Abstract
Highly variable within and across species, patterns of sperm use not only are often driven by post-copulatory sexual selection but can also be impacted by experimental design. In investigations of paternity bias using competitive double matings, the inter-mating interval is a temporal factor that can affect sperm use patterns if the first male’s sperm is used or lost at an appreciable rate between matings or if its viability or relative competitiveness is influenced by the time since ejaculation. Rapid loss of first-male sperm within the female after mating has been established in the seed beetle (Callosobruchus maculatus), a model system in sperm competition studies. However, our understanding of sperm precedence in this species, which disproportionately favors the last (second) male to mate, is based on long inter-mating intervals. Here, I determine the effect of a shortened inter-mating interval on second-male paternity (P 2) and, importantly, the extent to which females in this species are willing to remate immediately. I find that P 2 is significantly reduced when females remate immediately than when they remate 24 or 48 h after the first mating and that immediate remating is common, indicating that there is a substantial potential for female remating decisions to influence the intensity of sperm competition within species. To understand the variation in inter-mating intervals from a female perspective, I further identify key differences between females that did and did not remate at three inter-mating intervals (0, 24, and 48 h after the initial mating) and discuss potential mechanisms for the observed variation in female refractoriness.
Significance statement
Females often mate with multiple males, and in insects it is common for the last male to mate to obtain the greatest share of paternity. One possible explanation for this bias towards last-male sperm use is the passive loss of sperm from the first male with time since mating. Here, I examine the effect of different intervals between matings in a seed beetle species to determine if first-male paternity is increased when females remate sooner. My results show that first males do achieve higher paternity when females remate sooner, which is more common than expected in this species. I also show the differences between females that do and do not remate, which can help explain potential causes for variation in female remating behavior. Together, these results show the significant impact that female remating decisions can have on male competition and, ultimately, their reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Female multi-male mating within a single reproductive cycle is the rule, not the exception, across animal taxa (Walker 1980; Thornhill and Alcock 1983; Eberhard 1996). A commonly observed outcome of this female behavior in species with internal fertilization is sperm precedence, the phenomenon of non-random fertilization success among consecutive mates (Lewis and Austad 1990; Simmons 2001). Such fertilization bias may be examined within a particular species by observing the average proportion of eggs fertilized by the last (second) male to mate in competitive double matings, denoted as P 2 (Boorman and Parker 1976). Previous empirical studies have not only documented species averages for P 2 but have also revealed a high degree of variation in these values both within and across species, with sperm precedence patterns either apportioned equally according to numerical sperm representation among the males (but see Manier et al. 2013) or disproportionately favoring either the first or, more commonly among insects, the last male to mate (Parker 1970a; Lewis and Austad 1990).
The variation in sperm use patterns has been attributed to a number of different factors (Table 1), but non-mutually exclusive sources for this variation are thought to have arisen via an arms race in which female and male interests are expressed via post-copulatory inter- and intra-sexual selection. In the former case, runaway processes such as those involved in cryptic female choice for the preferred male’s sperm or stimulating genital morphologies are thought to be important (Eberhard 1996); in the latter case, male traits and/or behaviors that function in offense or defense of sperm competition are thought to be important (Parker 1970a; Simmons 2001). While these two post-copulatory mechanisms and their relative importance in driving sperm precedence patterns are difficult to disentangle and are likely species-specific, studies attempting to elucidate these causes can be further complicated by factors in experimental design that also lead to intra-specific variation in sperm use patterns (Danielsson 1998).
In investigations of paternity bias using competitive double matings, the inter-mating interval is a temporal factor that has been demonstrated to affect sperm precedence (Birkhead and Møller 1992). While most post-copulatory sexual selection studies control for the inter-mating interval, studies in which two distinct intervals were imposed or the interval was allowed to vary naturally based on female remating decisions have exposed changes in sperm precedence patterns with varying intervals between matings (Dickinson 1988; Yamagishi et al. 1992; Arnaud et al. 2001; Drnevich 2003; Blyth and Gilburn 2005). Whereas some studies have revealed a reduction in P 2 with increased time between matings (Radwan 1997), many have revealed an increase in P 2 with an increase in the inter-mating interval (reviewed in Simmons 2001). Results from these studies illustrate that the inter-mating interval is a critical component of paternity outcomes and that by controlling it to draw conclusions about species-specific sperm precedence patterns, we may be masking important variations in P 2 (Eberhard 1996). A more informative approach may be to highlight the variation in sperm precedence values that exists within species and across contexts, which may allow us to elucidate mechanisms for sperm precedence patterns in the process (Eberhard 1996; Cook et al. 1997).
The inter-mating interval is likely to be salient for species in which sperm viability declines rapidly after copulation or over time while in storage within the female, the relative competitiveness of males’ sperm is influenced by the time since ejaculation, or the first males’ sperm are used or lost at an appreciable rate between matings. Indeed, one proposed explanation for the observed bias favoring last-male sperm use is the “passive sperm loss” hypothesis, which posits that as the interval between matings increases, passive loss of the first male’s sperm from the female reproductive tract leads to a disproportionate use of the second male’s sperm (Lessells and Birkhead 1990; Birkhead and Biggins 1998). Thus, a prediction of this hypothesis is that P 2 should increase as the inter-mating interval is increased, as shown in numerous species (reviewed in Simmons 2001).
The seed beetle, Callosobruchus maculatus, is a widely used model system in sperm competition studies, and previous investigations of its sperm precedence patterns have revealed high (83%) last-male sperm precedence (Eady 1991). However, the conclusion of last-male sperm precedence and other conclusions about sperm competition and relative male fertilization success in this species are disproportionately based on inter-mating intervals of 24 or 48 h after the initial mating (Eady 1991, 1995; Eady et al. 2004; Hotzy and Arnqvist 2009). Previous empirical work in this species has revealed not only that sperm are rapidly reduced in both long-term and short-term sperm storage sites (i.e., the spermatheca and bursa copulatrix) within the female reproductive tract but also that this passive loss of first-male sperm occurs as early as 4 h after the initial mating (Eady 1994b). Thus, first-male sperm are likely to be so greatly reduced at longer inter-mating intervals that they are unable to numerically compete for fertilizations and, as is observed, second males’ sperm will be disproportionately favored. Whether this sperm precedence pattern is upheld when the time between matings is decreased below 24 h has never been established.
Here, I use the seed beetle to test an indirect prediction generated by the passive sperm loss hypothesis that last-male sperm precedence, measured as the proportion of offspring sired by the second male to mate (P 2), will be reduced with a shorter inter-mating interval. Using competitive double matings, I measured P 2 at three inter-mating intervals (0, 24, and 48 h after the initial mating) to examine how the timing of female remating influences sperm use patterns. Importantly, I ask how prevalent female remating behaviors are at a shorter inter-mating interval (i.e., when females remate immediately) in comparison to longer inter-mating intervals (i.e., when females wait 24 or 48 h to remate). Lastly, to better understand inter-mating intervals from a female perspective, I identify key differences between females that did and did not remate at each inter-mating interval to reveal potential mechanisms for variation in female remating decisions. If the inter-mating interval is a critical determinant of P 2 and there is a significant variation in female propensity to remate, then most studies of sperm competition in this model system have likely led to an overestimation of last-male sperm precedence and an underappreciation of the females’ role in governing sperm use patterns and, ultimately, sperm competition intensity. Furthermore, examining the effect and prevalence of a short inter-mating interval for the first time in a system with previously established last-male sperm precedence patterns can help us infer female decision rules about remating so that we may functionally interpret the adaptive evolution of male and female traits or behaviors involved in reproduction (Eberhard 1996).
Methods
Study system
All seed beetles used in this study originated from southern India and were provided by Dr. Frank Messina of Utah State University. Detailed descriptions of their culturing and maintenance of stock cultures and family lines may be found in the electronic supplementary material (“Methods” section). Briefly, all seed beetles used in this experiment were reared in a laboratory growth chamber at Cornell University under constant conditions of 26 ± 1 °C, 10–50% RH, and a 12:12 light/dark cycle. All matings in the study were conducted between May and November of 2014, resulting in the use of beetles across multiple generations. To ensure that relatives did not mate, matrilines were initiated by isolating randomly infested seeds from a large, outbred population. Virgin males and females reared from these seeds were randomly paired for mating and assigned a unique matriline. The offspring from these matings served as focal individuals in the present study, were reared individually from a single seed to ensure that they were virgins and, upon their adult eclosion, were provided a unique identification number (ID), which included their generation and matriline. Moreover, their egg lay date, eclosion date, death date, sex, and group ID (based on the time of the year in which the matings were conducted) were recorded.
Mating observations
All mating trials were performed in the afternoon and were staged within the females’ 35-mm Petri dish. Copulation in seed beetles begins with the male drumming the female’s back with his antennae prior to mounting her; once he has successfully inserted his aedeagus, he leans back and remains relatively motionless while he transfers his ejaculate. Sperm are transferred to the female via a spermatophore, which first gets deposited in the bursa copulatrix before the sperm migrate to and enter the spermatheca, which reaches capacity approximately 0.5 to 2 h after insemination (Eady 1994a, 1995). At some interval after the onset of copulation, the female begins kicking the male, at which point a struggle ensues until his aedeagus is successfully dislodged from her genitalia. Kicking latency was calculated as the time the male leaned back until the time the female began to kick, and kicking duration was calculated as the time the female started to kick until the pair was separated. Copulation duration was calculated as the time from when the male leaned back to when he removed his aedeagus (i.e., pair separation). Each of these mating behaviors was recorded within the nearest 10 s. All individuals were weighed immediately before and after mating using a Sartorious MP1601 microbalance to the nearest ±0.1 mg. Individuals were weighed twice and the resulting weights were averaged; values that differed by more than 0.1 mg were re-measured, and the average weight was taken using all three measurements. Male ejaculate size was calculated by subtracting his post-mating weight from his pre-mating weight.
Mating trial protocol
The mean (±SE) age of focal females was 2.7 ± 0.1 days, and the mean age of all focal males was 3.2 ± 0.1 days. Focal females (n = 115) were weighed, randomly paired with a virgin male, and continuously observed until they successfully mated. Females that failed to mate by 20 min after introduction were discarded from the study (n = 2, 1.7%). Females that did successfully mate were re-weighed and immediately provided the opportunity to mate with a new virgin male. Females that were unwilling to remate at this time point exhibited several resistance behaviors that prohibited successful copulation—they ran away, kicked, or moved their abdomen to make their genitalia inaccessible to approaching males. The proportion of females remating within 25 ± 10 min was recorded to measure receptivity. Females that did not remate within this time frame were separated from the male and provided a single seed for oviposition until the next mating opportunity. Because resource availability in this species has been shown to affect both female remating propensity and P 2 (Eady et al. 2004), females were given only a single seed to attempt to constrict their use of first-male sperm and minimize the number of eggs laid between matings. Further mating opportunities were provided to focal females 24 h after the initial mating and again 48 h after the initial mating. The same male was used in each subsequent mating trial to remove variation in female remating due to preferences for particular males. Females that failed to remate within the trial that occurred 24 h post mating were provided the same single seed for oviposition, while females that failed to remate within the trial that occurred 48 h of their first mating were recorded but excluded from paternity analyses since they only mated once (n = 44, 38.9% of all females that mated once in the trial). Hence, female remating propensity was allowed to vary in this experiment, and this variation corresponded to an inter-mating interval of 0, 24, or 48 h after the initial mating. Successfully double-mated females (n = 69) were transferred to a new Petri dish containing clean seeds as oviposition substrate every 24 h until their natural death. The seed quantities provided each day were changed mid-experiment (from 50, 25, 15, and 10 to 40, 20, 10, and 5 provided each day, respectively) but still exceeded average daily oviposition rates and did not affect the numbers of eggs laid by females. Female IDs were used so that eggs could be blindly counted and scored to determine sperm precedence without knowledge of the inter-mating interval or sterilization order.
Sperm precedence
The sterile male technique was used to assign paternity. Only one of the two males was randomly selected for sterilization by exposure to 70 Gy of gamma radiation from a cesium source at Cornell University. A balanced design was used to control for sterilization order; thus, approximately half of all matings were NR matings, in which the first male to mate was normal (N) and the second male to mate was sterile (R), and half were RN matings. Using the Boorman and Parker (1976) formula to correct for rates of hatch failure due to natural infertility and incomplete sterilization (see Electronic supplementary material, Table S1), P 2 was calculated for each focal female. The proportions obtained from this formula were multiplied by the number of eggs laid after the second mating and then rounded to a whole number to quantify the absolute number of eggs fertilized by each male sire. Females that laid an unusually low number of eggs (<10) after the second mating (n = 2) were removed from the analysis. Moreover, one case in which P 2 was 100% and eggs laid between matings could not be quantified was excluded from the paternity analysis to remove the possibility of an unsuccessful mating (e.g., no sperm transferred or male infertility in the first mating). Hence, sperm precedence was analyzed for 66 females in total.
Female remating propensity
To examine how prevalent female remating behaviors are at shorter compared to longer inter-mating intervals, I determined the proportion of females that successfully remated at each inter-mating interval.
Key differences between females that did and did not remate
To compare females that did and did not remate, all focal females were marked as either remated (“1”) or not (“0”) at each inter-mating interval. Females that did not remate at 0 h (n = 87) were given additional opportunities at 24 h or, if necessary (n = 62), 48 h after the initial mating. The ages and weights of focal females and second males were documented at the start of each interval since these varied with time. The amount of weight lost between matings was also calculated for females with an inter-mating interval >0 h.
Statistical analyses
All statistical analyses were conducted using R version 3.1.2 (R Development Core Team 2014). All means are presented as ±1 SE.
Sperm precedence
Last-male sperm precedence (P 2) was analyzed using a generalized linear mixed model (GLMM) using the glmer function with a logit link function from the “lme4” R package (Bates et al. 2014). The binomial response was the number of eggs fertilized by the second male, and the total number of eggs laid after the second mating was the binomial denominator (n = 66). In the initial statistical model, the residual deviance was observed to be larger than the residual degrees of freedom, which is an indication of overdispersion (Crawley 2013). An observation-level random effect (OLRE) was used as a random factor in all subsequent analyses to control for overdispersion (Harrison 2014). The experimental group and nested matrilines and patrilines within a generation number for all focal individuals were included as random factors in the initial model, but only those effects that contributed to residual variability were included in the final model. These random factors were then used in bivariate analyses for variables that could potentially explain paternity bias, including the females’ age and weight and the absolute differences between second and first male demographics and mating variables (age at time of mating, pre-mating weight, ejaculate size, kicking latency, kicking duration, and copulation duration). Predictors that had a p value of 0.20 or below were considered for the final model, and these predictors were screened for collinearity with other significant predictors and removed when collinearity was present so that only the one with greater relative significance was included in the final GLMM. Because the data used in this study came from an experiment meant to test a separate hypothesis (the sexy sperm hypothesis), the mothers of focal males presented here came from different mating backgrounds (monandry or polyandry). Mating order was reciprocally balanced such that half of the double matings were MP matings, in which sons from monandrous (M) females mated first and sons from polyandrous (P) females mated second, and half were PM matings; thus, mating order was included as a predictor in statistical analyses, although it was not directly relevant to the present study. Sterilization order (NR or RN matings) was also considered as a predictor in statistical analyses. Non-significant terms were dropped one at a time, and models were compared using Akaike information criterion (change in AIC < 2). Only the best fitting model is reported here. The final model included female generation and OLRE as random effects; the mating order, inter-mating interval, sterile order, absolute ejaculate size, and copulation duration differences between first and second males were included as fixed effects. Post hoc comparisons were made among the inter-mating intervals using the Tukey HSD adjustments for multiple comparisons with the “LSmeans” R package (Lenth 2016).
Female remating propensity
The number of females that remated at each inter-mating interval was divided by the total number of females to obtain the proportions of females remating at each interval, which were then compared through a proportions test.
Key differences between females that did and did not remate
To investigate the differences between females that did and did not remate at each inter-mating interval, I used separate binomial GLMMs and GLMs with the response being whether focal females remated (“0” for no, “1” for yes) for each inter-mating interval. For the 0-h inter-mating interval, I used a GLMM, and the only random effect that contributed to variation in the response variable was the second male generation. Because none of the random effects could explain a significant amount of the variability in the response variable for the 24- or 48-h inter-mating intervals, I used GLMs instead. Predictors that were considered for all models included female traits (age at the time of first mating, pre- and post-mating weight, and ejaculate uptake), first male traits (age at the time of first mating, pre-mating weight, and ejaculate size), first mating behavioral variables (mating latency, kicking latency, kicking duration, and copulation duration), and second male traits (age, pre-mating weight, and absolute age and weight differences between him and the first male at the remating interval). The female’s age at the time of the trial and their weight loss between matings were predictors considered for the 24- and 48-h remating intervals. Additional predictors that were considered for the 24-h inter-mating interval included the number of eggs laid and the proportion of eggs laid (as a function of the total number of eggs laid across the female’s lifetime) during the inter-mating interval. This predictor was not considered for the 48-h inter-mating interval, however, since these eggs were not quantified for females that did not remate beyond a 48-h interval. Because females that lay more eggs lose more weight between matings (unpublished data), this factor has been indirectly taken into account by including female weight loss over the 48-h remating interval as a covariate. For the binomial GLMM, a bivariate analysis was conducted separately for each predictor of interest with the second male generation as a random effect. For the binomial GLMs, bivariate analyses were conducted separately for each predictor of interest with no random effects. From these bivariate analyses for the GLMM and GLMs, predictors that had a p value of 0.20 or below were considered for each final model (with the exception of the sterilization order and mating order, which were included as candidate predictors in all initial models regardless of significance). Pairwise comparisons using a linear model were used to screen all significant candidate predictors for collinearity. Whenever collinearity was present, only the predictor with the greater relative significance was included in the initial model. Non-significant terms were then dropped one at a time, and models were compared using Akaike information criterion (change in AIC < 2). Only the best fitting model is reported here. Fixed effects included within all final models may be found in Table 2.
Results
Sperm precedence
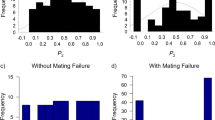
The mean (±SE) P 2 was 0.54 ± 0.05 for a 0-h inter-mating interval, 0.69 ± 0.05 for a 24-h inter-mating interval, and 0.72 ± 0.04 for a 48-h interval. P 2 was significantly lower at an inter-mating interval of 0 h compared to longer inter-mating intervals (binomial GLMM: n = 57; 0–24 h p = 0.007; 0–48 h p = 0.02; Fig. 1, Table 2). Pairwise comparisons adjusted for multiple comparisons using LSmeans show that P 2 did not significantly differ between 24- and 48-h inter-mating intervals (p = 1.00). Fitted values of P 2 using LSmeans were 0.55 ± 0.06, 0.74 ± 0.04, and 0.74 ± 0.05 for 0-, 24-, and 48-h inter-mating intervals, respectively (Fig. 1).
Proportion of offspring sired by the second male to mate (P 2) across three inter-mating intervals—0, 24, and 48 h after the first mating (sample sizes in parentheses); the white dots represent the actual median values, the black bars represent the inter-quartile range, and the gray areas represent the full distribution of the data
Female remating propensity
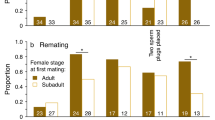
Out of 113 focal females, 26 remated immediately (23.0%), 25 remated 24 h after their first mating (22.1%), and 18 remated 48 h after their first mating (15.9%; Fig. 2). In total, 45.1% of females remated within a day of the first mating, and 61.1% of females remated within 2 days of the first mating. Forty-four females (38.9%) failed to remate within 2 days of the first mating. Of the females that did remate, the proportion of females remating did not significantly differ among the inter-mating intervals (proportions test: χ 2 = 1.14, df = 2, p = 0.57). These results suggest that inter-mating intervals that are shorter than the standard intervals typically used in this species (e.g., 24 and 48 h after the initial mating) are prevalent.
Key differences between females that did and did not remate
Females that remated immediately spent a significantly shorter duration of time kicking their first mate than females that did not remate at this time point (Remated = 103.3 ± 15.4 s, Didn’t Remate = 232.7 ± 22.7 s; binomial GLMM: n = 108, p = 0.01; Table 3). Females that did and did not remate at this time interval did not significantly differ in their pre-mating age or weight or post-mating weight gain, nor were there any differences in the traits of their first or second mates (i.e., absolute or relative ages and weights of males at the remating interval or the first ejaculate size) or the first mating behavioral variables (i.e., mating latency or copulation duration). The first mating kicking latency did significantly differ between these females, but this trait was collinear with the first-male ejaculate size, the latter of which was included in the final model.
Females that remated 24 h after the initial mating laid a significantly smaller percentage of their total eggs between their matings (Remated = 34.3 ± 2.6%, Didn’t Remate = 54.5 ± 5.1%; GLM: n = 32, p = 0.009) than females that did not remate at this time point. These females also weighed significantly less at the time of the second mating trial (Remated = 5.53 ± 0.12 mg, Didn’t Remate = 5.82 ± 0.10 mg; GLM: p = 0.02) compared to females that did not remate (Table 3). These females did not differ in any other traits (either her own or her mates’) or the first mating behavioral variables, however. Females that remated 48 h after the initial mating were significantly younger (Remated = 4.2 ± 0.2 days, Didn’t Remate = 4.9 ± 0.2 days; GLM: n = 48, p = 0.013), weighed significantly more at the time of their first mating (Remated = 6.39 ± 0.22 mg, Didn’t Remate = 6.14 ± 0.09 mg; GLM: p = 0.04), and lost more weight between matings (Remated = 1.79 ± 0.10 mg, Didn’t Remate = 1.35 ± 0.09 mg; GLM: p = 0.02) than females that did not remate at this time point (Table 3). For the 24-h inter-mating interval, females that lost more weight between their matings laid more eggs during this time interval (LM: F 1,30 = 13.8, p = 0.001). Thus, it is reasonable to assume the same for females that waited 48 h to remate, which suggests that females that did remate at this time point laid more eggs during the interval between matings than females that did not remate. Females that did and did not remate at the 48-h remating interval did not differ in any other traits (either her own or her mates’) or in the first mating behavioral variables.
Discussion
The purpose of this study was to determine if last-male sperm precedence observed in the seed beetle, C. maculatus, is upheld at a shorter inter-mating interval. Because passive loss of first-male sperm in the female reproductive tract is expected to lead to disproportionate use of second-male sperm as the time between matings increases, I predicted that P 2 would be diminished with a shorter time interval between matings. I found that P 2 is significantly reduced when females remate immediately than when they remate 24 or 48 h after the initial mating. Surprisingly, I found no difference in the proportions of females remating at each inter-mating interval, which indicates that short inter-mating intervals are relevant to this species, despite the traditional use of longer inter-mating intervals in experimental designs aimed at examining sperm competition and sperm precedence (Eady 1991, 1995; Eady et al. 2004; Hotzy and Arnqvist 2009). Lastly, I found that the differences between females that did and did not remate changed across the inter-mating intervals. Specifically, females that waited a day to remate were smaller and laid fewer eggs between matings, whereas females that waited two days to remate were younger, larger, and lost more weight between their matings when compared to females that did not remate at these time intervals. Together, these results indicate that female remating decisions and, consequently, sperm precedence are more variable than previously appreciated in this species (but see Eady et al. 2004). More generally, this study highlights the substantial potential for female remating decisions to influence the intensity of sperm competition within species.
The sperm precedence patterns observed with varying inter-mating intervals in the present study are consistent with studies in other taxa that have revealed an increase in P 2 by experimentally increasing the interval between matings (reviewed in Simmons 2001, but see Ala-Honkola et al. 2013). For example, P 2 increased from 83% at a 1-day inter-mating interval to 99% at a 14-day inter-mating interval in Drosophila melanogaster (Boorman and Parker 1976). In the milkweed leaf beetle (Labidomera clivicolli), the average P 2 was close to 50% when matings were successive but increased to 87% with a 5-day inter-mating interval (Dickinson 1988). Similarly, average P 2 values were close to 50% for successive matings but increased as the inter-mating interval was increased in the melon fly, Bactrocera cucurbitae (Yamagishi et al. 1992). Moreover, whereas two separate studies in C. maculatus have been used to suggest that P 2 increases from a 24- to 48-h inter-mating interval (Eady 1994a, 1995), this study reveals that P 2 does show an increasing trend in line with these previous studies but ultimately does not significantly differ between these intervals. This result is perhaps unsurprising given that sperm numbers in both the bursa copulatrix and spermathecae most precipitously decline between a 0- and 24-h inter-mating interval but decline less steeply between a 24- and 48-h inter-mating interval (Eady 1994b). Although it was beyond the scope of the present study, it would be interesting to assess sperm precedence patterns on an even finer scale (e.g., every 2 h from 0 to 10 h after the initial mating) to compare the results with what we know about sperm storage and decline within the female reproductive tract over time.
One possible explanation for why paternity is more evenly shared at a 0-h inter-mating interval is that first-male sperm have not yet moved into the spermatheca and so are more numerically and equally represented when being selected to fertilize ova; thus, the mechanism of sperm precedence at this time point does not depend on passive sperm loss. This explanation seems likely given what we know about passive sperm loss in the spermatheca in this species—sperm numbers decline from 6000 to 4000 to 2000 for each 24-h time interval since the first mating (Eady 1994b). Another possibility is that first-male sperm are more equally competitive when females remate immediately, but their relative competitiveness declines with time since ejaculation. While there were several instances in which P 1 was actually higher at longer inter-mating intervals (e.g., P 1 ranged from 2 to 96% for the 24-h inter-mating interval and 4 to 71% for the 48-h inter-mating interval), it was beyond the scope of the present study to assess other sperm traits that might contribute to their relative competitiveness (i.e., length or motility) and so this explanation cannot be ruled out. Another possible explanation is that first-male sperm are more viable when females remate immediately, but sperm viability declines over time while in storage within the female (Orr and Brennan 2015). Whether sperm mortality naturally occurs or can be accelerated by active movement by the female into certain sperm storage sites that increase sperm mortality remains unknown (Lessells and Birkhead 1990; Eberhard 1996). Further empirical studies are warranted to distinguish among these possibilities.
Yet another non-mutually exclusive explanation for the observed reduction in P 2 is that females did not oviposit between matings for the shortest inter-mating interval and so had more first-male sperm in storage to use. In a previous study that examined the effects of oviposition on the patterns of sperm use in the seed beetle, females given fewer oviposition sites during 24- and 48-h inter-mating intervals had a lower P 2 when they did remate, which the authors conclude is because they laid fewer eggs between matings and thus used fewer sperm from their first mate (Eady et al. 2004). Because females with longer inter-mating intervals were given only a single seed for oviposition between matings in the present study, they were restricted in their use of first-male sperm. Thus, this explanation for a lower P 2 based on differences of first-male sperm in storage seems unlikely for females remating at different intervals. However, sperm and ova were not directly counted in the present study or in the Eady et al. (2004) study, which is required to definitively test this assumption.
In addition to demonstrating that the inter-mating interval is a critical determinant of P 2, this study surprisingly reveals that immediate remating by females is common in this species, which differs from previous findings (Edvardsson et al. 2008; den Hollander and Gwynne 2009). It is possible that female remating behaviors differ among population strains based on subtle genetic differences and unintentional selection regimes in the lab. The female inter-mating interval has been shown to be quite variable but significantly heritable in Drosophila (Lüpold et al. 2013) and, through artificial selection, was shown to be significantly heritable within several generations in C. maculatus (Eady et al. 2004). The current study further demonstrates variation in female remating propensities on which selection can act, which corroborates findings in other seed beetle species (Harano and Miyatake 2005; Maklakov et al. 2005). Although female mating strategies are likely to be complex and depend on a number of species-specific factors (e.g., life history, sperm longevity, female reproductive tract complexity, timing of sperm storage, and mechanism for sperm precedence), what females gain from the timing of their remating decisions should be a point of focus for future studies.
Moreover, a common practice is to use the remating interval as a means for understanding female preferences for certain males (e.g., female choice of the second male relative to the first male). For example, a study in D. melanogaster observed whether females adjust their remating interval based on mating opportunities with males of varying sizes and found that females remated more quickly when courted by large (i.e., higher quality) second males (Pitnick 1991). Moreover, two separate studies in D rosophila simulans used copulation latency as a measure for assessing female preference and revealed that attractive males preferred by females were more successful in sperm competition when second to mate (Hosken et al. 2008) and that females use their first mate as a basis for evaluating the quality of their second mate (Ala-Honkola and Manier 2016). In the present study, if immediate remating is an indication of female preference for the second male over the first male, then it is unclear why females do so given that it lowers the second male’s reproductive success. One possible explanation for this puzzling outcome is that males that are preferred in a pre-copulatory context are not intrinsically better sperm competitors and so perform poorly in a post-copulatory context, as has been observed in D. simulans (Ala-Honkola and Manier 2016; but see Hosken et al. 2008). Such a contradictory outcome merits further investigation.
Furthermore, examining key differences between females that did and did not remate at each inter-mating interval reveals potential mechanisms for female refractory behavior, including risk of sperm depletion. Females that remated at a 24-h remating interval laid a significantly smaller proportion of their eggs before their second mating, which may be because they did not have an adequate sperm supply and so remated to obtain more sperm. It cannot be ruled out, however, that these females may have had a lower-quality first mate and waited to lay the majority of their eggs after “trading up” with a second mate (Thornhill and Alcock 1983), which would support female remating based on male quality. Similarly, females that remated at the 48-h remating interval were younger and weighed significantly more prior to their first mating than females that did not. Post hoc analyses reveal a trend of younger and larger females being more fecund, which makes these females more likely to run the risk of sperm depletion. Furthermore, females that remated at 48 h lost more weight between their matings. Given that female weight loss is highly correlated with the number of eggs laid during this time interval, these females necessarily used more of the first males’ sperm and so risk sperm depletion. Another intriguing possibility is that it is the presence of sperm itself within the female reproductive tract that inhibits female remating, as has been found in the almond moth, Cadra cautella (McNamara et al. 2008). Although quantifying sperm was beyond the scope of this study, an interesting follow-up would be to dissect the spermatheca of females that become receptive again to determine if they are sperm-depleted.
Female remating behavior may also have been based on her condition, since females that remated at a 24-h remating interval weighed significantly less than females that did not remate at that time point, and females that delayed remating by another day (i.e., with a 48-h remating interval) weighed significantly more prior to their first mating than females that did not remate at that time point. However, it is unclear why the female’s condition would not have been an important distinguishing factor for females at a 0-h interval. That smaller, less fecund females would be more likely to remate at a 24-h interval is perplexing and contradicts results from previous investigations of female remating propensities in D. melanogaster and the almond moth (Pitnick et al. 2001b; McNamara et al. 2008). Further analyses revealed that these smaller females did not differ in the number of eggs laid between matings or the size of the ejaculate obtained from their first mating, both of which make sperm deficiency in these females unlikely. One possibility is that these smaller females were of inherently lower quality and so remated to acquire more resources through a second ejaculate, which supports previous findings in the seed beetle (Edvardsson 2007). This result does seem unlikely, however, given that it was not consistent for all females across remating intervals and females that remated at 48 h were relatively larger prior to mating.
Interestingly, females that did not remate immediately kicked for a significantly longer duration of time during the first mating. Corroborating other studies of C. maculatus, this unanticipated result has two potential explanations, both of which involve the males’ spiny genitalia that have been shown to cause exceptional damage to the female reproductive tract (Crudgington and Siva-Jothy 2000). One supported function of these genital spines is that they increase the rate at which male seminal products are transferred into the female’s circulatory fluid, since females that mated with males with longer spines had a larger proportion of the males’ ejaculate move from their reproductive tract into their body (Hotzy et al. 2012). From this study, it was suggested that the increased absorption of seminal products benefits males by affecting the pattern of sperm use by females. Although the kicking phase of copulation has been shown to reduce female reproductive tract damage (Crudgington and Siva-Jothy 2000; but see Dougherty and Simmons 2017), results from the present study suggest that the females’ reproductive tract may undergo cumulative damage during kicking and that females that kick longer incur more damage. Hence, one hypothesis for why female remating propensity differed based on kicking duration is that females that kicked for a longer duration more quickly absorbed manipulative seminal products (Yamane et al. 2008) that immediately reduced their receptivity. Further studies are warranted to test this hypothesis given these conflicting results with those previously published as well as the known effects of male seminal fluids on sperm precedence, female receptivity, and egg production in C. maculatus (Yamane et al. 2015).
Another non-mutually exclusive hypothesis is that females that kick for a longer duration of time wait to remate to avoid the cost of more reproductive tract damage. If damage from kicking is cumulative, then males that can induce female refractoriness by inflicting harm can reduce the benefit to females for remating (Johnstone and Keller 2000). Whether the harm caused by genital spines in male seed beetles is adaptive or “collateral” remains unknown, but such harmful male tactics are suggested to be more likely in species with a greater last-male mating advantage (Johnstone and Keller 2000). Furthermore, why the influence of kicking on female remating would be significant for only the shortest remating interval is unclear, but perhaps it coincides with the time it takes for damaged female tissues to heal or represents the threshold of when the benefits for female remating exceed the costs. One obvious hypothesis that emerges from these results is that the time the female spends kicking during copulation is a primary cause of reproductive tract damage. A recent investigation of female wounding in relation to mating duration and female kicking revealed that significant wounding is present prior to the onset of kicking and yielded no support for the hypothesis that kicking increases the rate of wounding (Dougherty and Simmons 2017). However, because this study assessed wounding at only two time points after kicking but did not do so for uninterrupted matings, we cannot rule out that further wounding occurs with extended kicking durations. Further studies are warranted to investigate these relationships between the female kicking duration, reproductive tract damage and repair, and the timing and extent of ejaculate component absorption within female bodily tissues.
One surprising result is that the ejaculate size itself did not significantly affect female remating at any remating interval. The large ejaculate size in this species is presumed to reduce female propensity to remate (Edvardsson 2007), and previous studies have confirmed this (Eady 1995; Savalli and Fox 1999). However, these latter studies manipulated ejaculate size by varying male mating status, whereas in the present study, male mating status was controlled and ejaculate sizes naturally varied among virgin males. That the results between these studies and the present study differ suggests that there are confounding effects of ejaculate components that likely vary by male mating status rather than ejaculate size. The result that no differences in female remating were observed based on ejaculate size in the present study suggests that that there are no dosage-dependent effects (Eberhard 1996). This result in combination with previous results implicates the importance of male mating status and suggests that virgin males either produce more of these manipulative compounds or their refractory effects are more potent. It also seems plausible that these compounds vary among males, which may be capable of tailoring these compounds to their advantage based on environmental or social contexts (e.g., female mating status and/or male mating role; Sirot et al. 2011). Males are assumed to control female mating frequency through the ejaculate by varying its size and hydration benefits contained within it, thus changing how beneficial remating is for the female (Edvardsson 2007), but the results from the present study do not support this assumption. Future studies should look into the ejaculate components and how these differ among and within males across contexts.
Conclusions that have been made about sperm use patterns in different taxa are likely to be dramatically impacted by experimental design (Danielsson 1998). For example, the last-male sperm precedence pattern found in a species of pseudoscorpion was discredited by a simple change in protocol from two to three matings (Zeh and Zeh 1994). Moreover, as investigators, we often discard females that are unwilling to remate at a designated inter-mating interval (but see Harano et al. 2006). Might we be missing important information here? While it is important to control for variables that can potentially affect sperm use and storage, discarding females unwilling to remate may mean the dataset includes only females that have lower thresholds for remating or lower-quality first mates. If so, then females with high remating thresholds or higher-quality first mates will never be screened for differential sperm use, which necessarily limits our understanding of the consequences of female behavioral decisions on sperm use patterns. Careful consideration is needed in future studies for factors in experimental designs that can influence sperm use patterns.
Conclusions
In summary, I found that P 2 typical in the seed beetle is significantly reduced when females remate immediately than when they wait to remate 24 or 48 h after the first mating. Intriguingly, I also found that immediate remating is common, indicating that there is a substantial potential for female remating decisions to influence the intensity of sperm competition in this and other species more generally. I further identified key differences between females that did and did not remate at three distinct inter-mating intervals and found support for several mechanisms for female refractoriness, including enhanced direct benefits (i.e., adequate sperm supplies and nutrient acquisition) and reduced costs (i.e., cumulative damage incurred during mating).
Change history
09 December 2017
The electronic supplementary file from the original article contained track changes and line numbers. Everything else in the wording and/or copy is completely fine. A clean electronic supplementary file was uploaded.
References
Ala-Honkola O, Hosken DJ, Manier MK, Lüpold S, Droge-Young EM, Berben KS, Collins WF, Belote JM, Pitnick S (2013) Inbreeding reveals mode of past selection on male reproductive characters in Drosophila melanogaster. Ecol Evol 3:2089–2102. doi:10.1002/ece3.625
Ala-Honkola O, Manier MK (2016) Multiple mechanisms of cryptic female choice act on intraspecific male variation in Drosophila simulans. Behav Ecol Sociobiol 70:519–532. doi:10.1007/s00265-016-2069-3
Arnaud L, Gage MJ, Haubruge E (2001) The dynamics of second-and third-male fertilization precedence in Tribolium castaneum. Entomol Experim et Appl 99:55–64. doi:10.1046/j.1570-7458.2001.00801.x
Arnqvist G, Danielsson I (1999) Copulatory behavior, genital morphology, and male fertilization success in water striders. Evolution 53:147–156
Bates D, Mächler M, Bolker B, Walker S (2014) Fitting linear mixed-effects models using lme4. arXiv preprint arXiv:14065823. doi:10.18637/jss.v067.i01
Birkhead TR, Møller AP (1992) Sperm competition in birds: evolutionary causes and consequences. Academic Press, London
Birkhead TR, Biggins JD (1998) Sperm competition mechanisms in birds: models and data. Behav Ecol 9:253–260. doi:10.1093/beheco/9.3.253
Blyth JE, Gilburn AS (2005) The effect of an inversion system and the time interval between matings on postcopulatory sexual selection in the seaweed fly, Coelopa frigida. Heredity 95:174–178. doi:10.1038/sj.hdy.6800713
Boorman E, Parker GA (1976) Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol Entomol 1:145–155. doi:10.1111/j.1365-2311.1976.tb01217.x
Cook PA, Harvey IF, Parker GA (1997) Predicting variation in sperm precedence. Philos Trans R Soc Lond Ser B Biol Sci 352:771–780. doi:10.1098/rstb.1997.0061
Crawley MJ (2013) The R book, Second edn. Wiley, England
Crudgington HS, Siva-Jothy MT (2000) Genital damage, kicking and early death. Nature 407:855–856. doi:10.1038/35038154
Danielsson I (1998) Mechanisms of sperm competition in insects. In Annales Zoologici Fennici 35:241–257
den Hollander M, Gwynne DT (2009) Female fitness consequences of male harassment and copulation in seed beetles, Callosobruchus maculatus. Anim Behav 78:1061–1070. doi:10.1016/j.anbehav.2009.06.036
Dickinson J (1986) Prolonged mating in the milkweed leaf beetle Labidomera clivicollis clivicollis (Coleoptera: Chrysomelidae): a test of the “sperm-loading” hypothesis. Behav Ecol Sociobiol 18:331–338. doi:10.1007/BF00299664
Dickinson J (1988) Determinants of paternity in the milkweed leaf beetle. Behav Ecol Sociobiol 23:9–19. doi:10.1007/BF00303052
Dougherty LR, Simmons LW (2017) X-ray micro-CT scanning reveals temporal separation of male harm and female kicking during traumatic mating in seed beetles. Proc R Soc Lond B 284:20170550. doi:10.1098/rspb.2017.0550
Drnevich JM (2003) Number of mating males and mating interval affect last-male sperm precedence in Tenebrio molitor L. Anim Behav 66:349–357. doi:10.1006/anbe.2003.2219
Eady PE (1991) Sperm competition in Callosobruchus maculatus (Coleoptera: Bruchidae): a comparison of two methods used to estimate paternity. Ecol Entomol 16:45–53. doi:10.1111/j.1365-2311.1991.tb00191.x
Eady PE (1994a) Intraspecific variation in sperm precedence in the bruchid beetle Callosobruchus maculatus. Ecol Entomol 19:11–16. doi:10.1111/j.1365-2311.1994.tb00384.x
Eady PE (1994b) Sperm transfer and storage in relation to sperm competition in Callosobruchus maculatus. Behav Ecol Sociobiol 35:123–129. doi:10.1007/BF00171502
Eady PE (1995) Why do male Callosobruchus maculatus males inseminate so many sperm? Behav Ecol Sociobiol 36:25–32. doi:10.1007/BF00175725
Eady PE, Rugman-Jones P, Brown DV (2004) Prior oviposition, female receptivity and last-male sperm precedence in the cosmopolitan pest Callosobruchus maculatus (Coleoptera: Bruchidae). Anim Behav 67:559–565. doi:10.1016/j.anbehav.2003.07.003
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton
Edvardsson M (2007) Female Callosobruchus maculatus mate when they are thirsty: resource-rich ejaculates as mating effort in a beetle. Anim Behav 74:183–188. doi:10.1016/j.anbehav.2006.07.018
Edvardsson M, Arnqvist G (2000) Copulatory courtship and cryptic female choice in red flour beetles Tribolium castaneum. Proc R Soc B 267:559–563. doi:10.1098/rspb.2000.1037
Edvardsson M, Rodríguez-Muñoz R, Tregenza T (2008) No evidence that female bruchid beetles, Callosobruchus maculatus, use remating to reduce costs of inbreeding. Anim Behav 75:1519–1524. doi:10.1016/j.anbehav.2007.10.005
Harano T, Miyatake T (2005) Heritable variation in polyandry in Callosobruchus chinensis. Anim Behav 70:299–304. doi:10.1016/j.anbehav.2004.10.023
Harano T, Yasui Y, Miyatake T (2006) Direct effects of polyandry on female fitness in Callosobruchus chinensis. Anim Behav 71:539–548. doi:10.1016/j.anbehav.2005.05.017
Harrison XA (2014) Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ 2:e616. doi:10.7717/peerj.616
Hosken DJ, Taylor ML, Hoyle K, Higgins S, Wedell N (2008) Attractive males have greater success in sperm competition. Curr Biol 18:R553–R554. doi:10.1016/j.cub.2008.04.028
Hotzy C, Arnqvist G (2009) Sperm competition favors harmful males in seed beetles. Curr Biol 19:404–407. doi:10.1016/j.cub.2009.01.045
Hotzy C, Polak M, Rönn JL, Arnqvist G (2012) Phenotypic engineering unveils the function of genital morphology. Curr Biol 22:2258–2261. doi:10.1016/j.cub.2012.10.009
Johnstone RA, Keller L (2000) How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am Nat 156:368–377
LaMunyon CW, Eisner T (1994) Spermatophore size as determinant of paternity in an arctiid moth (Utetheisa ornatrix). PNAS 91:7081–7084
Lenth RV (2016) Least-squares means: the R package lsmeans. J Stat Softw 69:1–33. doi:10.18637/jss.v069.i01
Lessells CM, Birkhead TR (1990) Mechanisms of sperm competition in birds: mathematical models. Behav Ecol Sociobiol 27:325–337. doi:10.1007/BF00164003
Lewis SM, Austad SN (1990) Sources of intraspecific variation in sperm precedence in red flour beetles. Am Nat 135:351–359
Lüpold S, Manier MK, Berben KS, Smith KJ, Daley BD, Buckley SH, Belote JM, Pitnick S (2012) How multivariate ejaculate traits determine competitive fertilization success in Drosophila melanogaster. Curr Biol 22:1667–1672. doi:10.1016/j.cub.2012.06.059
Lüpold S, Pitnick S, Berben KS, Blengini CS, Belote JM, Manier MK (2013) Female mediation of competitive fertilization success in Drosophila melanogaster. PNAS 110:10693–10698. doi:10.1073/pnas.1300954110
Maklakov AA, Kremer N, Arnqvist G (2005) Ageing and the evolution of female resistance to remating in seed beetles. Biol Lett 2:62–64. doi:10.1098/rspb.2005.3240
Manier MK, Lüpold S, Pitnick S, Starmer WT (2013) An analytical framework for estimating fertilization bias and the fertilization set from multiple sperm-storage organs. Am Nat 182:552–561. doi:10.1086/671782
McNamara KB, Elgar MA, Jones TM (2008) Seminal compounds, female receptivity and fitness in the almond moth, Cadra cautella. Anim Behav 76:771–777. doi:10.1016/j.anbehav.2008.04.018
Orr TJ, Brennan PL (2015) Sperm storage: distinguishing selective processes and evaluating criteria. Trends Ecol Evol 30:261–272. doi:10.1016/j.tree.2015.03.006
Parker GA (1970a) Sperm competition and its evolutionary consequences in the insects. Biol Rev 45:525–567. doi:10.1111/j.1469-185X.1970.tb01176.x
Parker GA (1970b) Sperm competition and its evolutionary effect on copula duration in the fly Scatophaga stercoraria. J Insect Physiol 16:1301–1328. doi:10.1016/0022-1910(70)90131-9
Pitnick S (1991) Male size influences mate fecundity and remating interval in Drosophila melanogaster. Anim Behav 41:735–745. doi:10.1016/S0003-3472(05)80340-9
Pitnick S, Miller GT, Reagan J, Holland B (2001a) Males’ evolutionary responses to experimental removal of sexual selection. Proc R Soc Lond B 268:1071–1080. doi:10.1098/rspb.2001.1621
Pitnick S, Brown WD, Miller GT (2001b) Evolution of female remating behaviour following experimental removal of sexual selection. Proc Biol Sci 268:557–563
Radwan J (1997) Sperm precedence in the bulb mite, Rhizoglyphus robini: context-dependent variation. Ethol Ecol Evol 9:373–383. doi:10.1080/08927014.1997.9522879
Development Core Team R (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Simmons LW, Parker GA (1992) Individual variation in sperm competition success of yellow dung flies, Scatophaga stercoraria. Evolution 46:366–375
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton University Press, Princeton
Savalli UM, Fox CW (1999) The effect of male mating history on paternal investment, fecundity and female remating in the seed beetle Callosobruchus maculatus. Funct Ecol 13:169–177. doi:10.1046/j.1365-2435.1999.00287.x
Sirot LK, Wolfner MF, Wigby S (2011) Protein-specific manipulation of ejaculate composition in response to female mating status in Drosophila melanogaster. PNAS 108:9922–9926. doi:10.1073/pnas.1100905108
Siva-Jothy MT, Tsubaki Y (1989) Variation in copulation duration in Mnais pruinosa pruinosa Selys (Odonata: Calopterygidae): 1. Alternative mate-securing tactics and sperm precedence. Behav Ecol Sociobiol 24:39–45. doi:10.1007/BF00300052
Thornhill R, Alcock J (1983) The evolution of insect mating systems. Harvard University Press, Cambridge
Walker WF (1980) Sperm utilization strategies in nonsocial insects. Am Nat 115:780–799
Wedell N, Cook PA (1998) Determinants of paternity in a butterfly. Proc R Soc B 265:625–630. doi:10.1098/rspb.1998.0340
Wilson N, Tubman SC, Eady PE, Robertson GW (1997) Female genotype affects male success in sperm competition. Proc R Soc Lond B 264:1491–1495. doi:10.1098/rspb.1997.0206
Yamagishi M, Itô Y, Tsubaki Y (1992) Sperm competition in the melon fly, Bactrocera cucurbitae (Diptera: Tephritidae): effects of sperm “longevity” on sperm precedence. J Insect Behav 5:599–608. doi:10.1007/BF01048007
Yamane T, Goenaga J, Rönn JL, Arnqvist G (2015) Male seminal fluid substances affect sperm competition success and female reproductive behavior in a seed beetle. PLoS One 10:e0123770. doi:10.1371/journal.pone.0123770
Yamane T, Miyatake T, Kimura Y (2008) Female mating receptivity after injection of male-derived extracts in Callosobruchus maculatus. J Insect Physiol 54:1522–1527. doi:10.1016/j.jinsphys.2008.09.001
Zeh JA, Zeh DW (1994) Last-male sperm precedence breaks down when females mate with three males. Proc R Soc Lond B 257:287–292. doi:10.1098/rspb.1994.0127
Acknowledgements
I thank my committee members—Drs. Janis Dickinson, Hudson Kern Reeve, Linda Rayor, and Mike Webster—and fellow departmental colleagues for critical discussion and comments; Frank Messina for providing the stock population of beetles, extra oviposition substrate, and advice on culture maintenance; Ken Kemphues for help with access to and use of the irradiator; Pat Sullivan and Andy Clark for their input and help with experimental design; Abigail Giancola and Hannah Yoo for beetle culturing assistance; Lynn Johnson for statistical advice; Lars Washburn for use of a microbalance; family and friends for their support; the city of Austin for their tacos and sunshine; and two anonymous referees for their comments that greatly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Data availability
The datasets generated and/or analyzed during the current study are available in the Dryad Digital Repository, [http://dx.doi.org/10.5061/dryad.7h7h3].
Funding
This research was supported by grants from the Department of Neurobiology and Behavior at Cornell, ATHENA Fund and the Linda and Samuel Kramer Graduate Student Fellowship at the Cornell Lab of Ornithology, the Cornell Sigma Xi Student Research Grant, the American Museum of Natural History, and the National Science Foundation Graduate Research Fellowship (Cornell NSF Grant DGE-1144153).
Conflict of interest
The author declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Communicated by D. J. Hosken
A correction to this article is available online at https://doi.org/10.1007/s00265-017-2413-2.
Electronic supplementary material
ESM 1
(DOCX 90 kb).
Rights and permissions
About this article
Cite this article
Hook, K.A. Female remating decisions and a shorter inter-mating interval diminish last-male sperm precedence. Behav Ecol Sociobiol 71, 121 (2017). https://doi.org/10.1007/s00265-017-2350-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2350-0