlids distinguished between stationary images and their mirror image counterparts when reflected vertically but not horizontally. Overall performance was poor though, with only three out of eight cichlids learning the task. It was hypothesized by the authors that horizontally reflected images look too much alike to be easily discriminated, a finding that was confirmed in a subsequent experiment (transfer task), in which one individual correctly chose a horizontally reflected image in place of the training stimulus (indicating that they looked “the same” or similar) while a vertically reflected training stimulus presumably looked too different to be selected. If present results are interpreted with these findings in mind, they in fact provide additional support for the assumption that vertically inverted stimuli in general, regardless if they are moving or stationary, are not easily recognized as variations of the training stimulus and therefore not chosen significantly more often over an alternative stimulus. Interestingly though, in the present study, the fish did not choose according to chance but instead selected the vertically inverted alternative significantly more often than the inverted positive stimulus. So, while the inverted positive stimulus was not recognizable as the rewarded training stimulus anymore, it was still distinguishable from the alternative. In this case, the (unlikely) reason for choosing the alternative significantly often could have been that the inverted alternative may have resembled the rewarded training stimulus (walking human) more closely than the actual inverted human, at least in the form of dot numbers in the lower and upper body half. As it is not supported by the remaining results that fish based their distinction on single figural elements such as dot position, this may simply have been a replacement strategy.
Considering that cichlids did not successfully distinguish between stationary images and their horizontally mirrored counterparts (Gierszewski et al. 2013), the performance of cichlids in E4 was in fact unexpectedly high, with two out of eight fish distinguishing between a right- vs. a left-walking human (horizontal mirror images of one another) when speed was 1.0 and five out of eight fish when speed was reduced to 0.4. Movement obviously presents a crucial cue for fish to base recognition on; however, the poor results of both studies still suggest that identification of mirror image counterparts is unlikely a biologically relevant task for cichlids and probably fish in general (probably owing to the rare occurrence of reflections in aquatic environments) and as such not easily learned by most individuals.
Surprisingly, one of the poorest performances was achieved in the introductory tasks (E1a, E1b), in which only single dots moving in two different directions had to be distinguished from one another. These tasks were accomplished by only five and six (out of eight) individuals, respectively. The only other experiment seeing individuals fail was E4, in which PLDs of right- vs. left-walking humans were shown. All three tasks shared a common feature, i.e., they were the only ones in which the two stimuli within a pair were identical (dots or PLD of human walking sideways), varying only in the direction of movement. This may have been a problem for some individuals. Even though unlikely, it is possible, that participation in previous experiments caused a preference in a few of the experienced fish for some movement cues over others. A small indication of this is provided by the fact that the only naive fish (trained in the “horizontal” group) and all (experienced) fish in the “vertical group” mastered E1 straight away (5 and 4, 6, 12, 22 sessions, respectively), whereas the three experienced fish in the “horizontal group” did not learn the task. It can be speculated that the movement in the vertical plane was more conspicuous and thereby preferred by fish that had previously been trained to choose (for example) a moving stimulus over a stationary one, or a faster moving stimulus over a slower one (i.e., always choosing “more” movement over less). Two of the three unsuccessful individuals in E1a were also the unsuccessful ones in E1b and in E4, but they succeeded in all other tasks.
In the last transfer test in E4, it was tested if cichlids could correctly identify the training stimulus when stimuli were shown walking backwards instead of forwards. Like rats (MacKinnon et al. 2010), cichlids were not able to perform this transfer. Later tests (E5–E8) suggest that at least one of the factors cichlids learned to distinguish stimuli based on was their characteristic movement, as fish were able to identify training stimuli when shown as PDs. This may help to explain why backwards (and inverted)-walking humans were not recognized anymore; the direction of movement had changed. While the alternative stimulus was now walking in the “correct” direction, movement of individual dots varied from the original training stimulus though, yielding fish to not prefer the alternative but choose according to chance level instead.
Discrimination of organisms
In the second part of the experiments, cichlids were successively trained to distinguish between four pairs of organisms. All fish reached the learning criterion in all four experiments, irrespective of an aquatic, an aerial, or a terrestrial nature of the displayed organisms, and group performance in transfer tests strongly indicates that cichlids memorized each stimulus based on several features and properties (including movement) as opposed to single figural elements. As a group, cichlids excelled in all transfer tests, recognizing previously rewarded training stimuli when size or color (only E5) was altered, shown from a new perspective or in the form of a point-light display. Looking at individual results (Table 2) gives very similar results. Even when individuals chose according to chance (< 15 times out of 20), they still chose the “correct” stimulus more often than the alternative one in all but two cases. Most mistakes were made in transfer tasks in which the eagle* was enlarged, trout* and human* were size reduced, and human* and dog were shown from a new perspective or in which eagle* and bat were shown in the form of a PD. Judging from these results, there was no trend as to one type of transfer task being more challenging for fish than another or regarding the identification of terrestrial organisms being harder than that of aquatic ones (Table 2). While cichlids in a previous study were unable to recognize (the same) videos of eel* and trout when shown as PDs (Schluessel et al. 2015), four out of seven cichlids in the current study excelled at this task while another three chose according to chance level; however, they still chose the eel* most often. This shows that while negative results are important and should be reported, (over-)interpretation of null results should be considered with caution as intraspecific differences as well as training level may influence ability and performance of animals quite significantly. The performance of cichlids in E7 and E8 transfer tests, in which organisms were shown as PDs, was especially impressive, as organisms and their respective movements looked very similar, sometimes even making recognition hard for the experimenter.
In a comparative study (Fuss et al. 2017), bamboo sharks were presented with the same videos of organisms but performance differed quite dramatically from the cichlids, with sharks performing poorly in almost all transfer tests with the exception of those in the eel* and trout experiment. Few individuals exceeded group performance and ironically bamboo sharks did specifically poorly in the dolphin* vs. shark experiment. While potentially confounding factors were considered that could have negatively influenced the results, no actual cause was identified and no other comparable study exists on sharks to elucidate the data further.
Cichlids had already shown in another study (Schluessel et al. 2014b) that three-dimensional objects were correctly identified when rotated in different planes, indicating an ability of Pseudotropheus for form constancy. As expected, cichlids in the present study did extremely well recognizing organisms when shown from different perspectives, yielding significant group results in all four experiments and individuals achieving significant results in 21 out of 28 tasks.
Looking at the session numbers fish needed to reach each learning criterion throughout all tasks, intraspecific variation as well as general trends are observable. As in all previously mentioned studies on cognitive abilities of cichlids and other fish, individuals varied greatly in their ability to solve two alternative forced choice tasks, with fish ranging between 3 (minimum) and 30 (maximum) sessions to reach the learning criterion. Usually, the session number decreases after the first experiment within a series, as fish know by then how to proceed and what to expect. This was observed in the second part of the experiment, were fish needed on average 13 sessions to distinguish between the first organismic pair eel and trout, followed by only seven sessions for the second, six for the third, and three for the fourth (dolphin vs. shark) experiment. An alternative, but less likely, interpretation is that cichlids learned more easily to distinguish between organisms shown from the front (E7 and E8) than from top (E5) or from the side (E6). Additionally, the difficulty of the task influences the session number. For example, in the first task in which dot patterns instead of single dots had to be distinguished (E3, random vs. biological), the session number almost doubled to 23, from 10 in the first (E1) and 14 in the second experiment. In the subsequent experiment (E4), it decreased again to 13.
It is difficult to speculate on the ecological significance of the stimuli used, but if there is any, it is likely to be low. However, as explained previously, it was not important to use ecologically meaningful stimuli for the outcome of this study, as we did not test for a certain behavior in a natural setting but for a generalized application of a cognitive ability instead. Judging from the results, it seems reasonable to assume that ecologically better-suited stimuli would have resulted in an even better performance of fish. There is no reason to presume that unnatural stimuli would elicit a stronger reaction than meaningful ones.
Functional significance
Cognitive abilities underlie natural selection, as do any other behavioral, physiological, or morphological trait. While competing with energy demands for other vital systems such as reproduction and foraging (Striedter 2005), cognition is still a significant driver of fitness, enabling individuals to respond appropriately to threats and challenges as well as to make situation-dependent decisions on a wide variety of topics. Being able to not only perceive and detect motion but to assess it cognitively on a higher level seems advantageous to just about any individual subjected to pressure from predators or living in social groups or communities. It may enable individuals to quickly identify approaching animals and classify them according to their movement pattern, possibly reducing potentially dangerous encounters or situations under conditions where other visual cues (such as body coloration for example) may be harder to make out, e.g. at low light levels. Unfortunately, results of this study do not provide any information about a potential fitness gain for Pseudotropheus by possessing cognitive abilities such as the ones studied here. African cichlids, such as Pseudotropheus, provide the most diverse extant animal radiations (Seehausen 2006) featuring a unique system of animals to study cognition, potential ecological drivers, and fitness tradeoffs, while controlling for phylogeny and lifestyle. While the current study only focused on the presence and the extent of cognitive skills in an individual species in regard to a very specific task, future studies should assess ecological and phylogenetic relationships in more detail. One way could be to assess cognitive abilities and brain size in cichlids with different environmental demands comparatively. Studies across several avian and mammalian species have shown that overall brain size and cognitive abilities usually correlate positively (e.g., Reader and Laland 2002; Sol et al. 2005; Deaner et al. 2007) and that closely related species occupying different niches may feature distinct differences in the size of certain brain areas, related to differences in cognitive abilities (Tebbich and Bshary 2004). Even within species, such as guppies, where individual selection lines were bred for larger brain size, cognitive differences were observed (Kotrschal et al. 2013a, b, 2014). In cases of known ecological demands, phylogenetic signals as well as cognitive abilities and brain anatomy, one could discuss the potential fitness gain to be obtained for individuals of a particular species by possessing a specific ability in much greater detail than under current conditions.
In conclusion, cichlids can learn to distinguish between movements in different directions (horizontal vs. vertical; diagonal vs. diagonal), biological vs. random or scrambled motion, and horizontal mirror-image movements. While stimulus alterations do not significantly affect performance, inverted or backwards-moving stimuli are not recognized. Finally, cichlids can successfully recognize familiar organisms based on their movement alone and when shown from new perspectives.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abaid N, Spinello C, Laut J, Porfiri M (2012) Zebrafish (Danio rerio) responds to images animated by mathematical models of animal grouping. Behav Brain Res 232:406–410
Agrillo C, Miletto Petrazzini ME, Bisazza A (2014) At the root of math: numerical abilities in fish. In: Geary DC, Berch DB, Mann Koepke K (eds) Evolutionary origins and early development of basic number processing. Academic Press, London, pp 3–27
Baldauf SA, Kullmann H, Thünken T, Winter S, Bakker TCM (2009) Computer animation as a tool to study preferences in the cichlid Pelviachromis teaniatus. J Fish Biol 75:738–746
Beardsworth T, Buckner T (1981) The ability to recognize oneself from a video recording of one’s movements without seeing one’s body. Bull Psychon Soc 18:19–22
Blake R (1993) Cats perceive biological motion. Psychol Sci 4:54–57
Brown J, Kaplan G, Rogers LJ, Vallortigara G (2010) Perception of biological motion in common marmosets (Callithrix jacchus): by females only. Anim Cogn 13:555–564
Brown C, Laland K, Krause J (2011) Fish cognition and behavior, 2nd edn. Wiley-Blackwell, Cambridge
Chang DHF, Troje NF (2008) Perception of animacy and direction from local biological motion signals. J Vis 8:3–310
Clark DT (1981) Visual responses in developing zebrafish. University of Oregon Press, Eugene
Cutting JE, Kozlowski LT (1977) Recognizing friends by their walk: gait perception without familiarity cues. Bull Psychon Soc 9:353–356
Darmaillacq AS, Dickel L, Rahmani N, Shashar N (2011) Do reef fish, Variola louti and Scarus niger, perform amodal completion? Evidence from a field study. J Comp Psychol 125:273–277
Deaner RO, Isler K, Burkart J, van Schaik C (2007) Overall brain size, and not encephalization quotient, best predicts cognitive ability across non-human primates. Brain Behav Evol 70:115–124
Dittrich WH, Lea SEG (1993) Motion as a natural category for pigeons—generalization and a feature-positive effect. J Exp Anal Behav 59:115–129
Dittrich WH, Lea SEG, Barrett J, Gurr PR (1998) Categorization of natural movements by pigeons: visual concept discrimination and biological motion. J Exp Anal Behav 70:281–299
Douglas RH, Eva J, Guttridge N (1988) Size constancy in goldfish (Carassius auratus). Behav Brain Res 30: 37–42
Foley AG, Gannon S, Rombach-Mullan N, Prendergast A, Barry C, Cassidy AW, Regan CM (2012) Class I histone deacetylase inhibition ameliorates social cognition and cell adhesion molecule plasticity deficits in a rodent model of autism spectrum disorder. Neuropharmacology 63:750–760
Fox R, McDaniel C (1982) The perception of biological motion by human infants. Science 218: 486–487
Frech B, Vogtsberger M, Neumeyer C (2012) Visual discrimination of objects differing in spatial depth by goldfish. J Comp Physiol A 198:53–60
Fuss T, Russnak V, Stehr K, Schluessel V (2017) World in motion: perception and discrimination of movement in grey bamboo sharks (Chiloscyllium griseum). Anim Behav Cogn 4:223–241
Gerlai R, Fernandes Y, Pereira T (2009) Zebrafish (Danio rerio) responds to the animated image of a predator: towards the development of an automated aversive task. Behav Brain Res 201:318–324
Gierszewski S, Bleckmann H, Schluessel V (2013) Cognitive abilities in Malawi cichlids (Pseudotropheus sp.): matching-to-sample and image/mirror-image discriminations. PLoS One 8:e57363
Gori S, Agrillo C, Dadda M, Bisazza A (2014) Do fish perceive illusory motion? Sci Rep 4:6443
Herman LM, Morrel-Samuels P, Pack AA (1990) Bottlenosed dolphin and human recognition of veridical and degraded video displays of an artificial gestural language. J Exp Psychol Gen 119:215–230
Herter K (1953) Die Fischdressuren and ihre sinnesphysiologischen Grundlagen. Berlin: Akademie
Hirai M, Chang DHF, Saunders DR, Troje NF (2011a) Body configuration modulates the usage of local cues to direction in biological-motion perception. Psychol Sci 22:1543–1549
Hirai M, Saunders DR, Troje NF (2011b) Allocation of attention to biological motion: local motion dominates global shape. J Vis 11:4
Johannson G (1973) Visual perception of biological motion and a model for its analysis. Percept Psychophys 14:201–211
Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Brannstrom I (2013a) Artificial selection on relative brain size in the guppy reveals costs and benefits of evolving a larger brain. Curr Biol 23:168–171
Kotrschal A, Rogell B, Bundsen A, Svensson B, Zajitschek S, Immler S (2013b) The benefit of evolving a larger brain: big-brained guppies perform better in a cognitive task. Anim Behav 86:e4–e6
Kotrschal A, Corral Lopez A, Amcoff M, Kolm N (2014) A larger brain confers a benefit in a spatial mate search learning task in male guppies. Behav Ecol 26:527–532
Kozlowski LT, Cutting JE (1977) Recognizing the sex of a walker from a dynamic point light display. Percept Psychophys 21:575–580
MacKinnon LM, Troje NF, Dringenberg HC (2010) Do rats (Rattus norvegicus) perceive biological motion? Exp Brain Res 205:571–576
Mather G, Murdoch L (1994) Gender discrimination in biological motion displays based on dynamic cues. Proc R Soc Lond B 258:273–279
Mehlis M, Thünken T, Bakker TCM, Frommen JG (2015) Quantification acuity in spontaneous shoaling decisions of three-spined sticklebacks. Anim Cogn 18:1125–1131
Nakayasu T, Watanabe E (2014) Biological motion stimuli are attractive to medaka fish. Anim Cogn 17:559–575
Neri P (2012) Feature binding in zebrafish. Anim Behav 84:485–493
Newport C, Wallis G, Temple SE, Siebeck UE (2013) Complex, context-dependent decision strategies of archerfish, Toxotes chatareus. Anim Behav 86:1265–1274
Orger MB, Smear MC, Anstis SM, Baier H (2000) Perception of Fourier and non-Fourier motion by larval zebrafish. Nat Neurosci 3:1128–1133
Ortega JL, Stoppa K, Güntürkün O, Troje NF (2009) Vision during head bobbing: are pigeons capable of shape discrimination during the thrust phase? Exp Brain Res 199:313–321
Parron C, Deruelle C, Fagot J (2007) Processing of biological motion point-light displays by baboons (Papio papio). J Exp Psychol Anim Behav Process 33:381–391
Pavlova M, Krähgeloh-Mann I, Birbaumer N, Sokolov A (2002) Biological motion shown backwards: the apparent-facing effect. Perception 31:435–443
Reader SM, Laland KN (2002) Social intelligence, innovation, and enhanced brain size in primates. Proc Natl Acad Sci USA 99:4436–4441
Regolin L, Tommasi L, Vallortigara G (2000) Visual perception of biological motion in newly hatched chicks as revealed by an imprinting procedure. Anim Cogn 3:53–60
Schaller A (1926) Sinnesphysiologische und Psychologische Untersuchungen an Wasserkäfern und Fischen. Z Vgl Physiol 4:370–464
Schiemenz F (1924) Über den Farbsinn der Fische. Z Vgl Physiol 1:175–220
Schluessel V (2015) Who would have thought that ‘Jaws’ also has brains? Cognitive functions in elasmobranchs. Anim Cogn 18:19–37
Schluessel V, Fricke G, Bleckmann H (2012) Visual discrimination and object categorization in the cichlid Pseudotropheus sp. Anim Cogn 15:525–537
Schluessel V, Beil O, Weber T, Bleckmann H (2014a) Symmetry perception in sharks (Chiloscyllium griseum) and cichlids (Pseudotropheus sp.) Anim Cogn 17:1187–1205
Schluessel V, Kraniotakes H, Bleckmann H (2014b) Visual discrimination of rotated 3D objects in Malawi cichlids (Pseudotropheus sp.): a first indication for form constancy in fishes. Anim Cogn 17:359–371
Schluessel V, Kortekamp N, Ortiz Cortes J, Klein A, Bleckmann H (2015) Perception and discrimination of movement and biological motion patterns in fish. Anim Cogn 18:1077–1091
Schuster S, Rossel S, Schmidtmann A, Jäger I, Poralla J (2004) Archerfish learn to compensate for complex optical distortions to determine the absolute size of their aerial prey. Curr Biol 14:1565–1568
Seehausen O (2006) African cichlid fish: a model system in adaptive radiation research. Proc R Soc Lond B 273:1987–1998
Shashar N, Rosenthal GG, Caras T, Manor S, Katzir G (2005) Species recognition in the blackbordered damselfish Dascyllus marginatus (Rüppell): an evaluation of computer-animated playback techniques. J Exp Mar Biol Ecol 318:111–118
Siegel RM, Andersen RA (1988) Perception of the three-dimensional structure from motion in monkey and man. Nature 331:259–261
Simion F, Regolin L, Bulf H (2008) A predisposition for biological motion in the newborn baby. Proc Natl Acad Sci USA 105:809–813
Sol D, Duncan RP, Blackburn TM, Cassey P, Lefebvre L (2005) Big brains, enhanced cognition, and response of birds to novel environments. Proc Natl Acad Sci USA 102:5460–5465
Sovrano VA, Bisazza A (2008) Recognition of partly occluded objects by fish. Anim Cogn 11:161–166
Striedter GF (2005) Principles of brain evolution. Sinauer Associates, Sunderland
Sumi S (1984) Upside-down presentation of the Johansson moving light-spot pattern. Perception 13:283–286
Tebbich S, Bshary R (2004) Cognitive abilities related to tool use in the woodpecker finch, Cactospiza pallida. Anim Behav 67:689–697
Thompson B, Hansen BC, Hess RF, Troje NF (2007) Peripheral vision: good for biological motion, bad for signal noise segregation? J Vis 7:12–12.7
Tomonaga M (2001) Visual search for biological motion patterns in chimpanzees (Pan troglodytes). Psychologia 44:46–59
Troje NK (2008) Retrieving information from human movement patterns. In: Shipley TF, Zacks JM (Eds.) Understanding Events: How Humans See, Represent, and Act on Events. Oxford University Press, Oxford pp 308–334
Troje NF (2002) Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. Journal of Vision, 2:371–387
Troje NF, Aust U (2013) What do you mean with “direction”? Local and global cues to biological motion perception in pigeons. Vis Res 79:47–55
Troje NF, Chang DHF (2013) Shape-independent processes in biological motion perception. In: Johnson KL, Shiffrar M (eds) People watching: social, perceptual, and neurophysiological studies of body perception. Oxford University Press, Oxford, pp 82–100
Troje NF, Westhoff C (2006) Inversion effect in biological motion perception: evidence for a “life detector”? Curr Biol 16:821–824
Turnell ER, Mann KD Rosenthal GG, Gerlach G (2003) Mate choice in zebrafish (Danio rerio) analyzed with video-stimulus techniques. Biol Bull 205:225–226
Vallortigara G, Regolin L (2006) Gravity bias in the interpretation of biological motion by inexperienced chicks. Curr Biol 16:R279–R280
Vallortigara G, Regolin L, Marconato F (2005) Visually inexperienced chicks exhibit spontaneous preference for biological motion patterns. PLoS Biol 3:e208
Vangeneugden J, Vancleef K, Jaeggli T, Van Gool L, Vogels R (2010) Discrimination of locomotion direction in impoverished displays of walkers by macaque monkeys. J Vis 10:22
Wyzisk K, Neumeyer C (2007) Perception of illusionary surfaces and contours in goldfish. Vis Neurosci 24:291–298
Acknowledgments
We would like to thank Timo Thuenken for help with the statistical analysis, Sven Kappmeyer for help generating the videos and point displays used in E6 and E7, Adrian Klein for designing the stimuli used in experiments E1 and E2, and Slawa Braun for help with animal caretaking, maintenance, and repairs. We specifically thank N.F. Troje for allowing us to use the Program “Biomotionlab” and for helpful comments on the manuscript. We would also like to thank two anonymous reviewers for their constructive criticism of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
This article is a contribution to the Topical Collection From Sensory Perception to Behavior—Guest Editors: Theo C. M. Bakker, Horst Bleckmann, Joachim Mogdans, Vera Schluessel
The experimental work presented herein was conducted by these authors as part of their bachelor theses.
Electronic supplementary material
ESM 1
(MP4 7144 kb)
Rights and permissions
About this article
Cite this article
Schluessel, V., Hiller, J. & Krueger, M. Discrimination of movement and visual transfer abilities in cichlids (Pseudotropheus zebra). Behav Ecol Sociobiol 72, 61 (2018). https://doi.org/10.1007/s00265-018-2476-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-018-2476-8