Abstract
Long-term monogamy is a key characteristic of family living across animals. The evolutionary maintenance of long-term monogamy has been suggested to be facilitated by increased reproductive coordination as a result of mate familiarity, leading to increased reproductive success. However, such effects can be compromised if females mate outside the pair bond (e.g. female polyandry), introducing conflicts of interest between the male and female. Here, we experimentally test the effects of both mate familiarity and female polyandry on agonistic behaviour and reproduction in a family living lizard, Liopholis whitii. We found that mate familiarity did not decrease the level of aggression between pairs whereas reducing female polyandry did. However, we did not find an effect of either mate familiarity or female polyandry on female reproductive output. These results suggest that male behavioural responses to female polyandry may influence pair stability in Liopholis whitii, providing support for the growing appreciation of the multiple ways in which female polyandry can influence the stability of family living.
Significance statement
Family living is underpinned by social pair bonds between adults (i.e. stable social monogamy). Therefore, key to understanding the emergence and maintenance of family living is identifying factors influencing pair bonds. We manipulated both female polyandry and mate familiarly in replicated enclosure experiment using social lizards to test their role in mediating within-pair aggression and ultimately the coordination of reproductive behaviour and hence reproductive output. We found that polyandry but not mate familiarity influenced levels of aggression between pairs but this did not transmit into concomitant effects on reproductive output.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Family living is characterised by the presence of long-term pair bonds between adults (hereafter stable social monogamy) and prolonged adult-offspring associations. The evolutionary maintenance of stable social monogamy has been suggested to be favoured because partners that have been together for an extended period of time may be more coordinated in their reproductive behaviour, resulting in increased reproductive output (the ‘mate familiarity’ hypothesis – Black 1996). However, while there is empirical evidence that reproductive investment increases with the length of the pair bond (e.g. Black 2001; Pyle et al. 2001; van de Pol et al. 2006; Adkins-Regan and Tomaszycki 2007; Griggio and Hoi 2011; Sánchez-Macouzet et al. 2014), the majority of studies have been unable to separate out the effects of pair stability from that of male and female breeding age (van de Pol et al. 2006; Sánchez-Macouzet et al. 2014). To address this, we need studies in which we can manipulate pair familiarity in an experimental context and examine the consequences for pair coordination and ultimately reproductive success.
The benefits of social monogamy will not only depend on the length of the pair bond, but also on a number of other social behaviours. One behaviour that can disrupt pair bond stability is when males and females pursue and/or accept copulations from individuals outside the pair bond (e.g. female polyandry; Taylor et al. 2014). Female polyandry has been shown to increase intersexual aggression between males and females (Valera et al. 2003), increase the risk on infaticide by the social male (e.g. Robertson 1990; Osorio-Beristain and Drummond 2001) and reduce paternal investment in care, including male desertion (e.g. Griffin et al. 2013). In the long term, persistent female polyandry can result in the evolutionary dissolution of social monogamy and instead promote the emergence of a more promiscuous social and mating system (Kokko 1999).
Here, we experimentally examined the effects of pair familiarity and female polyandry on male-female behaviour and female reproductive output in a family living lizard, Liopholis whitii. L. whitii belong to the Egernia group of lizards characterised by large diversity in both their social and mating behaviour, from solitary species through to those that form large communal family groups (reviewed by Chapple 2003; Gardner et al. 2015; While et al. 2015). Importantly, social organisation across the group is underpinned by stable social monogamy and relatively low levels of extra-pair paternity (Chapple 2003). In some species, pairs have been recorded to last more than 25 years (e.g. in the sleepy lizard Tiliqua rugosa; Leu et al. 2015). It has been suggested that the maintenance of long-term monogamy in this and other species in the Egernia group is driven by increased coordination of reproductive behaviour resulting in increased reproductive success (Bull 2000; Leu et al. 2015). Female polyandry, on the other hand, is expected to decrease pair stability and compromise the maintenance of long-term monogamy via the introduction of conflicts of interest between males and females within the pair bond (While et al. 2009a; see also Valera et al. 2003). To test these hypotheses, we experimentally manipulated pair familiarity and female polyandry in a fully factorial design and examined the extent to which this influenced (a) levels of aggression between male and female partners and (b) the consequences of this for female reproductive output.
Methods
Study species
White’s skink (L. whitii) is a medium-sized (up to 100 mm snout vent length, SVL) viviparous skink distributed throughout a wide altitudinal range (0–1600 m) and broad habitat types in south-eastern Australia (Chapple 2003; Wilson and Swan 2013; Cogger 2014). We used L. whitii from a population on the east coast of Tasmania, Australia (42° 57′ S, 157° 88′ E). Individuals at this study site are found in discrete patches of open grassland in close proximity to excavated burrows or rock crevices that are used as retreat sites. L. whitii reproduce annually, with mating occurring during the austral spring (September–October) (While et al. 2009b). Gestation spans 3–4 months and birth of offspring occurs in the austral summer (January–February). Tasmanian populations of L. whitii live in stable social groups typically consisting of a single female and her male partner, often along with a cohort of 1–3 juvenile or sub-adult individuals (While et al. 2009b). Approximately 70% of adults exhibit stable long-term pair bonds (While et al. 2009b), with pair bonds lasting up to 10 years (GMW et al. unpublished data). Levels of extra-pair paternity are moderate within this population, with extra-pair offspring comprising about 30% of the offspring born each year (While et al. 2009b).
Field and experimental methods
We captured a total of 120 adult L. whitii (72 males and 48 females) at the start of the breeding season (early September) in 2015. Lizards were captured using mealworm fishing and noosing techniques (as outlined in While et al. 2014). At their time of capture, individuals were weighed (± 1 mg), measured (snout-vent length, total length ± 0.5 mm) and toe clipped for permanent identification. Toes were kept for DNA analysis to allow later assignment of paternity (see below). Lizards were then released into small (1 m diameter) outdoor enclosures at the University of Tasmania’s animal compound. Each enclosure was supplied with a brick block for basking, a 30 × 15 cm steel sheet for shelter, along with water and food (Tenebrio larvae) provided ad libitum. Each of 48 enclosures housed a male-female pair. The remaining 24 un-partnered males were also housed in these enclosures, but individually and used as extra-pair males for the polyandrous treatment (see below).
We manipulated pair familiarity and female polyandry in a 2 by 2 factorial design. To manipulate pair familiarity, we constructed male female pairs from either lizards that had been caught in the same burrow system (n = 24) or by constructing male-female pairs from lizards that had been caught in separate burrows (n = 24). Shared burrow use by a single male and his female partner is the key characteristic of L. whitii pairs, which rely on these permanent burrow sites to undertake the majority of their basking, foraging and social behaviours (While et al. 2009a, 2011, 2014; see also Chapple 2003; Chapple and Keogh 2006). We crossed our manipulation of pair familiarity with a manipulation of female polyandry, by creating monogamous and polygynous treatment groups. To achieve this, we gave females access to either only their social partner or their social partner and additional males during a 3-week mating period (mating period in L. whitii goes from mid-September to mid-October; McEvoy et al. 2013). Specifically, for the monogamous treatment, females were given access to only their social partner for the duration of the mating period. In contrast, females in the polyandrous treatment had their social male partner removed and replaced with an extra-pair male (from one of the 24 un-partnered males). The extra-pair male was with the female for 2 days of the week before being removed and replaced with the female’s original male partner. The females in the monogamous treatment also had their social male partner removed during the same period (as a control), but without a male replacing him during his absence. Three male removal and return cycles occurred over 3 weeks, up until the completion of the mating season (mid-October). Each female in the polyandrous treatment had access to two extra pair males in total (one male in weeks 1 and 2, and a second, different male in week 3). The extra pair males chosen for each female were selected based on size (such that size differences in SVL were minimised) and genetic structure; only extra pair males caught between 40 and 200 m from a given female were mated, to avoid any female mating biases based on inbreeding or outbreeding effects (see While et al. 2014; Bordogna et al. 2016).
To quantify levels of male-female aggression between pair members, we recorded pair interactions between each male and female pair once a day for a 4-week period following the mating season using GoPro cameras (Hero3+, California, USA). One-hour periods were filmed of the female and her social partner. Filming occurred between 0900 and 1200, when temperatures are most suitable for high levels of lizard activity. From the footage, we could record three key variables associated with aggression and conflict between adults: chasing, biting and fleeing (see McEvoy et al. 2013 for a detailed description of aggression in these lizards). Biting describes one individual biting their partner, chasing describes the action of one individual aggressively chasing their partner without contact being made, and fleeing describes the action of an individual attempting to escape an enclosure by scrambling in the enclosure’s periphery (independently of being chased by their partner). We recorded both total number of aggressive interactions between partners as well as, for a subset of videos in which we could identify the identity of the aggressor (n = 27), the extent of male to female vs. female to male aggression. All behavioural observations were collected by two observers (TBJ and JS) and videos were scored blind with regard to treatment to minimise observer bias. A subset (n = 12) of videos were scored by both observers to confirm inter-observer reliability, which was found to be high in all cases (Cohen’s kappa (k) greater than 0.75 for each variable; k biting = 1.00, k chasing = 1.00, k fleeing = 0.79; Kaufman and Rosenthal 2009).
At the end of female gestation (mid-January), individuals were moved into the indoor terrestrial ecology facilities at the University of Tasmania, where they were housed individually in plastic terraria as described above. Female containers were checked at 2-h intervals daily for the birth of offspring. For each offspring, the date of birth, weight (± 1 mg), SVL and total length (± 0.5 mm) were recorded. Offspring were toe clipped for permanent identification, with toes kept to allow DNA analysis for later assignment of paternity (see below). Male and female pairs were then released along with their offspring at the females site of capture.
Parentage assignment and confirmation of polyandry manipulation success
All individuals included in the study were genotyped for three microsatellite loci (EST1, EST2, EST4: Gardner et al. 1999) using standard molecular techniques with DNA extracted from tail tip samples (see While et al. 2011 for further details). We only used three microsatellites because (a) the low number of potential dads (n = 3) meant a limited number of microsatellites were required to distinguish between potential fathers and (b) the aim of parentage assignment was simply to confirm that our manipulation of polyandry resulted in mating between the female and the extra pair male. Paternity was assigned using the computer program CERVUS 3.0 (Marshall et al. 1998) using the following simulation parameters: 10,000 cycles, 95% of candidate parents sampled, 95% loci typed, and a genotyping error rate of 1% (calculated in CERVUS from our data). The ‘one known parent’ option was used, with all adult males released into the same enclosure as the mother included as possible fathers. Paternity was assigned to the male with the highest male–female–offspring trio LOD score and the lowest number of mismatches (0 or 1) (e.g. Gardner 2002; Chapple and Keogh 2005; While et al. 2011).
Data analyses
Data were analysed using a combination of ANOVAs, Generalized Linear Models (GLMs), and Generalized Linear Mixed Models (GLMMs) fit by maximum likelihood. These were run in R version 3.1.0 (R development core team 2015) using the ‘aov’ function for ANOVA models, the ‘glm’ function for the GLM, and the ‘glmer’ function for GLMMs (Bates et al. 2016). For GLMMs, Laplace approximation was used to estimate model parameters, as it is a more accurate technique than the simpler and widely used pseudo quasi-likelihood estimation method (Bolker et al. 2009). Estimates of fixed effects for all models were obtained with the ‘car’ package (Fox and Weisberg, 2011). We report results for models containing all main effects and significant interactions following backward elimination of non- significant interactions. Results are reported as means, with standard errors as the measure of variability. All data were checked for violation of assumptions, and no violations were found.
We examined differences in the level of aggression between males and females within their pairs as a function of the two treatments using GLMMS with a poisson distribution. Specifically, three models were run separately with frequency of biting, chasing and fleeing behaviours as dependent variables. Pair familiarity and polyandry treatment were entered as fixed factors and time was included as a covariate (to account for some pairs being filmed for slightly longer overall than others). Models were initially overdispersed, but this was corrected by including a subject level random effect to account for overdispersion. For 27 videos, we could identify the male and female. For these videos, we ran three additional GLMMS (with a poisson distribution) to examine differences between males and females in the frequency of biting, chasing and fleeing behaviours. Additionally, changes in female weight were analysed (non-pregnant females were used, to avoid confounding weight changes with clutch mass) from the start to the finish of the mating season to assess whether our treatments had any consequences for females in terms of reduced body condition. This was analysed using a type III ANOVA.
To examine the consequences of the two treatments, and the subsequent differences in conflict between the two treatments on female reproductive traits, we ran several models. First, we used a GLM with the binomial family specified to test for any differences between the treatments in successfully producing offspring. Secondly, we analysed whether there were any differences between treatments in female reproductive output (relative clutch mass; Shine 1980), average birthdate of offspring, average offspring mass and average offspring condition). Offspring condition measurements were calculated by dividing mass by SVL (Green 2001). These analyses were conducted using type III ANOVAs. Each model included litter size as a covariate.
Results
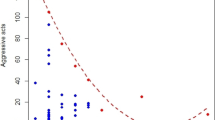
In total, 500 h of adult interaction footage were recorded, giving us 2302 independent observations of aggression and avoidance behaviours between males and females. There was no effect of mate familiarity on the frequency of fleeing, biting or chasing within pairs. However, males and females chased each other and fled more in the polyandrous treatment compared to the monogamous treatment. (Table 1, Fig. 1). Males were the main instigators of aggressive interactions in almost all instances. Indeed, males were observed chasing (4.66 ± 1.07 chases/h) and biting (3.59 ± 0.60 bites/h) females significantly more often than females were observed chasing (0.81 ± 0.81 chases/h) and biting (1.4 ± 0.79 bites/h) males (chases, χ2 = 80.74, p < 0.001; bites, χ2 = 25.56, p < 0.001). There was no difference in the number of times males and females were observed trying to flee (7.92 ± 2.95 flees/h vs 7.18 ± 3.42 flees/h; χ2 = 0.98, p = 0.32). There was no significant effect of treatment on female weight change from the start to the end of the mating period (Table 1).
Fourteen out of the 42 females (33%) recaptured at the end of the mating period gave birth, resulting in a total of 35 offspring. Paternity analysis confirmed that our polyandrous treatment resulted in a successful manipulation of extra pair paternity, with 6 out of 7 clutches (86%) produced by polyandrous females containing at least one extra-pair offspring. There was no difference in the likelihood of giving birth between either treatment (Table 2). We also found no significant difference between either familiar and unfamiliar pairs or monogamous and polyandrous pairs in birth date, relative clutch mass, average offspring mass or average offspring condition (Table 2).
Discussion
There is a considerable interest in understanding the evolution and maintenance of stable social monogamy (reviewed in Reichard and Boesch 2003). One of the key explanations for the maintenance of stable monogamous pair bonds is that it enhances reproductive performance via increased reproductive coordination (Black 1996). Our study represents the first examination of pair dynamics and reproductive output in response to experimental manipulation of mate familiarity in a reptile (and, to the best of our knowledge, a non-avian species more generally). Furthermore, we combined this manipulation with a manipulation of polyandry, which can create conflicts of interest between the female and her social partner (e.g. Griffin et al. 2013), leading to a disruption of stable social monogamy. We showed that polyandry, but not mate familiarity, resulted in a significant exaggeration of agonistic behaviours between males and females within a pair, indicative of increased within-pair conflict. However, we found no effect of either mate familiarity or female polyandry on female reproductive output, albeit with limited statistical power to detect smaller effect sizes. Below, we discuss potential explanations for these results and their broader implications for understanding the evolution of social monogamy.
One of the primary explanations for the maintenance of stable social monogamy, including in Egernia lizards, is that mate familiarity enhances reproductive output through enhanced pair coordination (Bull 2000; see also Black 1996). For example, recent research on stable social monogamy in Tiliqua rugosa showed that long-term pairs mated earlier in the mating season than shorter term pairs, which may enhance offspring survival if earlier mating correlates with earlier offspring birth date (Bull 1988; Leu et al. 2015). In contrast, we found little evidence that mate familiarity influences pair aggression/coordination in L. whitii, nor did we not find evidence that it increases female reproductive output. These conflicting results may reflect the nature of the mating system in L. whitii. Specifically, in contrast to the majority of other systems used to explore the mate familiarty hypothesis (e.g. Black 2001; Pyle et al. 2001; van de Pol et al. 2006; Adkins-Regan and Tomaszycki 2007; Griggio and Hoi 2011; Sánchez-Macouzet et al. 2014), including T. rugosa (Leu et al. 2015), L. whitii live with their social partner for the duration of the breeding and non-breeding period (Chapple and Keogh 2006; While et al. 2009b). As a result, pair familiarity may be of less functional significance for the finding, re-acquainting and priming of social partners in L. whitii compared to other systems. However, it is important to note that the moderate number of females who went through reproduction (33%, on average 60% of female L. whitii go through reproduction in a year in the wild; While et al. 2009a, b) within our experimental set up limited our ability to tease apart more subtle differences in reproductive investment between familiar and unfamiliar partners. Irrespective of this, the lack of differences in aggressive interactions and the lack of a difference in the likelihood of giving birth between familiar and unfamiliar pairs still suggests limited support for the mate familiarity hypothesis.
In contrast to mate familiarity, we did find a significant effect of polyandry on intra-pair aggression. Specifically, pairs in the polyandrous treatment exhibited increased aggression toward one another compared to pairs in the monogamous treatment, despite that male removal occurred in both treatments. Furthermore, aggression was primarily directed toward the female by the male. This suggests that males are able to assess the risk of polyandry directly via chemical recognition mechanisms, as has been shown for other species (e.g. the sand lizard Lacerta agilis; Olsson et al. 2004). This could occur through a female’s partner either recognising chemical cues left by a rival male directly in his territory, or indirect cues left in the female during mating. Regardless of the specific mechanism, this result provides further evidence that kin recognition functions in a wide number of contexts in the Egernia (e.g. mate choice; While et al. 2014; parental care, While et al. 2009a). Further, the increased aggression associated with mating outside the pair bond is consistent with theoretical suggestions that aggression may serve as a male adaptation to punish females for undertaking extra-pair copulations (Johnstone and Keller 2000). However, despite substantial work on extra-pair paternity, this study represents one of the few empirical examples that inferred risk of paternity increases aggression between pair members (see also Valera et al. 2003).
Increased aggression as a result of polyandry could have significant implications for female fitness given that increased inter-sexual aggression has been shown to influence reproductive output in female lizards (Le Galliard et al. 2005). However, we did not find any direct consequences of enhanced conflict between polyandrous pairs for female reproductive output. As with the mate familiarity hypothesis, whether the increased inter-sexual aggression resulting from polyandry has more subtle effects on female fitness requires additional work. For example, Le Galliard et al. (2005) found no effect of inter-sexual aggression on female reproductive output in the following reproductive event, they did find a negative effect of inter-sexual aggression of female reproductive output when measured across a female’s lifetime. Irrespective of the extent of direct or indirect effects of polyandry on female fitness, our results here suggest that extra pair mating by females may undermine the stability of pair bonds in this system through increased conflict. This supports previous research suggesting a negative effect of female polyandry on family stability, via its effects on reduced paternal investment in offspring tolerance in this system (While et al. 2009a). Female polyandry may, therefore, represent a trade-off for females between the benefits of undertaking extra-pair copulations in terms of inbreeding avoidance (see While et al. 2014) and the costs in terms of increased male aggression (this study) and reduce male tolerance of offspring (see While et al. 2009a).
In conclusion, we have shown through an experimental approach that polyandry but not mate familiarity influences within-pair aggression in a socially monogamous lizard. This adds to the growing body of work articulating the extent to which female mating behaviour can have fundamental implications for the maintenance and diversification of complexity sociality (e.g. Cornwallis et al. 2010; Griffin et al. 2013). Moving forward, more work is required to understand the factors responsible for the origins and maintenance of stable social monogamy in this system. We suggest that limited resource availability (mates and/or territories) may be important. Egernia lizard ecology is characterised by high habitat saturation, with strong territoriality and relatively long lifespans creating low breeder turn-over and intense competition over access to limited permanent crevice sites (O’Connor and Shine 2004; Langkilde et al. 2005; While et al. 2009b). This may elevate the risk of being left without a mate or territory when switching mates between breeding seasons (see also Choudhury 1995) resulting in selection on the maintenance of social monogamy between seasons. Data on the costs and benefits of long-term pairing in the wild and well-replicated experimental manipulations of habitat availability across seasons may help further our understanding of the role resources play in stable social monogamy (e.g. Halliwell et al. 2017).
References
Adkins-Regan E, Tomaszycki M (2007) Monogamy on the fast track. Biol Lett 3:617–619
Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, Dai B, Grothendieck G, Green P, Bolker MB (2016) Package ‘lme4’. https://cran.r-project.org/web/packages/lme4/lme4.pdf
Black JM (1996) Introduction: pair bonds and partnerships. In: Black JM (ed) Partnerships in birds: the study of monogamy. Oxford University Press, Oxford, pp 3–20
Black JM (2001) Fitness consequences of long-term pair bonds in barnacle geese: monogamy in the extreme. Behav Ecol 12:640–645
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24:127–135
Bordogna G, Cunningham GC, Fitzpatrick FJ, Halliwell B, MacGregor HEA, Munch KL, Wapstra E, While GMW (2016) An experimental test of relatedness-based mate discrimination in a social lizard. Behav Ecol Sociobiol 70:2139–2147
Bull CM (1988) Mate fidelity in an Australian lizard Trachydosaurus rugosus. Behav Ecol Sociobiol 23:45–49
Bull CM (2000) Monogamy in lizards. Behav Process 51:7–20
Chapple DG (2003) Ecology, life-history, and behavior in the Australian Scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol Monogr 17:145–180
Chapple DG, Keogh JS (2005) Complex mating system and dispersal patterns in a social lizard, Egernia whitii. Mol Ecol 14:1215–1227
Chapple DG, Keogh JS (2006) Group structure and stability in social aggregations of white’s skink, Egernia whitii. Ethology 112:247–257
Choudhury S (1995) Divorce in birds: a review of the hypotheses. Anim Behav 50:413–429
Cogger H (2014) Reptiles and amphibians of Australia, 7th edn. CSIRO Publishing, Melbourne
Cornwallis CK, West SA, Davis KE, Griffin AS (2010) Promiscuity and the evolutionary transition to complex societies. Nature 466:969–972
Fox J, Weisberg S (2011) An {R} companion to applied regression, 2nd edn. Sage Publications, Thousand Oaks
Gardner MG, Cooper SJB, Bull CM, Grant WN (1999) Isolation of microsatellite loci from a social lizard, Egernia stokesii, using a modified enrichment procedure. J Hered 90:301–304
Gardner MG, Bull CM, Cooper SJB (2002) High levels of genetic monogamy in the group-living Australian lizard Egernia stokesii. Mol Ecol 11:1787–1794
Gardner MG, Pearson SK, Johnston GR, Schwarz MP (2015) Group living in squamate reptiles: a review of evidence for stable aggregations. Biol Rev 91:925–936
Green AJ (2001) Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82:1473–1483
Griffin AS, Alonzo SH, Cornwallis CK (2013) Why do cuckolded males provide paternal care? PLoS Biol 11:e1001520
Griggio M, Hoi H (2011) An experiment on the function of the long-term pair bond period in the socially monogamous bearded reedling. Anim Behav 82:1329–1335
Halliwell B, Uller T, Wapstra E, While GM (2017) Resource distribution mediates social and mating behavior in a family living lizard. Behav Ecol 28:145–153
Johnstone RA, Keller L (2000) How males can gain by harming their mates: sexual conflict, seminal toxins, and the cost of mating. Am Nat 156:368–377
Kaufman AB, Rosenthal R (2009) Can you believe my eyes? The importance of interobserver reliability statistics in obserations of animal behaviour. Anim Behav 78:1487–1491
Kokko H (1999) Cuckoldry and the stability of biparental care. Ecol Lett 2:247–255
Langkilde T, Lance VA, Shine R (2005) Ecological consequences of agonistic interactions in lizards. Ecology 86:1650–1659
Le Galliard JF, Fitze PS, Ferriere R, Clobert J (2005) Sex ratio bias, male aggression, and population collapse in lizards. Proc Natl Acad Sci USA 102:18231–18236
Leu ST, Burzacott D, Whiting MJ, Bull CM (2015) Mate familiarity affects pairing behaviour in a long-term monogamous lizard: evidence from detailed bio-logging and a 31-year field study. Ethology 121:760–768
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
McEvoy J, While GM, Sinn DL, Wapstra E (2013) The role of size and aggression in intrasexual male competition in a social lizard species, Egernia whitii. Behav Ecol Sociobiol 67:79–90
O’Connor DE, Shine R (2004) Parental care protects against infanticide in the lizard Egernia saxatilis (Scincidae). Anim Behav 68:1361–1369
Olsson M, Madsen T, Ujvari B, Wapstra E (2004) Fecundity and MHC affects ejaculation tactics and paternity bias in sand lizards. Evolution 58:906–909
Osorio-Beristain H, Drummond H (2001) Male boobies expel eggs when paternity is in doubt. Behav Ecol 12:16–21
Pyle P, Sydeman WJ, Hester M (2001) Effects of age, breeding experience, mate fidelity and site fidelity on breeding performance in a declining population of Cassin’s auklets. J Anim Ecol 70:1088–1097
R Development Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org/
Reichard UH, Boesch C (2003) Monogamy: mating strategies and partnerships in birds, humans and other mammals. Cambridge University Press, Cambridge
Robertson RJ (1990) Tactics and counter-tactics of sexually selected infanticide in tree swallows. In: Blondel J, Gosler A, Lebreton JD, McCleery R (eds) Population biology of passerine birds: an integrated approach. Springer, Berlin, pp 381–390
Sánchez-Macouzet O, Rodríguez C, Drummond H (2014) Better stay together: pair bond duration increases individual fitness independent of age-related variation. Proc R Soc B 281:20132843
Shine R (1980) “Costs” of reproduction in reptiles. Oecologia 46:92–100
Taylor ML, Price TA, Wedell N (2014) Polyandry in nature: a global analysis. Trends Ecol Evol 29:376–383
Valera F, Hoi H, Krištín A (2003) Male shrikes punish unfaithful females. Behav Ecol 14:403–408
van de Pol M, Heg D, Bruinzeel LW, Kuijper B, Verhulst S (2006) Experimental evidence for a causal effect of pair-bond duration on reproductive performance in oystercatchers (Haematopus ostralegus). Behav Ecol 17:982–991
While GM, Uller T, Wapstra E (2009a) Family conflict and the evolution of sociality in reptiles. Behav Ecol 20:245–250
While GM, Uller T, Wapstra E (2009b) Within-population variation in social strategies characterize the social and mating system of an Australian lizard, Egernia whitii. Aust Ecol 34:938–949
While GM, Uller T, Wapstra E (2011) Variation in social organization influences the opportunity for sexual selection in a social lizard. Mol Ecol 20:844–852
While GM, Uller T, Bordogna G, Wapstra E (2014) Promiscuity resolves constraints on social mate choice imposed by population viscosity. Mol Ecol 23:721–732
While GM, Chapple DG, Gardner MG, Uller T, Whiting MJ (2015) Egernia lizards. Curr Biol 25:R593–R595
Wilson S, Swan G (2013) Complete guide to reptiles of Australia, 4th edn. New Holland, Sydney
Acknowledgements
We thank two anonymous reviewers for insightful comments on earlier versions of the MS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The work was funded by the Australian Research Council (DP150102900 to GMW, TU, DGC and MGG and DE150100336 awarded to GMW) and the Holsworth Wildlife Research Fund (to TBJ).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All work was carried out with approval from the Animal Ethics Committee at the University of Tasmania (Ethics Approval Number A0015058).
Data availability statement
All data associated with this paper is available on dryad (doi:10.5061/dryad.rm95m).
Additional information
Communicated by S. J. Downes
For consideration in Behavioral Ecology and Sociobiology
Rights and permissions
About this article
Cite this article
Botterill-James, T., Sillince, J., Uller, T. et al. Experimental manipulation suggests effect of polyandry but not mate familiarity on within-pair aggression in the social skink, Liopholis whitii . Behav Ecol Sociobiol 71, 71 (2017). https://doi.org/10.1007/s00265-017-2302-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00265-017-2302-8