Abstract
Many vertebrates use vocalizations to communicate about the presence of predators, and some encode information about predator type or behavior. A fast-approaching predator typically elicits a “flee alarm call,” prompting others to escape to safety. In a field experiment, we presented gliding models of a predatory bird to several species representing four families of passerine, including our model species, the zebra finch (Taeniopygia guttata). All families presented with the glider gave a distinct call on at least one occasion, apart from the zebra finch, for which no specific alarm call was recorded. Following on from this unexpected result, we conducted an experiment in which we exposed captive zebra finches to video of a looming raptor. Results of the captive study showed that birds responded to the looming raptor with escape behavior and responded to less threatening stimuli with orienting or startle behavior. Despite this anti-predator response, birds did not give any distinct alarm call, and the distance calls of both males and females did not differ in structure or rate of delivery after exposure to a stimulus. Zebra finches are one of the most studied birds in the world, are gregarious, and have a rich vocal repertoire, yet their alarm communication has not been investigated experimentally. Our results are consistent with the hypothesis that zebra finches lack a flee alarm call and appear not to signal about immediate danger through a change in calling rate.
Significance statement
Many animals emit alarm calls when faced with a threatening event in order to communicate with nearby group members. Threatening events can be simulated with models or by presenting a video of a looming stimulus on a screen. In separate studies, we presented gliding models and computer animations of a hawk to zebra finches, a bird species used in studies around the world, in order to test if they gave alarm calls to warn others of approaching danger. Although birds fled in response to the simulated predators, they did not emit a distinct alarm call. The birds also did not change their rate of calling or the acoustic structure of their distance calls. Surprisingly for a social and highly vocal species, the birds appear to lack alarm calls warning flockmates of immediate danger.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals use a range of morphological, physiological, and behavioral strategies to avoid predation, and many vertebrates use alarm calls to warn of danger or deter attack (Klump and Shalter 1984; Caro 2005; Hollén and Radford 2009; Zuberbühler 2009). Alarm calls can be categorized according to their context, function, and acoustic structure (Magrath et al. 2015). Flee alarm calls warn nearby conspecifics of immediate danger from a fast-moving or flying predator and prompt others to freeze or flee to safety (Klump and Shalter 1984). They are often relatively pure tone in structure, and at least some, such as the “seeet” calls of passerines, appear designed to be difficult for predators to hear or locate (Marler 1955; Klump et al. 1986). Mobbing alarm calls are usually given to a terrestrial or stationary threat and prompt others to approach and harass the predator. They may also be directed at the predator itself, to communicate that it has been sighted and an attack would be unsuccessful (Klump and Shalter 1984). These calls are often lower in frequency, harsh, and broadband, with the caller easier to locate (Marler 1955; Bradbury and Vehrencamp 2011). In addition to these general categories, alarm calls can encode information on the predator type or urgency of threat (Zuberbühler 2009; Gill and Bierema 2013). For example, Diana monkeys (Cercopithecus diana) use different alarm calls for eagles and leopards (Zuberbühler 2000), while white-browed scrubwrens (Sericornis frontalis) and superb fairy-wrens (Malurus cyaneus) include more elements in their flee alarm calls when a predator is flying closer (Leavesley and Magrath 2005; Fallow and Magrath 2010). Meerkats (Suricata suricatta) combine information by producing distinct calls depending on the type of predator as well as varying acoustic structure to convey urgency information (Manser 2001).
Although alarm calls often have a distinct acoustic structure, call rate can also convey information about danger independently of call structure. For example, Wheatcroft (2015) found that a sample of 15 species of passerines produce calls with the same acoustic structure in social and predator contexts, but called at a higher repetition rate when a predator was present. Similarly, individuals of some species produce “sentinel” calls to signal that they are “on duty” watching for predators and can vary rate according to perceived risk. For example, both meerkats and pied babblers (Turdoides bicolor) give repeated sentinel calls when vigilant and no predators are present, and playbacks show that others reduce their vigilance and increase foraging when sentinel calls indicate minimal risk (Manser 1999; Hollén et al. 2008; Bell et al. 2009).
Alarm calling appears to be more likely in social species, or at least when kin or other conspecifics are nearby (Maynard Smith 1965; Caro 2005; Zuberbühler 2009). For example, female Belding’s ground squirrels (Urocitellus beldingi) are more likely to give alarm calls when offspring or other kin are nearby (Sherman 1977), and female Siberian jays (Perisoreus infaustus) have a higher chance of alarm calling when related rather than unrelated subordinates are close (Griesser and Ekman 2004). Individuals may also give alarm calls when mates are near, as seen in downy woodpeckers (Picoides pubescens; Sullivan 1985) and roosters (Gallus gallus; Wilson and Evans 2008). Furthermore, mixed-species flocks often coalesce around gregarious “nuclear” species (Sridhar et al. 2009; Srinivasan et al. 2010), and at least in some cases, these species are the first to give alarm calls when a predator appears.
Alarm calling and associated anti-predator behavior have been widely studied in a variety of taxa (Zuberbühler 2009). Ethical and practical reasons mean that experimental studies typically use models rather than live predators to prompt alarm calls. Commonly, physical models with various degrees of resemblance to real predators have been used successfully, including simple shapes, such as thrown sticks or hats, taxidermic or constructed stationary predators, and motorized or gliding predator models (e.g., Curio 1975; Blumstein and Armitage 1997; Goodale and Kotagama 2005; Magrath et al. 2007; Sloan and Hare 2008; Davies and Welbergen 2009). These methods have been shown to prompt anti-predator behavior and alarm calls analogous to those elicited by natural predators.
We investigated the anti-predator vocal behavior of zebra finches (Taeniopygia guttata), a highly vocal and gregarious native Australian passerine distributed throughout most of mainland Australia (Pizzey and Knight 2012). Overall flock size fluctuates depending on the reproductive status of the birds and the distribution of resources (Zann 1996), and during the non-breeding season, zebra finches are most commonly found in mixed-sex pairs or in small groups (McCowan et al. 2015). Wild zebra finches often form mixed-species flocks, especially during vulnerable activities like foraging (Higgins et al. 2006). Birds living in arid areas are vulnerable to predation by raptors, snakes, and occasionally dingoes, especially while visiting waterholes (Zann 1996). This species is used worldwide as a model to study topics such as behavior, physiology, and neurobiology (Griffith and Buchanan 2010). Their vocalizations have been studied in detail, and they are known to possess up to 11 distinct calls (Zann 1996); however, there is limited information on alarm communication. “Thuk” calls appear to be used to warn offspring of a potential nearby predator and get them ready to flee to safety (Zann 1996). However, a field study failed to detect any distinct flee alarm call given by adults in flocks (Giuliano 2012). The sexually dimorphic distance call, which is the longest and loudest call of this species, appears to be multi-functional, with one possible use to express alarm (Zann 1996). However, whether the distance call, which is stereotyped within individuals but shows great variation among individuals, can be varied to function as an alarm signal has never been studied. Given that this is a well-known social species with a rich vocal repertoire, the limited knowledge of alarm communication and possible absence of a flee alarm are both surprising.
We tested whether zebra finches have vocal alarm signals by carrying out both field and captive experiments. We first tested the vocal response of zebra finches to model gliding hawks in the field and compared their response to similarly sized birds from other families in the same habitat. We then tested a captive population for immediate and longer term vocal changes to computer-animated video of attacking hawks.
Field experiment
We conducted field work in semi-arid regions of southeastern Australia involving presentation of a realistic gliding model of a predatory bird. Our focus was on recording the anti-predator response of the zebra finch in comparison to similarly sized members of three other families known to form mixed-species flocks with, or live in the vicinity of, zebra finches. Apart from the zebra finch, all species tested are known to possess distinct alarm calls to warn adult conspecifics of nearby danger, which appears to be a common feature of Australian passerine vocal behavior (Higgins et al. 2001, 2006; Higgins and Peter 2002). The behavior of birds presented with the glider was scored, and it was noted whether or not the model successfully elicited an alarm call. We also conducted acoustic analyses on zebra finch distance calls to detect any changes in the structure of post-stimulus calls. We predicted that birds presented with the glider would flee and emit alarm calls to warn nearby conspecifics of the threat.
Study species and sites
Field work was undertaken at three main locations in southeastern Australia. Work at Fowlers Gap Arid Zone Research Station in New South Wales (31° 21′ S, 141° 40′ E) was conducted over a period of 2 weeks in November (spring) 2014. Various types of vegetation are present in this area, which covers 39,000 ha and is situated in the semi-arid zone. Most of the land consists of open chenopod shrubland with patches of acacia, while tall eucalypts can be found along the creekbeds. Murray-Sunset National Park in Victoria (34° 46′ S, 141° 51′ E) was visited for a week in April (autumn) 2015. This area is characterized by sandy soil, spinifex grasses, and mallee eucalypts. Finally, work in three areas in the Hume region of northern Victoria (Rutherglen 36° 03′ S, 146° 27′ E; Winton Wetlands 36° 28′ S, 146° 04′ E; Kinnairds Wetland 36° 04′ S, 145° 28′ E) occurred in December (summer) 2015 over a 2-week period. Most locations visited in Rutherglen were open farmland, with some areas consisting of swampy, dense vegetation. Winton Wetlands and Kinnairds Wetland are well-maintained public access areas. The forest at Winton Wetlands is more open than that at Kinnairds Wetland. These locations were chosen as they are known sites for zebra finches and the target families, most are on public land, and they are easily accessible by car and on foot.
Species recorded are shown in Fig. 1 and include members of four families: Estrildidae, Maluridae, Meliphagidae, and Acanthizidae. All species are small, native passerines and are vulnerable to predation by aerial predators. There is some overlap in the distribution of species targeted; however, not all species are found in each area. Alarm call behavior is well documented for all families, apart from Estrildidae for which little information is available (see “Introduction” section).
Photos of species recorded during the field experiment. a Zebra finch (Estrildidae: Taeniopygia guttata). b Variegated fairy-wren (Maluridae: Malurus lamberti). c Splendid fairy-wren (Maluridae: Malurus splendens). d White-winged fairy-wren (Maluridae: Malurus leucopterus). e Singing honeyeater (Meliphagidae: Gavicalis virescens). f White-plumed honeyeater (Meliphagidae: Ptilotula penicillatus). g Yellow-throated miner (Meliphagidae: Manorina flavigula). h Yellow-rumped thornbill (Acanthizidae: Acanthiza chrysorrhoa). i Chestnut-rumped thornbill (Acanthizidae: Acanthiza uropygialis). Photos a, e–g by Mark Hall; photo b by Richard Peters; photo c by José Ramos; photos d and i by Jordan de Jong; photo h by Rowan Mott
Experimental stimuli
We constructed gliding models of a predatory bird to present to our species in order to record their anti-predator response to a consistent threat. Models were based on a successful design used in previous studies of alarm calling in small passerines (e.g., Magrath et al. 2007). The body and wings of the gliders were shaped from thick foam to the approximate size of a female collared sparrowhawk (Accipiter cirrocephalus). This species occurs throughout Australia, feeds mainly on small birds it captures in flight, and is a known predator of zebra finches and members of the target families (Marchant and Higgins 1993; Pizzey and Knight 2012; Mainwaring and Griffith 2013). The gliders were painted and matched by eye to the bird’s feather patterns, and yellow eyes were affixed to the face of each glider to duplicate the distinctive appearance of this raptor. A fake tail made from a carbon rod with clear plastic stabilizers was added to aid control in flight.
Experimental procedure
This study was conducted on individuals or small single-species groups of fewer than 10 members. Work was conducted between 0700 and 1900 h on days with warm to hot temperatures and low wind. Nearly half (48%) of all trials were undertaken before 1200 h, and only 35% occurred during the hottest part of the day (between 1200 and 1600 h) as birds were less active and more difficult to find during this time. Target birds were found by searching suitable vegetation until an individual, pair, or flock was located. We either moved quietly through the habitat or waited for birds to appear; the latter method was particularly useful near water bodies. Several species, such as the zebra finch, fairy-wrens, and white-plumed honeyeaters, frequented certain types of vegetation and were therefore more locatable than the more elusive, less-predictable species. Only adult birds were targeted, and trials were not undertaken near known active nests. Upon locating a suitable target, we waited for at least 2 min to ensure that the birds were not disturbed by our presence or by any wild predators. If disturbance did occur, the trial was postponed for at least 10 min and until we were sure that the birds had resumed normal activities.
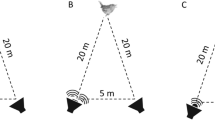
During a trial, one researcher presented the glider while the other filmed and recorded the target birds. For birds that were foraging on the ground, the glider was directed to one side and traveled approximately 5 m above the ground. This method follows that used by Magrath et al. (2007). Some birds were targeted while perched in a tree or shrub. In this case, the glider was pitched at a steeper angle so that it reached the apex of its flight above the height of the perched bird. Gliders thrown in this manner did not fly as far laterally, so the distance between us and the target bird was shorter. The distance between the glider initiation and recording equipment and the target individual or group ranged from 5 to 20 m. Birds perched higher than approximately 8 m aboveground were not targeted as it was likely that the glider would fly below them. Birds that reacted to the glider but did not flee the area returned to normal behaviors within 2 min of glider presentation. At sites where multiple species were present, only one species was targeted per trial. Thus, occasionally, several trials were conducted at one site; however, these were separated by a period of at least 20 min to avoid overly stressing the birds. We moved to a different location when all target species present at a particular site had been exposed to the glider.
The response of the birds was recorded with a high-definition video camera, either a Canon Legria HF21 or a Canon XA10, the latter with an attached Sennheiser ME66 shotgun microphone with K6 powering module. Separate audio was simultaneously recorded using a Roland R-26 portable recorder, also with a Sennheiser ME66 microphone. Audio recordings were sampled at 44.1 kHz and 16 bits.
Data and statistical analyses
Species were grouped according to family (Fig. 1) for analysis. Videos were examined and the response of the recorded birds scored using the following categories: fled (flew out of view of the camera), retreated (moved into cover of vegetation), approached (moved towards the glider after it landed on the ground or in vegetation), or showed no visible response. For some videos, the behavioral response of the birds was unable to be seen on film due to dense vegetation obscuring their movements. Audio analysis consisted of visualizing the recordings in Raven Pro 1.4 (Charif et al. 2010) followed by examination of the spectrograms for alarm calls. Spectrograms were visualized using a Blackman window function, 0.590 ms hop size with 95% overlap, and 43.1 Hz grid spacing. Potential alarm calls were matched to published descriptions and spectrograms (Jurisevic and Sanderson 1994a, b; Higgins et al. 2001, 2006; Higgins and Peter 2002). We examined the probability of alarm call by family using the Kruskal-Wallis test, with Wilcoxon-signed rank test for independent data used for pairwise comparisons involving zebra finches, in the R statistical environment (R Core Team 2013).
As far as we know, there are no records of a distinct alarm call given by zebra finches to warn conspecific adults of predator presence, and all calls given by zebra finches in this study were standard distance calls. However, to examine whether the vocal response post-stimulus was subtly different, we selected the last three calls given prior to glider presentation to compare with the first call given after presentation. Calls were first selected in Raven Pro 1.4 and filtered to 300 Hz to remove low-frequency background noise. We used an acoustic similarity score, the similarity function in Sound Analysis Pro 2011 (Tchernichovski et al. 2000), to assess any differences in call structure following a stimulus. Using this measurement, identical calls score close to 100% similarity and the less similar the calls, the lower the value. Therefore, if birds give distinct alarm calls in response to a threatening stimulus, there will be a much lower similarity score when comparing post-stimulus calls to pre-stimulus calls, than when comparing among pre-stimulus calls. This program was designed specifically for analyzing syllables in zebra finch song, but it can be modified to assess similarity in other vocalizations (Tchernichovski et al. 2000). We used the default settings, although we set the advance window to 0.50 ms to allow for clearer visualization of calls, and applied the boost amplitude function to increase the overall amplitude. Calls were individually windowed to include only the sound of interest before similarity scoring, which used the symmetric and mean value settings. In Sound Analysis Pro, symmetric and asymmetric options are available; however, asymmetric measures are used when a subject copies a template sound and a judgment on the quality of this imitation is required. Therefore, we used symmetric measurements, as neither call was acting as the model for the other. Scores for a given bird were averaged to provide a single measure of call similarity for pre-stimulus calls and a single measure of call similarity between pre-stimulus and post-stimulus calls.
Results (wild birds)
Zebra finches responded with anti-predator behavior to the gliding model hawk, but did not produce alarm calls. Individuals in all families presented with the model glider fled on at least one occasion (Fig. 2a), and overall, this was the most common response. Retreating was also demonstrated by most species in at least one trial, although some individuals in the family Meliphagidae approached the glider after it had landed. An alarm call was recorded from every family on at least one occasion, but never for zebra finches (Fig. 2b). The average similarity score between three pre-stimulus and the first post-stimulus calls ranged from 82 to 98% for zebra finches (Table 1), which indicates a high level of resemblance and shows that no distinct alarm call was emitted. Results of the Kruskal-Wallis rank sum test showed that the probability of alarm calling varied between family (Kruskal-Wallis chi-square = 15.101, df = 3, P = 0.0017). Pairwise comparisons showed that, when compared to zebra finches, the probability of alarm calling was significantly different for Maluridae (W = 59.5, Z score = −3.355, P = 0.0008), Meliphagidae (W = 150.5, Z score = −2.295, P = 0.022), and Acanthizidae (W = 21.0, Z score = −2.857, P = 0.004).
Results from the field experiment with sample sizes reported above the graphs. a Proportion of trials of each family that resulted in flight (black), retreat (white), approach (light gray), no behavior (dark gray), or unseen (stippled) behavior in response to the gliding model raptor. b Proportion of trials of each family that resulted in a distinct alarm call (black) or no distinct call (white) elicited from the target individual
Overall, the field experiment showed that all families presented with the glider responded with appropriate behavior and alarm called on at least one occasion, apart from our focal species, zebra finches. Zebra finches tended to flee from the glider, but they did not emit a distinct alarm call or change their distance call structure to indicate alarm. The lack of an alarm call by zebra finches is unusual, and perhaps related to the perceived degree of threat, so we turned then to a captive experiment using computer-animated predator models simulating an even greater threat.
Captive experiment
Following from the unexpected results of the field experiment, we exposed captive zebra finches to computer-generated animations of a looming hawk to quantify their response to imminent danger. Looming objects expand rapidly in angular size, and reliably prompt attention and avoidance responses in a variety of species (Schiff et al. 1962; Schiff 1965; Carlile et al. 2006; Bach et al. 2009). Video images are used increasingly in studies of animal behavior (review: Woo and Rieucau 2011), including to simulate looming raptors (Carlile et al. 2006). We predicted that the finches would show a strong anti-predator response to video animation of a looming raptor in contrast to control stimuli that did not feature a looming object. Birds were tested in social groups, so that there was the opportunity to communicate with conspecifics. To test for vocal communication about danger, we compared calls produced following each of the stimuli with calls given under baseline conditions. The greater number of individuals tested in this study allowed us to analyze the dimorphic male and female distance calls separately, which could potentially uncover any differences in alarm calling behavior between the sexes. We carried out acoustic analyses to test for either structurally distinct alarm calls or longer term changes in calling rate that might signal danger.
Study species
Zebra finches used in this experiment were adult (older than 100 days) captive-bred descendants of wild-caught birds obtained from a breeding colony housed at the La Trobe University zoology reserve. Birds used were between 8 and 12 generations removed from wild birds, which were captured in northern Victoria by Richard Zann in 2001. When not part of a trial, birds were kept in large (8000 × 3000 × 3500 mm) aviaries with free access to an indoor and outdoor section, the latter with the roof and walls constructed from wire. The aviary is surrounded by native vegetation, and the captive birds have visual contact with aerial predators flying overhead and potential visual exposure to terrestrial predators such as snakes. A total of 27 males and 27 females were used, and all birds were banded with two to four colored leg bands for identification.
Experimental stimuli
To examine the responses of finches to a potential threat, we used Maya 2014 (Autodesk Inc.) software to create computer animations resembling a collared sparrowhawk. We simulated the highest level of threat with an 8-s animation of a looming sparrowhawk (loom), beginning as a small bird that slowly unfolds and flaps its wings, rapidly increasing in size as it reaches the endpoint with raised talons (Fig. 3a). The animation was accompanied by wing flap sounds increasing in amplitude (obtained from www.freesound.org), as the bird appears to draw nearer. We created a lower threat animation of a sparrowhawk flying past at a distance (flyby). It involved a 2-s clip of a sparrowhawk flying from left to right without changing in size, accompanied by two wing flaps and with the sound remaining at the same amplitude throughout (Fig. 3b). The final stimulus had no video playback and consisted solely of the same wing flap sounds as the looming stimulus and lasted 6 s. This control stimulus was used to determine if the finches were responding more to the sound of the wings or the image on the screen.
Area in pixels occupied by the animated collared sparrowhawk in a the loom stimulus and b the flyby stimulus. Occurrence of the wing flap sounds is indicated on the graphs with arrows. Screenshots of the actual animations used in the study are included with frame number indicated below each picture. The frame rate equals 25 fps
Experimental procedure
The birds were housed in a large outdoor aviary at the La Trobe University zoology reserve in Bundoora, Victoria, for the duration of the experiment. The aviary was custom-designed and consisted of three connected areas that the birds could access through small feeder doors. The two outer sections, referred to as holding aviaries, were the same size (2400 × 900 × 1820 mm), while the middle section, the experimental aviary, was smaller (900 × 900 × 1710 mm). Birds were provided with ad libitum seed (an equal mix of red panicum, yellow panicum, and Japanese millet), water, shell grit, and cuttlebone, with endive given twice weekly. The birds were allowed access to all three areas to acclimate for at least 5 days before trials began. The design of the aviary allowed for a small group of experimental birds to be confined to a limited area while still surrounded by members of their social group in the holding aviaries. The birds in holding aviaries could not view the stimuli presented on screen (below). Keeping birds in groups rather than isolated meant that they retained their natural social and acoustic environment, which seems likely to allow a more natural response to the predator stimuli (McCowan et al. 2015). It is also possible that individuals may not call if alone, particularly if the function of their call is to warn nearby conspecifics.
Zebra finches were tested in four cohorts of between 12 and 16 birds. A 24-in widescreen monitor (Dell UltraSharp U2410) was placed on a table at perch height (approximately 130 cm from the ground) outside of the aviary behind a clear Perspex window. Speakers (Sony SRS-A27) were placed either side of the monitor. The monitor and speakers were connected to a laptop (Toshiba Satellite P50t-A) kept behind the left-hand wall of the first aviary and out of view of the birds. A high-definition video camera (Canon XA10) was also placed at perch height on a bracket inside the aviary facing directly towards the monitor. A Sennheiser ME66 shotgun microphone with K6 powering module was connected to the camera. Audio recordings were sampled at 44.1 kHz and 16 bits. Birds were viewed via a webcam (Microsoft Lifecam VX-2000) stationed outside the aviary and connected to the laptop. A barrier of thick shade cloth was attached to the inner walls of both holding aviaries during testing to prevent the non-test birds from viewing the images on the monitor. A small container of seed was attached to the perch in line with the monitor and the camera in order to encourage birds to congregate in this area.
After the acclimation period, all birds in the cohort were confined to holding aviary 1. Three birds were caught and placed into the experimental aviary with access to the other sections cut off by shutting the feeder doors. The group of birds being tested was always of mixed sex, as this resembles a common foraging group structure in the wild (McCowan et al. 2015). The test birds were left undisturbed for at least 30 min. After this acclimation period and once all three birds were situated in front of the monitor, the first stimulus was presented. The birds were allowed at least 30 min between each stimulus presentation and 30 min after the final stimulus presentation before being moved to the third section of the aviary. Once a trial was over, the tested birds were restricted to holding aviary 2, but the other birds in the cohort were allowed access to both holding aviary 1 and the experimental aviary for at least 2 days before the next trial began. Stimuli were played in a random order and counterbalanced across 18 groups.
Data and statistical analyses
We analyzed both the visual behavior and vocalizations of the birds. Our approach to the analysis of vocalizations addressed the following three questions: (1) Do birds have an acoustically distinct flee alarm call? (2) If not, or in addition, does a change in call rate signal danger? (3) Could longer term changes in call structure signal a change in assessment of background level of danger? Four of the 18 trials were excluded from analysis due to equipment failures during one of the stimulus presentations. It was not possible to score data blind as the stimulus presentations were recorded on camera, but close analysis of video allowed unambiguous categorization of behavior. Any bird absent from the video camera’s field of view at the beginning of the presentation was omitted from the behavioral analysis. Some individuals were also omitted from vocal analyses as they did not produce enough calls or their recorded calls were unclear. All statistical analyses were performed in the R statistical environment (R Core Team 2013).
Behavioral response
Behavior was analyzed using Observer XT 11 (Noldus Inc.). For each stimulus presentation, a video clip from 30 s before a stimulus started until 30 s after it ended was extracted from raw footage using Adobe Premiere Elements 12 (Adobe Systems Inc.). The following index of increasingly strong response was used to score the behavior of each bird immediately after stimulus onset:
-
0 = No response: no change in behavior.
-
1 = Orienting: increase in head movement rate or turning to face the monitor.
-
2 = Startle: a quick flinching movement or slicking back of feathers.
-
3 = Duck: lowering of the head and body.
-
4 = Jump: flapping wings and leaving the perch for no more than 2 s, then returning to perch.
-
5 = Flight: flapping wings and leaving the perch completely to land on the aviary walls or elsewhere out of the camera’s field of view.
If more than one behavior was observed in an individual, the value of the highest scoring behavior was recorded. The highest level of response was determined for each individual for each stimulus and compared using a generalized linear mixed model using the glmer function from the lme4 package (Bates et al. 2015), specifying a Poisson error structure with a log link function. Stimulus was used as a fixed effect and bird identity and group as random effects. We examined pairwise contrasts between levels of stimulus directly.
Presence of alarm calls
To determine whether birds gave a distinct alarm call, we selected calls recorded during the acclimation period (baseline) and compared these with the first call given after the start of the stimulus (first call). We sampled baseline calls from audio recordings of the 30 min prior to the presentation of the first stimulus (Fig. 4). This period was divided into 60 × 30 s samples, and for each individual bird, we selected three samples of 30 s using a random number generator. The first clear call from each sample chosen was used for analysis. Spectrograms were visualized in Raven Pro 1.4 using aforementioned parameters. The calls selected during the baseline block occurred before any stimulus presentation and in the absence of any disturbances. Calls given after the start of stimulus were only sampled as first calls if they occurred within 5 min. All calls were bandpass filtered to 300 Hz before analysis.
Representative timelines for two test groups. Stimuli presentations are marked with dark boxes. The dark circle indicates the occurrence of the first vocalization after a stimulus presentation. Note that the 30-min baseline period (gray box) occurred immediately before the first stimulus, while the timing of the 30 samples after each stimulus (white boxes) varied slightly depending on the interval between stimuli. We obtained 30-min blocks in between the presentation of stimuli 1 and 2 (after 1) and in between stimuli 2 and 3 (after 2). In each case, the 30-min period was selected from the onset of the following stimulus and working backwards in time. We also selected 30 min following the presentation of the third and final stimulus (after 3)
Call similarity was analyzed in a similar way to that of calls recorded during the field study. For each bird, we calculated the similarity between pairs of baseline calls and between each baseline and first call in the similarity function in Sound Analysis Pro 2011 using aforementioned settings. For each bird, scores were averaged to obtain a single measure of call similarity during the baseline and a single measure of call similarity between baseline calls and first calls. This process was repeated for each of the three stimuli, and a linear mixed-effect model (lme function from the nlme package; Pinheiro et al. 2013) was used to compare statistically the baseline vs. first call similarity scores, fitting sex and stimulus as fixed effects and bird identity and group as random effects. Similarity scores were converted to proportions and transformed using the logit function prior to analysis.
Immediate changes in call rate
Even if birds do not have acoustically distinct alarm calls, they might signal immediate danger by changing the rate of the multi-functional distance calls, so we calculated the number of distance calls given by each individual in the 30 s directly before and after each stimulus presentation. The zebra finches did make other vocalizations during the experiment, mostly the two short, soft calls referred to as “tets” and “stacks” (Zann 1996); however, these were not included in any analyses as they were often too quiet to be clearly recorded by the equipment. Distance calls given while birds were in view of the camera could be assigned directly to individuals, and we used individual variation in distance call structure to assign calls to individuals in the rare event that they were temporarily out of view. Calls recorded when a bird was out of view were compared by ear and with spectrograms to known calls from individuals and assigned if they matched. We then compared the call rate in the 30 s before and after the stimulus using a linear mixed-effect model (lme), fitting sex, stimulus, and time period as fixed effects and bird identity and group as random effects. Call rates were transformed using the logit function prior to analysis.
Longer term changes in call structure
In addition to signaling immediate danger through alarm calls or changes in call rate, there might be ongoing changes in calls that signal a higher level of alertness or arousal. We therefore examined the call structure in a 30-min sample after each stimulus (Fig. 4). Three randomly selected calls were chosen from each 30-min sample in the same way that we selected baseline calls. We then examined variation in call structure across the experiment by comparing baseline vs. after and first call vs. after. Similarity scores were averaged to obtain a single value for each comparison for each bird. Linear mixed-effect models (lme) were used to statistically compare similarity scores, fitting sex and stimulus as fixed effects and bird identity and group as random effects and transforming response data using the logit function prior to analysis. We were unable to perform similar analyses for call rate in the 30-min periods due to the experimental setup with the combined vocalizations of the large social group of conspecifics present in the surrounding aviaries interfering with call identification.
Results (captive finches)
Behavioral response
The zebra finches responded most strongly to the most threatening stimulus (Fig. 5). The looming hawk stimulus usually prompted birds to fly from the perch, while the other two stimuli typically produced only orientation or startling (Fig. 5; mean ± SD response score: looming 4.06 ± 1.41, flyby 2.47 ± 1.25, sound only 2.09 ± 1.49). Pairwise contrasts from the model showed that behavioral responses to the looming stimulus were higher when compared to both the flyby (Z = −5.18, P < 0.001) and sound only (Z = −6.49, P < 0.001) stimuli. There was no significant difference in response between the sound only and flyby stimuli (Z = 1.423, P = 0.155).
Proportion of individuals in the captive study that displayed flight (black), jump (white), duck (light gray), startle (dark gray), or orienting (stippled) behavior in response to the three stimuli. The score of “no response” was not recorded for any trial. Sample sizes are reported above and vary slightly between stimuli as some birds were excluded from analysis as they were not visible on camera at stimulus onset
Presence of alarm calls
The birds did not give an acoustically distinct alarm call. The similarity among calls in the baseline period was no greater than that between the baseline and first call after the stimulus, showing that the first call after the stimulus did not differ from the undisturbed distance calls in the baseline period (Tables 2 (a) and 3 (a)). Females had slightly more variable calls than males, but there was no interaction between stimulus type and sex (Table 3 (a)). Figure 6 shows representative spectrograms of baseline calls from male and female birds, paired with first calls given by the same individuals after the looming hawk stimulus.
Representative spectrograms of male and female zebra finch contact calls recorded during the captive experiment. A male call before any stimuli presentations (a) and the first call from the same male given after the loom stimulus (b). The similarity score of these calls was 99.2%. A baseline female call (c) and the first call after the looming stimulus from the same female (d). The similarity score of these calls was also 99.2%
Immediate changes in call rate
Birds did not change their rate of calling in response to any of the three stimuli. The call rate in the 30 s before the stimulus did not differ from the 30-s period after for any stimulus (Fig. 7; Table 4). Females had a slightly lower call rate than males, but there was no interaction of sex with stimulus type or time period (Table 4; P > 0.3 for all comparisons).
Longer term changes in call structure
We found no long-term change in call structure. Similarity scores for baseline and after calls exceeded 95% similarity regardless of stimulus (Table 2 (b)), as did comparisons between first call and after calls (Table 2 (c)). There was no significant difference in similarity scores as a function of sex, stimulus type, or an interaction between sex and stimulus type for baseline vs. after and first call vs. after (Table 3 (b) and (c), respectively).
General discussion
Although zebra finches responded with anti-predator behavior to both the model hawk and the video playback stimuli, they did not produce any vocal signals of alarm. In the field, zebra finches usually fled from the model. Similarly, in the captive study, finches typically fled from the looming animated raptor and oriented or startled to the less intense stimuli. Despite this response, they did not give distinct alarm calls in the field or captive study, and an investigation into vocalizations produced during the playback experiment showed that they also did not change their rate of calling. Furthermore, distance call structure remained unchanged in the longer term period after stimuli compared to an earlier period of undisturbed calling. The lack of a detectable flee alarm call, or communication about immediate danger, is surprising in a social species like the zebra finch.
We can find no report of distinct flee alarm calls in the literature. There is limited evidence for any type of alarm call in zebra finches, and we found no support for the suggestion that distance calls can signal alarm. Incubating zebra finches left their nest earlier in response to an approaching person if their partner was perched nearby (Mainwaring and Griffith 2013), which might mean that signals or cues from the partner provided a warning. However, there were no recordings, and no alarm call was detected. Similarly, although thuk calls appear to alert young to potential danger near the nest (Zann 1996), as far as we know, they have not been tested experimentally. Lastly, a previous field study using similar methods resulted in no distinct alarm calls recorded from wild zebra finches after 31 glider presentations (C. Giuliano unpublished data). Clearly, there needs to be further investigation of anti-predator behavior in this well-studied species, including testing for potential communication about danger at the nest.
The methods used in our study were sufficient to prompt anti-predator responses in wild and captive birds in the absence of young, and so, we consider it unlikely that zebra finches give flee alarm calls to warn adult conspecifics of danger. In other field studies that provoked alarm calls, gliding model hawks were directed above or to the side of focal birds (Magrath et al. 2007), yet this method was also used in our field study and failed to elicit alarm calls from only the zebra finch. It is possible that the zebra finches did not perceive the glider as a high enough threat to warrant alarm calling, and they may be highly sensitive to approach direction (Wang and Frost 1992; Judge and Rind 1997; McMillan and Gray 2012). However, when we altered the approach direction, and thus the immediacy of the threat, the finches still did not emit an alarm call.
In addition, captive zebra finches did not alter their call rate directly after exposure to any stimulus. Even if these finches did not have a distinct flee alarm call, they might have signaled about immediate danger by changing the call rate, particularly as it has been suggested that distance calls serve multiple functions (Zann 1996). However, while there was considerable variation in distance call rate among individuals, there was no change in call rate in the 30 s following any type of stimulus compared to the 30 s before that stimulus. Several other species of passerine show an increased rate of general purpose calls after detecting predators (Wheatcroft 2015), and some species include more notes in alarm calls in situations of greater threat (Leavesley and Magrath 2005; Templeton et al. 2005; Fallow and Magrath 2010). Another possibility is that call rate could have dropped, and a decrease or cessation of calling might itself indicate danger. Zebra finches possess a large repertoire of calls, and individuals are constantly in vocal contact, so a sudden halt in vocalizations might be a cue of danger. Evidence of this exists in some other species (Dapper et al. 2011; Haff et al. 2014); however, in our study, we found no evidence to suggest that the finches were using a decrease or termination of calling to indicate predator presence.
Zebra finches are highly gregarious and extremely vocal, so the apparent lack of such a warning call is surprising and difficult to explain. Information on alarm calling in other Australian estrildid finches varies (Higgins et al. 2006). Some species (e.g., star finch, Neochmia ruficauda; red-browed finch, Neochmia temporalis) are known to possess a call distinct from other vocalizations which functions as an alarm call. Others (e.g., crimson finch, Neochmia phaeton) use both a specific call as well as a multi-functional distance call in alarm. Some estrildids are not known to alarm call; however, this could be due to little research conducted on the species’ vocalizations as a whole (e.g., double-barred finch, Taeniopygia bichenovii). Despite living in large flocks in the wild, zebra finches are not found in kin groups, which removes one benefit of calling. Perhaps personal risk during an attack is low in large flocks, thus reducing the net benefit of calling to warn others. Another possibility is that the wild zebra finches benefit further from joining mixed-species flocks, in which they gain advantages from group size effects and respond to alarm calls of heterospecifics. Several species of birds rarely give alarm calls while in mixed-species flocks, yet do respond to heterospecific calls (Goodale et al. 2010; Martínez and Zenil 2012; Magrath et al. 2015), and others respond to heterospecifics despite never alarm calling themselves (Goodale and Kotagama 2005, 2008; Griffin et al. 2005). Wild zebra finches do form mixed-species flocks, particularly during vulnerable activities like drinking and foraging (Higgins et al. 2006), but as far as we know, there have been no studies on the alarm calling dynamics of these mixed assemblages.
As well as not communicating about immediate danger, we detected no longer term change in call structure that might indicate a revised estimate of risk. According to Zann (1996), the zebra finch distance call is multi-functional and is used for simple contact between individuals and to communicate location, identity, and alarm. Whether or how this call is altered to express alarm has never been studied, and our results suggest that that the birds do not use any subtle changes in acoustic properties to communicate about perceived risk levels. The possibility that zebra finches do not rely on audible cues, despite being highly vocal with a vast repertoire, must be considered as well. This species is almost always found in pairs or groups, so it is possible that they rely on the visual cue of flockmates fleeing as an indicator of predator presence (e.g., Lima 1995).
Overall, we found that zebra finches exposed to simulated predators responded with appropriate behavior, but neither gave alarm calls nor altered call rate to signal immediate danger. Results from our studies suggest that the finches do not have a distinct call to warn adult conspecifics about predators, and the thuk alarm call described in Zann (1996) is probably used solely in parent-offspring communication to warn of potential danger. Investigating the vocalizations of adults with dependent offspring in response to predator stimuli would help to determine if the thuk call is specific to that context. The zebra finch is the most-studied bird in Australia and is a widely used model system (Griffith and Buchanan 2010); thus, it is surprising that very little is known about their anti-predator defenses. Future research on interactions with predators in the wild, including in mixed-species flocks, could provide insight into anti-predator behavior and the puzzling lack of flee alarm calls in this well-studied species.
References
Bach DR, Neuhoff JG, Perrig W, Seifritz E (2009) Looming sounds as warning signals: the function of motion cues. Int J Psychophysiol 74:28–33
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48
Bell MBV, Radford AN, Rose R, Wade HM, Ridley AR (2009) The value of constant surveillance in a risky environment. Proc R Soc Lond B 276:2997–3005
Blumstein DT, Armitage KB (1997) Alarm calling in yellow-bellied marmots: I. The meaning of situationally variable alarm calls. Anim Behav 53:143–171
Bradbury JW, Vehrencamp SL (2011) Principles of animal communication. Sinauer, Sunderland
Carlile PA, Peters RA, Evans CS (2006) Detection of a looming stimulus by the Jacky dragon: selective sensitivity to characteristics of an aerial predator. Anim Behav 72:553–562
Caro T (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Charif RA, Waack AM, Strickman LM (2010) Raven Pro 1.4 user’s manual. Cornell Lab of Ornithology, Ithaca
Curio E (1975) The functional organization of anti-predator behaviour in the pied flycatcher: a study of avian visual perception. Anim Behav 23:1–115
Dapper AL, Baugh AT, Ryan MJ (2011) The sounds of silence as an alarm cue in túngara frogs, Physalaemus pustulosus. Biotropica 73:380–385
Davies NB, Welbergen JA (2009) Social transmission of a host defense against cuckoo parasitism. Science 324:1318–1320
Fallow PM, Magrath RD (2010) Eavesdropping on other species: mutual interspecific understanding of urgency information in avian alarm calls. Anim Behav 79:411–417
Gill SA, Bierema AM-K (2013) On the meaning of alarm calls: a review of functional reference in avian alarm calling. Ethology 119:449–461
Giuliano C (2012) Do wild zebra finch (Taeniopygia guttata) respond to aerial alarm calls of sympatric heterospecifics? Honours thesis, La Trobe University, Melbourne, Australia
Goodale E, Kotagama SW (2005) Alarm calling in Sri Lankan mixed-species bird flocks. Auk 122:108–120
Goodale E, Kotagama SW (2008) Response to conspecific and heterospecific alarm calls in mixed-species bird flocks of a Sri Lankan rainforest. Behav Ecol 19:887–894
Goodale E, Beauchamp G, Magrath RD, Nieh JC, Ruxton GD (2010) Interspecific information transfer influences animal community structure. Trends Ecol Evol 25:354–361
Griesser M, Ekman J (2004) Nepotistic alarm calling in the Siberian jay, Perisoreus infaustus. Anim Behav 67:933–939
Griffin AS, Savani RS, Hausmanis K, Lefebvre L (2005) Mixed-species aggregations in birds: zenaida doves, Zenaida aurita, respond to the alarm calls of carib grackles, Quiscalus lugubris. Anim Behav 70:507–515
Griffith SC, Buchanan KL (2010) The zebra finch: the ultimate Australian supermodel. Emu 110:v–xii
Haff TM, Horn AG, Leonard ML, Magrath RD (2014) Conspicuous calling near cryptic nests: a review of hypotheses and a field study on white-browed scrubwrens. J Avian Biol 45:1–14
Higgins PJ, Peter JM (2002) Handbook of Australian, New Zealand and Antarctic birds. Volume 6: pardalotes to shrike-thrushes. Oxford University Press, Melbourne
Higgins PJ, Peter JM, Steele WK (2001) Handbook of Australian, New Zealand and Antarctic birds. Volume 5: tyrant-flycatchers to chats. Oxford University Press, Melbourne
Higgins PJ, Peter JM, Cowling SJ (2006) Handbook of Australian, New Zealand and Antarctic birds. Volume 7: boatbill to starlings. Oxford University Press, Melbourne
Hollén LI, Radford AN (2009) The development of alarm call behaviour in mammals and birds. Anim Behav 78:791–800
Hollén LI, Bell MBV, Radford AN (2008) Cooperative sentinel calling? Foragers gain increased biomass intake. Curr Biol 18:576–579
Judge SJ, Rind FC (1997) The locust DCMD, a movement-detecting nurone tightly tuned to collision trajectories. J Exp Biol 200:2209–2216
Jurisevic MA, Sanderson KJ (1994a) Alarm vocalisations in Australian birds: convergent characteristics and phylogenetic differences. Emu 94:69–77
Jurisevic MA, Sanderson KJ (1994b) The vocal repertoires of six honeyeater (Meliphagidae) species from Adelaide, South Australia. Emu 94:141–148
Klump GM, Shalter MD (1984) Acoustic behaviour of birds and mammals in the predator context. Z Tierpsychol 66:189–226
Klump GM, Kretzschmar E, Curio E (1986) The hearing of an avian predator and its avian prey. Behav Ecol Sociobiol 18:317–323
Leavesley AJ, Magrath RD (2005) Communicating about danger: urgency alarm calling in a bird. Anim Behav 70:365–373
Lima SL (1995) Collective detection of predatory attack by social foragers: fraught with ambiguity? Anim Behav 50:1097–1108
Magrath RD, Pitcher BJ, Gardner JL (2007) A mutual understanding? Interspecific responses by birds to each other’s aerial alarm calls. Behav Ecol 18:944–951
Magrath RD, Haff TM, Fallow PM, Radford AN (2015) Eavesdropping on heterospecific alarm calls: from mechanisms to consequences. Biol Rev 90:560–586
Mainwaring MC, Griffith SC (2013) Looking after your partner: sentinel behaviour in a socially monogamous bird. PeerJ 1:e83
Manser MB (1999) Response of foraging group members to sentinel calls in suricates, Suricata suricatta. Proc R Soc Lond B 266:1013–1019
Manser MB (2001) The acoustic structure of suricates’ alarm calls varies with predator type and the level of urgency. Proc R Soc Lond B 268:2315–2324
Marchant S, Higgins PJ (1993) Handbook of Australian, New Zealand and Antarctic birds. Volume 2: raptors to lapwings. Oxford University Press, Melbourne
Marler P (1955) Characteristics of some animal calls. Nature 176:6–8
Martínez AE, Zenil RT (2012) Foraging guild influences dependence on heterospecific alarm calls in Amazonian bird flocks. Behav Ecol 23:544–550
Maynard Smith J (1965) The evolution of alarm calls. Am Nat 99:59–63
McCowan LSC, Mariette MM, Griffith SC (2015) The size and composition of social groups in the wild zebra finch. Emu 115:191–198
McMillan GA, Gray JR (2012) A looming-sensitive pathway responds to changes in the trajectory of object motion. J Neurophysiol 108:1052–1068
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Development Core Team (2013) nlme: Linear and nonlinear mixed effects models. R package version 3.1–111, http://CRAN.R-project.org/package=nlme
Pizzey G, Knight F (2012) The field guide to the birds of Australia. HarperCollins Publishers, Sydney
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, https://www.R-project.org/
Schiff W (1965) Perception of impending collision: a study of visually directed avoidant behavior. Psychol Monogr 79:1–26
Schiff W, Caviness JA, Gibson JJ (1962) Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science 136:982–983
Sherman PW (1977) Nepotism and the evolution of alarm calls. Science 197:1246–1253
Sloan JL, Hare JF (2008) The more the scarier: adult Richardson’s ground squirrels (Spermophilus richardsonii) assess response urgency via the number of alarm signallers. Ethology 114:436–443
Sridhar H, Beauchamp G, Shanker K (2009) Why do birds participate in mixed-species foraging flocks? A large-scale synthesis. Anim Behav 78:337–347
Srinivasan U, Raza RH, Quader S (2010) The nuclear question: rethinking species importance in multi-species animal groups. J Anim Ecol 79:948–954
Sullivan K (1985) Selective alarm calling by downy woodpeckers in mixed-species flocks. Auk 102:184–187
Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP (2000) A procedure for an automated measurement of song similarity. Anim Behav 59:1167–1176
Templeton CN, Greene E, Davis K (2005) Allometry of alarm calls: black-capped chickadees encode information about predator size. Science 308:1934–1937
Wang Y, Frost BJ (1992) Time to collision is signalled by neurons in the nucleus rotundus of pigeons. Nature 356:236–238
Wheatcroft D (2015) Repetition rate of calls used in multiple contexts communicates presence of predators to nestlings and adult birds. Anim Behav 103:35–44
Wilson DR, Evans CS (2008) Mating success increases alarm-calling effort in male fowl, Gallus gallus. Anim Behav 76:2029–2035
Woo KL, Rieucau G (2011) From dummies to animations: a review of computer-animated stimuli used in animal behavior studies. Behav Ecol Sociobiol 65:1671–1685
Zann RA (1996) The zebra finch: a synthesis of field and laboratory studies. Oxford University Press, New York
Zuberbühler K (2000) Referential labelling in Diana monkeys. Anim Behav 59:917–927
Zuberbühler K (2009) Survivor signals: the biology and psychology of animal alarm calling. Adv Stud Behav 40:277–322
Acknowledgments
The authors would like to thank Juan Hernandez and Jon Salisbury for help with constructing the gliders, and Tom Chandler and Chandara Ung at Monash University for creating the animations. Assistance in the field was provided by Melissa Van De Wetering, Bridie Clarke, Heather Maginn, José Ramos, Jordan de Jong, Travis Dutka, Jemima Connell, Kristian Bones Enger, Shannan Courtenay, Lauren Grimes, Tom Handley, and Kathryn Lyons. Thanks to Nicola Khan for helping with husbandry of the finches and Angela Simms for assisting with video analysis. Thanks to our anonymous reviewers for providing valuable feedback on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research was supported by funding from La Trobe University. Field work was supported by a Holsworth Wildlife Research Endowment awarded to NEB.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This article does not contain any studies with human participants by any of the authors. All experimental procedures were approved by La Trobe University’s Animal Ethics Committee (AEC protocol no. 12-39) and conducted under Victorian Government DELWP permit nos. 10007632 and 10007491 and NSW National Parks and Wildlife Service permit no. SL101447.
Data availability
The datasets generated during and/or analyzed during the current study are available in the La Trobe University FigShare repository and have the following DOI: 10.4225/22/592e4f968fe8d
Additional information
Communicated by P. A. Bednekoff
Rights and permissions
About this article
Cite this article
Butler, N.E., Magrath, R.D. & Peters, R.A. Lack of alarm calls in a gregarious bird: models and videos of predators prompt alarm responses but no alarm calls by zebra finches. Behav Ecol Sociobiol 71, 113 (2017). https://doi.org/10.1007/s00265-017-2343-z
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2343-z