Abstract
Many birds show a characteristic forward and backward head movement, while walking, running and sometimes during landing flight, called head bobbing. During the hold phase, the head of the bird remains stable in space, while during the thrust phase, the head is rapidly moved forward. Three main functions for head bobbing have been proposed: Head bobbing might have a biomechanical cause, it might serve depth perception via motion parallax, or it might be an optokinetic response that primarily serves image stabilization for improved vision during the hold phase. To investigate vision during the different phases and in particular to test for visual suppression during the saccadic thrust phase, we tested pigeons on a shape discrimination task, presenting the stimuli exclusively either in the hold phase, thrust phase or at random times. Results clearly demonstrate that shape discrimination is as good during the thrust phase as it is during the hold phase.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pigeons, chickens, moorhens, partridges, storks, crows, ibises and many other birds show a characteristic head movement while walking (Dunlap and Mowrer 1930; Friedman 1975a; Dagg 1977; Frost 1978; Davies and Green 1988; Cronin et al. 2005; Friedman 1975b). In pigeons, the head moves backward and forward with respect to the moving body with a frequency that ranges from about 2 to 10 Hz (Troje and Frost 2000). Head bobbing is characterized by a hold phase and a thrust phase. During the hold phase, the head of the bird remains stable in space (Frost 1978; Troje and Frost 2000), whereas during the thrust phase, the head is rapidly moved forward, thus catching up with the constantly moving body. In pigeons and chickens, head-bobbing movement has been observed during walking, landing after flight (Davies and Green 1991), prior to pecking (Goodale 1983) and when actively observing its environment (Dawkins 2002). In walking birds, head bobbing is synchronized with the motion of the feet (Dunlap and Mowrer 1930). The amplitude of one head-bobbing cycle is thus identical to the bird’s step length, and the head-bobbing frequency equals step frequency.
Head bobbing was first described in 1930 by Dunlap and Mowrer. Since then, three main functions have been proposed, one being primarily biomechanical, and two relating to vision: depth perception through motion parallax, and image stabilization. Dagg (1977) discusses the possibility that head bobbing is used to help shift the centre of gravity forward, thus serving a similar function as arm swing does during human walking. However, it soon became obvious that head bobbing was controlled visually. Dunlap and Mowrer (1930) had already noticed that blindfolded birds do not head bob. Friedman (1975a) showed that head bobbing can be elicited independently of active locomotion by optic flow. He also observed that birds walking in place on a treadmill did not show head bobbing. Another indication that head bobbing is under visual control is the fact that during the hold phase, the head is not completely stabilized, but slightly slips, thus providing the error signal required to stabilize the head (Frost 1978; Troje and Frost 2000).
Based on these observations, it was suggested that head bobbing is similar to other optokinetic behaviours and basically provides a mechanism to stabilize the retinal image (Frost 1978). Stabilizing the retinal image facilitates object recognition and may help the visual system to distinguish between self-induced visual motion and the motion of objects in the visual environment (Frost 1978; Davies and Green 1988; Troje and Frost 2000). An additional finding that also seems to support this view is that eye movements, at least in pigeons and chickens, seem to occur exclusively during the thrust phase (Pratt 1982; Wallman and Letelier 1993). Wohlschläger et al. (1993) observed some exceptions, but report that the occasional saccades during the hold phase were of very small amplitude compared to the ones observed during the thrust phases. Based on electrophysiological recordings in response to rapid image movements, which mimic retinal stimulation during saccadic eye movements, Brooks and Holden (1973) suggested saccadic suppression during eye movements in pigeons. Taken together, this evidence suggests that head bobbing serves image stabilization during the hold phase, while visual processing is suppressed during the thrust phase.
However, there are also other observations which do not fit this image very well. For instance, for running and landing pigeons, the hold phase is replaced with a flexion phase. As during a proper hold phase, the head is retracted relative to the body, but since the latter moves much faster, the retraction of the head can no longer compensate for the forward motion of the body, and the head and eyes are not stabilized (Green et al. 1994; Green and Cheng 1998). Depending on the situations and environmental demands, head bobbing may serve other functions in addition to image stabilization. Some authors suggested that head bobbing might help to monocularly derive depth information through motion parallax (Frost 1978; Pratt 1982; Green et al. 1994; Necker 2007). With the exception of predatory birds, most birds have laterally placed eyes with relatively small binocular fields (Wallman and Letelier 1993; Martin and Katzir 1999). Therefore, stereo vision as a cue for depth perception can play a role only in a small part of the visual field. Since interocular distance is generally rather small, stereo acuity is limited and unlikely to yield reliable distance measures beyond distances of a few metres.
Another argument against the idea of visual suppression during the thrust phase is based on the fact that the thrust phase takes up about half of the time of a walking bird (Wohlschläger et al. 1993; Troje and Frost 2000). It seems unreasonable to shut the visual system completely down for such a large proportion of the bird’s walking time on the ground.
In this study, we employed a paradigm we had developed for an earlier one (Jimenez Ortega et al. 2008) in which freely walking pigeons had to visually discriminate between two different geometric shapes, by pecking on one of two response keys. In the present study, we presented the stimuli either only during the hold phase, only during the thrust phase or at random times during the head-bobbing cycle. Our experiment was designed such that we could present the visual stimuli either in a lateral position where the motion of the walking pigeon would induce a large amount of retinal optic flow during the head thrust, or in a frontal position, where retinal optic flow would be much smaller. If we were assuming complete or partial visual suppression during the thrust phase, we should expect that: (a) the pigeons’ ability to solve the task is absent or at least substantially reduced during the thrust phase, and (b) if at all possible, visual pattern discrimination during the thrust phase should be easier in the frontal visual field as compared to the lateral visual field.
Methods
Subjects
Six rock pigeons (Columba livia), males and females, aged between 3 and 7 years obtained from the aviary of the Biopsychology Department of Ruhr-University in Bochum participated in the experiment. During the experiments, they were kept in individual cages on a 12-h light–dark cycle. They had ad libitum access to drinking water. Food was restricted to keep their weight at 85% of their free-feeding weight.
Material
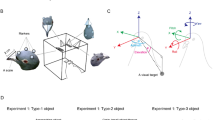
An experimental arena of 125-cm length and 54-cm width was constructed (Figs. 1, 2), with a food hopper and two pecking keys on either end. The two pecking keys (2.5 cm in diameter) were placed at each side of the feeder (2 cm in diameter). The lower edge of each key was 5 cm above the floor, and the two keys were spaced 23 cm apart. At one end of the arena (front end), either one or two 15″ LCD screens were mounted on a track surrounding the arena, which permitted an easy displacement of the screens around it. On the other end (back end) of the arena, a light barrier was installed consisting of an LED light and a photo cell set up to detect the bird walking between the two feeders. The arena was placed in a symmetric experimental chamber of 190-cm length, 100-cm width and 80-cm height. On one end of the experimental chamber, a video camera was placed to monitor the pigeons’ behaviour in the experiment.
Scaled drawing of the experimental arena. The lateral screen was located at 31 cm, perpendicular to the frontal screen. The bold line indicates the position of the light barrier close to the back end of the arena. Two high-speed cameras were located at the backside of the arena to capture the motion of the pigeons’ head movements
A single spherical marker (diameter 15 mm) covered with retro-reflective material was mounted to the pigeon’s head. Its movements were tracked by means of an optical motion capture system consisting of two high-speed CMOS cameras (MV-D752-80 from Photon Focus, 120 frames/s, 600 × 440 pixels), each equipped with an array of 30 ultra bright red LEDs, which flashed in synchrony with the camera shutters, and two frame grabber cards (Data Translation DT 3145) for image digitization. The system provided the three-dimensional position of the marker in real time at a sampling rate of 120 Hz and with a spatial resolution in the sub-millimetre range. Marker locations were stored in a computer that also controlled the stimulus presentation on the screen, delivered food as a reward or emitted a sharp sound as punishment, as required for the operant conditioning procedure.
Stimuli
The pigeons’ task was to discriminate between two 5 × 5 cm shapes: “stimulus A” and “stimulus B” (Fig. 3), by pecking once on one of the two keys. Individual stimuli were presented on a single LCD screen that was initially located in a central position right behind the box that contained a food hopper and two pecking keys at the front end of the arena. Later, the screen was moved into a lateral position (Fig. 1). The centre of the stimulus was located in the middle of the screen, 16 cm above the floor level.
During training, the stimuli were displayed continuously on the screen. During the catch trials, however, which were introduced in the subsequent test phase, stimuli were displayed only during the hold phase, only during the thrust phase or at random times.
Careful analysis of the recorded head movements during pilot trials showed that the hold and thrust phase of the head-bobbing cycle could be safely identified by assuming that the velocity of the head was less than 240 mm s−1 during the hold phase, and faster than 480 mm s−1 during the thrust phase. Therefore, a stimulus presentation during the hold phase was initiated when the speed of the head was less than 240 mm s−1. Stimulus presentation during the thrust phase was triggered when the speed of the head was larger than 480 mm s−1. For the random catch trials, stimuli were randomly presented according to two distributions of the hold and thrust phase duration, each parameterized by its mean and standard deviation. The distributions were calculated based on a total amount of 1,907 head-bobbing cycles captured from ten sessions for each pigeon. Duration of a hold phase was on average 91 ms (standard deviation 5.2 ms). The average duration of the thrust phase was 120 ms (standard deviation 4.3 ms).
The delay of the stimulus presentation in relation to the real-time motion capture system was measured to be in a range from 8 to 17 ms, mainly due to the refresh rate of the screen (120 Hz) and the temporal resolution of the motion capture system (120 Hz). Taking into account the hold and thrust phase durations and the system delay, we chose a stimulus duration of 66 ms, thus ensuring that stimulus presentation would never exceed the target phase (Fig. 4).
Samples of head motion data of a pigeon. The light grey bars represent the hold (a) and thrust (b) phases determined as described in the text. The patterned areas represent the 66 ms stimulus presentations. The upper panels show the raw displacement data as measured by the motion capture setup. The lower panel shows the velocity of the head over time derived from the displacement data as used to trigger stimulus presentation
Procedure
The animals were divided randomly into two groups of three pigeons each. One group of birds was trained to peck the right key when “stimulus A” was presented and the left key when “stimulus B” was presented. For the other group, this pattern was reversed.
After a peck on one of the two response keys, the screen went blank. In order to initiate a new trial, the bird had to walk first to the back end of the arena. The new stimulus was turned on when the pigeon passed the light barrier as it walked back to the front end where the pecking keys were. Training was conducted in four steps. In the first step, by alternately activating the two feeders at the front and back end of the arena, the pigeon learnt to walk back and forth between them to get food. In the second step, the birds learnt to peck on the keys at the front end of the arena to obtain food. After 2 s of food access in the front feeder, the pigeon walked to the feeder on the back end, where it received food for another 2 s without having to peck. During the third stage of training, the walking pigeon now had to peck on one of the keys at the front feeder, contingent on the shape presented on a screen also located at the front end of the arena (Fig. 5). A correct response was rewarded with 2 s of food access. An incorrect response triggered a loud, high-pitched noise, which lasted for 2 s. Finally, pigeons were also trained to respond to the stimulus presented in the lateral visual field (at 90º with respect to the frontal screen) by gradually moving the screen in ten consecutive steps from the frontal to the right lateral position. Once they had learnt to discriminate between stimuli in that position, we added a second screen to the left lateral position showing the same stimulus as on the right lateral screen.
Training sessions consisted of 96 trials per day divided into four consecutive sub-sessions with 24 trials each. Pigeons proceeded to the testing phase if they scored more than 70% correct responses in three consecutive sessions.
The testing sessions were identical to the training sessions, except that in each sub-session, 6 trials, randomly chosen out of the 24 test trials, were replaced with non-reinforced catch trials. In two of the six catch trials in each sub-session, the stimulus was presented exclusively during the hold phase, in two catch trials the stimulus was presented only during the thrust phase and in the remaining two catch trials the stimulus was turned on and off randomly. The order of the six catch trials was randomized in each sub-session. In the remaining 18 trials, the stimulus was constantly visible and responses were reinforced as during training.
Testing was continued until a total of 90 catch trials were collected for each bird, first for lateral stimulus presentation and then for frontal stimulus presentation. Sessions during which a bird scored below 70% correct on the training trials were excluded from the analysis and later repeated.
Results
For the three different kinds of catch trials (hold, thrust, random) and the two different viewing conditions (frontal, lateral), we evaluated the percentage of correct responses. In addition, we quantified the kinematics of head-bobbing motion in terms of the duration of the head-bobbing cycle, the duration of the hold phase as a percentage of the total cycle duration, the amplitude of the head-bobbing cycle in mm, and the amount of slip during the hold phase as a percentage of that amplitude.
One of the pigeons showed very unstable behaviour when solving the task and we were not able to collect sufficient number of sessions with performances above the criterion. Therefore, data from this pigeon were not considered in the data analysis. All other birds learnt the task well and eventually satisfied the criterion.
Percentage of correct responses
When the stimuli were presented laterally, the average percentage of correct responses for the hold, thrust and random presentations were 80, 77 and 84%, respectively. Presenting the stimulus in the frontal visual field, the percentage of correct responses for the hold, thrust and random presentation was 73, 80 and 82%, respectively (Fig. 6). All these values differ significantly from chance level (p < 0.01 for all). A 3 × 2 repeated-measures ANOVA was calculated with factors presentation (hold, thrust, random) and position (lateral, frontal). Neither of the two main effects nor the interaction between them was significant (p > 0.3 for all).
Head-bobbing kinematics
The individual head-bobbing motion of each pigeon during hold, thrust and random trials were analysed for frontal and lateral visual field presentation.
The head trajectory was divided into phases according to the velocity of the head. A thrust phase was defined when head velocity was equal to or larger than 240 mm s−1 in three or more consecutive frames (25 ms). A hold phase occurred when the speed of the head was less than 240 mm s−1 in three or more consecutive frames. A hold phase was considered valid only if it was followed by a valid thrust phase. Likewise, a thrust phase was considered valid only if it was followed by a valid hold phase. If a head-bobbing cycle was not complete, for instance due to fast fluctuations in velocity or because of missing frames (e.g. marker occlusion by the pigeon body), it was excluded from data analysis. Hold or thrust phases longer than 32 frames (260 ms) were considered to be artefacts and were also excluded from the analysis.
For each pigeon, and for each of the 3 × 2 conditions, the amplitude and duration of the head-bobbing cycle and the overall contributions of the hold phase were averaged based on 200 randomly picked, valid head-bobbing cycles obtained from the 30 trials. That is, a total of 1,200 head-bobbing cycles were used to analyse head-bobbing kinematics for each pigeon.
Durations of hold and thrust phases
For lateral stimulus presentation, the average durations of a full head-bobbing cycle for the hold, thrust and random catch trials were 195.4, 194.3 and 197.1 ms, respectively, whereas for presentations in the frontal visual field the durations were 171.4, 173.7 and 169.4 ms, respectively (Fig. 7a). A 3 × 2 repeated-measures ANOVA revealed that the head-bobbing cycle duration was longer for lateral visual field presentation than for frontal visual field presentations (F(1,4) = 9.65, p = 0.03). Therefore, the head-bobbing frequency was significantly higher in the frontal visual field (5.8 Hz) than in the lateral visual field (5.1 Hz). The phase during which the stimulus was presented had no effect and there was no interaction between the two factors either.
During lateral stimulation, pigeons spent 47.7, 47.7 and 47.9% of the overall head-bobbing time in the hold phase, for the hold, thrust and random phase stimulation, respectively. During frontal visual field stimulation, the hold phase lasted 45.1, 45.4 and 44.7% of the total time for the hold, thrust and random phase stimulation, respectively. A 3 × 2 repeated-measures ANOVA revealed no significant differences for any factor and their interactions. The fact that the percentage of the hold phase decreased with the overall duration of the head-bobbing cycle reflects that the time of the thrust phase was only slightly shorter for frontal view presentations as compared to lateral view presentations. The difference in the overall duration is mainly due to shortened hold phases.
Because we felt that due to the low number of subjects we might have lost interesting information in the between-subjects analysis, we also ran individual 3 × 2 ANOVAs for each pigeon. These ANOVAs were based on 180 trials (30 in each experimental condition). Four of the five birds showed significantly longer head-bobbing cycles (F(1,174) > 12, p < 0.01) and also a relatively longer hold phase duration in the lateral visual field than in the frontal visual field (F(1,174) > 15, p < 0.01) (Fig. 8a).
Amplitudes of hold and thrust phases
During lateral visual field stimulation, the average amplitudes of the head-bobbing cycle for the hold, thrust, and random catch trials were 83.7, 82.2 and 84.41 mm, respectively. During frontal visual field stimulation, they were 77.5, 80.3 and 78.8 mm, respectively (Fig. 7b). The repeated-measures ANOVA revealed no effects of visual field (frontal vs. lateral), phase of stimulation (hold, thrust or random) or their interaction.
Individual analysis of the pigeons’ head-bobbing amplitude revealed that only one bird (pigeon 251) showed significant differences in visual field (F(1,174)) = 57.8, p < .001) (Fig. 8b). The amplitude of the head-bobbing cycle was larger when the stimulus was presented in the lateral visual field. For the same bird, we also observed a main effect of the presentation phase (F(2,174) = 6.5, p < .005). The amplitude was largest when the stimulus was presented in the hold phase, and shortest when presented during the thrust phase.
When stimuli were presented laterally, the hold phase contribution to the head-bobbing amplitude was 3.4, 3.1 and 3.0%, for the hold, thrust and random phase conditions, respectively. During frontal visual field stimulation, they were 2.8, 2.8 and 2.8%, respectively. Data analysis revealed no significant differences for any factor or their interactions. It should be mentioned here, however, that our distinction between hold and thrust phase was somewhat conservative on the side of the thrust phase. We wanted to make sure that the birds definitely did not get any visual stimulation during the hold phase in the condition in which we were trying to stimulate only during the head thrust. As a consequence, the hold phase as defined here may already contain some head motion. This would explain why in our data, the slip velocity (about 0.013 m s−1) is about twice as large as previously reported (Frost 1978; Troje and Frost 2000).
The data show that head-bobbing durations are a little longer for lateral stimulus presentation as compared to frontal presentation. Head-bobbing amplitudes, on the other hand, show basically no effects. As a consequence, it seems that the difference in the kinematics between the two viewing conditions is due to different walking speeds. We tested this explicitly. The average walking speed was 0.44 m s−1 during lateral stimulus presentation and 0.48 m s−1 during frontal stimulus presentation. The difference was not significant when testing across birds. However, in two out of the five birds this differences was quite large and statistically significant (bird 051: F(1,174) = 28.3, p < 0.001; bird 347: F(1,174) = 15.5, p < 0.001, see Fig. 8c).
In summary, pigeons are well able to solve the pattern discrimination task even when the stimuli are shown either only during the hold or only during the thrust phase. Performance in both cases is about the same and also equal to the case in which stimuli are turned on and off at random times. We find a small effect of the location of presentation on the duration of the head-bobbing cycle that is slightly longer when the stimuli are presented laterally. Since head-bobbing amplitudes are less affected, and since head-bobbing amplitudes are identical with step amplitude, this difference basically translates into a difference in walking speed. At least some of the pigeons are a little slower, when the stimulus is presented laterally as compared to frontal stimulus presentation.
Discussion
During the hold phase of the head-bobbing cycle, the head is approximately stabilized in space (Frost 1978; Davies and Green 1988; Troje and Frost 2000; Friedman 1975b) and eye movements have rarely been observed (Pratt 1982; Bloch et al. 1984; Wohlschläger et al. 1993). It is generally believed that head bobbing is primarily an optokinetic response, which aids in pattern recognition by stabilizing the retinal image during the hold phase (Frost 1978; Davies and Green 1988). In accordance with this view, it has been proposed that during the thrust phase there is probably no visual input and the sensitivity of the visual system is reduced by saccadic suppression (Brooks and Holden 1973; Pratt 1982). Therefore, we expected reduced performance in the discrimination task when the stimulus presentation occurred during the thrust phase as compared to stimulation during the hold phase or when they were presented at random times. Surprisingly, we did not find such differences. Contrary to our initial predictions, the animals were able to discriminate between the stimuli when they were presented during the thrust phase as successfully as when they were presented during the hold phase, or when presented at random times. The amount of retinal optic flow during the thrust phase is much larger when the stimulus is located in the lateral visual field as compared to frontal view presentation. Therefore, for thrust phase presentations, we had also expected relatively better performance when the stimulus was shown frontally rather than laterally. However, no such differences were found. We took great care to strictly control the timing of the stimulus presentation to make sure that it could only be seen during one phase. Particularly, we were conservative in the definition of the thrust phase and presented stimuli only for a fraction of the thrust phase to exclude any contamination. Therefore, the data clearly show that pigeons are capable of shape discrimination during the trust phase.
The generally good performance in all conditions in the present experiment stands in contrast to results from other experiments in which we had used a similar task (Jimenez Ortega et al. 2008). In these experiments, walking pigeons learnt to discriminate between the same geometric shapes, which, however, were shown continuously. The results showed that pigeons could very well learn that task and eventually scored even a little higher than in the present experiment (80–100% correct during continuous presentation), but that they had substantial difficulties generalizing it to new stimulus positions. Moving the stimulus within the same lateral field of view caused significant performance drops, and switching presentation from one lateral field to the contralateral visual field reduced discrimination performance to chance level in most of the birds.
Geometric pattern recognition is an easy task for pigeon and, under normal skinner box conditions, they can reach performances close to 100% (e.g. Hodos and Karten 1966). Even some of the walking birds in Jimenez Ortega et al. (2008) previous work were able to reach performances of between 90 and 100% correct responses. We assume that the somewhat lower performance rates observed in the present experiment were due to the intermittent nature of the flickering stimulus. The important finding is that the performance rate during the thrust phase presentations was not lower than the one during the hold phase presentations. While this is strong evidence against the occurrence of suppression of visual processing during head thrusts, our experiments cannot entirely exclude that some forms of suppression may affect more subtle visual discrimination tasks.
Our results also cannot exclude the possibility of saccadic suppression during eye movements. Not all head thrusts are accompanied by eye movements and, if they are, the eye movements may not last for the whole duration of the head saccade (Pratt 1982; Wohlschläger et al. 1993). Occasional head thrusts without eye movement or even the short periods before and after eye movements may provide enough time to discriminate between the two shapes. The large size of the patterns, the strong contrast that defined them and the fact that we picked two very different shapes may also have masked poorer visual processing during the thrust phase.
The analysis of the kinematics of the pigeons’ head movements revealed differences in the motion pattern between the frontal and the lateral stimulus presentation. Pigeons moved generally a little slower when they had to attend to a visual stimulus in lateral presentation. In the lateral field of view, optic flow is much more pronounced than in the frontal view. Retinal image motion (or alternatively, compensating eye movements) is much larger as the head moves forward. The somewhat slower pace and the longer head-bobbing cycle time may be an attempt to reduce motion blur during the thrust phase. Interestingly, one bird (251) seemed to compensate the lower step frequency during lateral stimulus presentation with an increased head-bobbing amplitude, thus allowing longer thrust and hold phases, without having to reduce the overall speed. Similar behaviour has been observed in chickens. When confronted with new objects, they tend to walk slower and also perform larger head movements (Dawkins 2002).
The analysis of individual birds also showed that during lateral stimulus presentation, the fraction of the head-bobbing cycle taken by the hold phase was a little larger. While the percentage of correct responses was similar for lateral and frontal stimulation, this may indicate that visual processing in the lateral field of view is somewhat more difficult and takes more time. One might expect that the additional time spent in the hold phase should be observed only when the stimulus was also presented during the hold phase. However, the observed effects were generally very small and the expected interaction may have escaped detection due to a lack of statistical power.
The question of why some birds bob their head remains unresolved. The fact that birds see reasonably well during the thrust phase challenges the view that the main goal of head bobbing is to maintain a stable fixation of head and eye for better object recognition. Other observations also seem to contradict the optokinetic explanation of head bobbing: Obviously, pigeons have reasonable vision during flight, that is, at speeds between 15 and 25 m s−1, which is much larger than the 0.5–1 m s−1 experienced during walking. Many other bird species, such as gulls, ducks and many more, demonstrate that life without saccadic head bobbing is not a problem. It seems like we still lack a convincing explanation for the phenomenon of avian head bobbing.
References
Bloch S, Rivaud S, Martinoya C (1984) Comparing frontal and lateral viewing in the pigeon. III. Different patterns of eye movements for binocular and monocular fixation. Behav Brain Res 13:173–182
Brooks B, Holden AL (1973) Suppression of visual signals by rapid image displacement in the pigeon retina: a possible mechanism for ‘saccadic suppression’. Vision Res 9:1247–1258
Cronin TW, Kinloch MR, Olsen GH (2005) Head-bobbing behavior in foraging whooping cranes favors visual fixation. Curr Biol 15:R243–R244
Dagg AI (1977) Running, walking and jumping: the science of locomotion. Wykeham, London
Davies MNO, Green PR (1988) Head bobbing during walking, running and flying: relative motion perception in the pigeon. J Exp Biol 138:71–91
Davies MNO, Green PR (1991) The adaptability of visuomotor control in the pigeon during landing flight. Zool Jb Physiol 95:331–338
Dawkins MS (2002) What are birds looking at? Head movements and eye use in chickens. Anim Behav 63:991–998
Dunlap K, Mowrer OH (1930) Head movements and eye functions of birds. J Comp Psychol 11:99–113
Friedman MB (1975a) Visual control of head movements during avian locomotion. Nature 255:67–69
Friedman MB (1975b) How birds use their eyes. In: Wright P, Caryl PG, Wowles M (eds) Neural and endocrine aspect of behaviour in birds. Elsevier, Amsterdam, pp 182–204
Frost BJ (1978) The optokinetic basis of head bobbing in the pigeon. J Exp Biol 74:187–195
Goodale MA (1983) Visually guided pecking in the pigeon (Columba livia). Brain Behav Evol 22:22–41
Green PR, Cheng P (1998) Variation in kinematics and dynamics of the landing flights of pigeons on a novel perch. J Exp Biol 201:3309–3316
Green PR, Davies MNO, Thorpe PH (1994) Head bobbing and head orientation during landing flights of pigeons. J Comp Physiol 174:249–256
Hodos W, Karten HJ (1966) Brightness and pattern discrimination deficits in the pigeon after lesions of nucleus rotundus. Exp Brain Res 2:151–167
Jimenez Ortega L, Stoppa K, Güntürkün O, Troje NF (2008) Limits of intraocular and interocular transfer in pigeons. Behav Brain Res 193:69–78
Martin GR, Katzir G (1999) Visual fields in short-toed eagles, Circaetus gallicus (Accipitridae), and the function of binocularity in birds. Brain Behav Evol 53:55–66
Necker R (2007) Head bobbing of walking birds. J Comp Physiol A 193:1177–1183
Pratt DW (1982) Saccadic eye movements are coordinated with head movements in walking chickens. J Exp Biol 97:217–223
Troje NF, Frost BJ (2000) Head-bobbing in pigeons: how stable is the hold phase? J Exp Biol 203:935–940
Wallman J, Letelier J-C (1993) Eye movements, head movements, and gaze stabilization in birds. In: Zeigler HP, Bischof H-J (eds) Vision, brain, and behaviour in birds. The MIT Press, Cambridge, pp 245–263
Wohlschläger A, Jäger R, Delius J (1993) Head and eye movements in unrestrained pigeons (Columba livia). J Comp Psychol 107:313–319
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiménez Ortega, L., Stoppa, K., Güntürkün, O. et al. Vision during head bobbing: are pigeons capable of shape discrimination during the thrust phase?. Exp Brain Res 199, 313–321 (2009). https://doi.org/10.1007/s00221-009-1891-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1891-5