Abstract
Split-brood field studies using the biparental, subsocial wood-feeding cockroach Cryptocercus punctulatus indicate that parental absence in families of three different age classes of nutritionally independent juveniles results in significantly slower growth when compared to sib groups allowed to remain with adults, even when nymphs are more than 2 years old when parents are removed. In one family that was divided when nymphs were 15 months old and followed for 4 years after treatment, 69 % of nymphs allowed to remain with parents reached maturity in the third year of the study, while those isolated from parents remained in the subadult stage at the 3-year mark. In the fourth year, measurements of wet weights and head capsule widths of the newly mature adults in the two treatments of this family were not significantly different. A parental vs. non-parental social environment, then, resulted in different rates of growth to reproductive maturity, but nymphs in both treatments ultimately reached similar adult sizes. The results indicate that parental effects can modify offspring ontogeny independently of the direct transfer of resources in the form of trophallactic food and symbionts. Because cockroaches in this genus are sister group to the developmentally plastic and juvenilized termites, further exploration of the role of the social environment on development in Cryptocercus is warranted.
Significance statement
Termites are eusocial cockroaches that exhibit weak or delayed post-embryonic development; the majority of colony members are arrested in the small, soft-bodied, altricial morphotype displayed by early juvenile stages of their subsocial sister group, Cryptocercus. Developmental dynamics in this cockroach genus can therefore provide insight into conditions that may have favored the origin of eusociality in the lineage. Here, we demonstrate that the parental social environment in Cryptocercus punctulatus modifies offspring development independently of the direct transfer of hindgut fluids and does so for more than 2 years after hatch. A system in which parent-offspring interactions modify juvenile ontogeny, whatever the mechanism, contributes to the unique set of circumstances that ultimately resulted in the evolution of termite eusociality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood-feeding cockroaches in the genus Cryptocercus are of interest not only because they are the only known oviparous cockroaches to live in biparental family groups in a nest (Nalepa and Bell 1997; Maekawa et al. 2008), but also because it is now well established that they are sister group to termites. Details of the family life of these insects, then, can supply key information regarding the origins of eusociality in termites, a group that is not only phylogenetically distant from other eusocial insects, but also uniquely different in their diet, life history, and development.
The life history of Cryptocercus is remarkable in that the cockroaches typically produce a single clutch of eggs, followed by an extensive period of parental care lasting until the death of adults at about 3 years after hatch; the female generally outlives the male (Seelinger and Seelinger 1983; Nalepa 1984, 1988a; Park et al. 2002). These cockroaches therefore may be classified as semelparous, but not the usual sense. While their entire lifetime reproduction is enacted in a single reproductive episode, adults do not undergo immediate post-reproductive death in the manner of salmon or bamboo.
Young nymphs of Cryptocercus are altricial, evident in their small size, blindness, fragile exoskeleton, and reliance on adults for nourishment and symbionts (Nalepa and Bell 1997; Nalepa et al. 2008; Nalepa 2011); the dry weight of nymphs increases by more than an order of magnitude between the first and second instar (Nalepa and Mullins 2009). Nymphs acquire their full complement of gut symbionts by the third instar, at which point they are nutritionally self-sufficient (Nalepa 1990); proctodeal feeding from adults is rarely observed after that point (Seelinger and Seelinger 1983; Park and Choe 2003). Nonetheless, additional sources of parentally provided food, including fecal pellets, cuticular secretions, and wood fragments torn free by adults, may continue to have an impact on nymphs older than the third instar, and non-provisioning parental care in the form of defense, sanitation, and gallery excavation continues until nymphs are significantly melanized and at least half grown. This occurs in Cryptocercus punctulatus at about 3 years of age, coinciding with the approximate post-reproduction longevity of parents (Nalepa and Grayson 2011).
Brood care in C. punctulatus is known to be costly in that it limits the future reproduction of females (Nalepa 1988b; 2015). The potential benefits of a parental social environment to nymphs beyond nutritional independence, however, are little explored. A notable study is by Park and Choe (2003), who demonstrated that the body weight and head width of young nymphs of Cryptocercus kyebangensis were significantly larger in groups of juveniles allowed to remain with parents when compared to sib groups that were isolated from them. The goal of the present study is to extend on the work of these authors; the social environment of young C. punctulatus was disrupted in a split-brood experimental design using families with three different age classes of nutritionally independent nymphs.
Materials and methods
Experimental design
The study was conducted at Mountain Lake Biological Station, Giles County, Virginia, USA (37.364° N, 80.519° W; elevation 1160 m). Pairs of adult C. punctulatus together with their offspring were collected from rotting logs on the grounds of the station using methods previously described (Nalepa 1984).
In general, oothecae of C. punctulatus in this location are deposited in the field during the months of June and July (Cleveland et al. 1934; Nalepa 1988a); August 1 was therefore designated as ‘hatch’ for the purpose of aging the nymphs used in this study. Nymphal development prior to winter is typically completed by October, at which time they have usually reached the third or fourth instar (Nalepa 1990); consequently, families were collected and field boxes were set up and opened and checked during the month of October. Families with three age classes of nymphs were used in the study: families with nymphs in their hatch-year and ∼3 months old (n = 5); families with nymphs hatched the previous year and ∼15 months old (n = 7); and families with nymphs hatched 2 years previously and ∼27 months old (n = 5). From the data given in their paper, it can be assumed that the nymphs used in the study of C. kyebangensis by Park and Choe (2003) were in a developmental stage similar to the ∼3 month old families used here; in that study, however, nymphs had already undergone their first winter (their experiments were set up in the month of April, in the year following hatch). Parental care in the form of supplying trophallactic food and symbionts is not obligate in any of the age classes used in this study; however, none of the juveniles used were fully melanized/sclerotized and thus were poorly prepared to venture outside the natal log (Nalepa and Grayson 2011).
All families used in the experiments had both male and female parents present, and treatments were set up within 3 days of field collection. Nymphs in each family were split into two equivalent groups that were placed into one of two treatments: one group was replaced with the parents and the other was separated from the parents.
Head capsule widths of early instars are non-overlapping (Nalepa 1990); young nymphs are thus easily separated into instar groups. Third and fourth instars predominated in the ∼3-month-old families and an equal number of each instar was placed into treatments. In the older families, nymphs of approximately equal body sizes were separated into treatment groups; their head capsule widths were measured and tested to assure no significant differences (t test; see supplementary material) prior to placing the treatments in the field.
Each treatment was placed into a box made of untreated pine that had been lined with fiberglass window screen and filled with pieces of the log from which that family was collected; the natural gallery system was preserved as much as possible. Boxes were tagged and wired shut, placed in the field abutting large logs, covered with leaf litter, and left undisturbed until a year later. At that time, the boxes were brought into the laboratory, opened, survivorship noted, and head capsule widths measured using a dissecting microscope fitted with an ocular micrometer; in one set of treatments (Family #10, 4th year), a Cahn Electrobalance Model 7500 scale was used to measure wet weights.
For most families, the experiment was concluded after 1 year; however, two of the 3-month families were placed back into the field in boxes replenished with wood and checked again 2 years after the initial set up. One 15-month family was followed for 4 years, until nymphs reached adulthood; the female parent in this family was marked in the third year by scratching distinct marks into the hollow of her heavily sclerotized pronotum with an insect pin to subsequently distinguish her from adult offspring.
A total of 17 families were initially collected, treated, and set in the field. At the 1-year check, all of the nymphs had died in one of the treatments in four families; these were excluded from further analysis (n = 1, 3-month family; n = 1, 15-month family; n = 2, 27-month families).
Survival in the remaining families is reported but not analyzed further. Confinement of treatments to pine boxes excluded predators and also prevented exodus of the cockroaches from deleterious environmental conditions. During rainy periods, for example, the insects could not escape water that may have seeped into boxes; under natural conditions, they typically move within their extensive gallery systems in the log to escape such threats. Consequently, it is unlikely survivorship in this experiment is indicative of natural mortality. In similar but shorter-term experiments with C. kyebangensis, Park and Choe (2003) found no significant difference in the survivorship of their treatment groups.
Statistics
The Shapiro-Wilk W test for normality was run individually for treatments within each family; the null hypothesis of a normal population distribution was rejected in some treatments. Non-parametric tests (Wilcoxon Rank Sums Test) were consequently used to compare head capsule widths using JMP Pro 12.0.1 (SAS, 2015). Head capsule width was the response variable, with presence or absence of parents as the explanatory variable. Combined analysis of families within an age group was addressed using a mixed model with family as the random effect and treatment as the fixed effect. Residuals from a mixed model were used to check assumptions, and response (head width) was transformed to ranks within each family if normality and/or homogeneity of variance did not hold. The relationship between head capsule width and wet weight of young adults was examined using analysis of covariance, with treatment as the fixed effect, family as the random effect factor, and wet weight as covariable.
Results
One year after experimental set up, the development of nymphs in all families but two (#6, 15-month nymphs (P = 0.055); #13, 27-month nymphs (P = 0.371)) was significantly affected by the absence of adults (Table 1). In the two experimental families checked again 2 years after the initial split (Families #1, #2), significant differences between treatments were retained into the second year, despite both parents having died in the interim in family #2. In general, the younger the family, the greater the effect of parental presence on the development of nymphs (Table 2). When families within an age group were pooled and analyzed as a group accounting for family effects, the differences between treatments were significant in the 3 month (nonparametric mixed model on ranks, P = 0.006) and 15 month (mixed model on head width, P = 0.012) age groups. The treatments were significantly different in two of the three 27-month families when analyzed individually, but there was no significant difference when they were analyzed as a group when accounting for family effects (nonparametric mixed model on ranks, P = 0.092). The latter outcome, however, was influenced by small sample sizes and differences in variation within families.
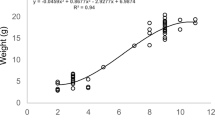
Family #10, where nymphs were already more than a year old when the family was divided, displayed a significant difference between the treatment groups at the 1-year check; this significance was lost after 2 years (P = 0.077). At year three, the difference in head capsule widths was again significant, likely because in those nymphs allowed to remain with parents, nine of the 13 (69 %) had become adults. In the treatment group without parents, all 17 nymphs were still subadults in the third year. In year four, all juveniles in both groups had reached maturity (Table 1, Fig. 1). Dissections of the newly mature females indicated that all seven in the ‘with parents’ treatment, and eight of 11 (73 %) of the females in the ‘alone’ group had sperm in their spermathecae, confirming that they were reproductively mature (note that mating between siblings is an artifact of their confined experimental conditions; most field collected pairs are outbred: Yaguchi et al. 2016). In year four, wet weight of the all these new adults was significantly related to head capsule width (P <0.001), with no interaction between head capsule width and treatment and no significant treatment effects (P = 0.94).
Discussion
As in Cryptocercus kyebangensis (Park and Choe 2003), the growth of early instar nutritionally independent nymphs in C. punctulatus was significantly affected by adult presence. Moreover, the current study indicates that the social environment provided by adults continued to have a significant effect on the growth of offspring in two of the three families split at more than 2 years after hatch. The long-term observation of family #10 (Fig. 1) suggests that these effects are embodied primarily in the length of development until reproductive maturity, as final adult size did not differ significantly between treatments. Thus, the primary effect of long-term parental presence as measured here lies in minimizing the prolonged developmental period of juveniles. This developmental effect is superimposed on natural variation in nymphal development within a family, much of which is rooted in asynchronous hatching of the oothecae and by brood size and associated early competition for parentally provided resources (Nalepa and Mullins 2009). Natural variation in development within a family was demonstrated in a previous study, where both subadults and adults were present in sib groups during the 4th year of development in C. punctulatus, during the 5th year in C. clevelandi (Nalepa et al. 1997, Table 2) and also in the nymphs of family #10 allowed to remain with adults in this study.
The parental social environment, then, has fitness effects on offspring that accounts for long-term brood care, in whatever form it takes, and for adult investment into their own somatic maintenance. Further work is required to tease out the relative effects of the male and female parent. Park and Choe (2003) demonstrated that the size of nymphs in field-captured biparental C. kyebangensis families was significantly larger than in single parent families (n = 8 with single parent families; n = 7 with an adult female, n = 1 with an adult male). In split brood studies with these single parents, their results were less clear, as only two of the seven families showed significant differences; in overall comparisons between the treatment groups, however, it was apparent that the presence of a single parent resulted in significantly larger offspring. Under field conditions similar to those used in the present study, Nalepa and Mullins (2011) showed that the physical presence of the male influenced the onset of female reproduction in C. punctulatus but did not affect the number or early development of nymphs.
Current evidence suggests that parental absence in Cryptocercus has consequences for offspring long after nymphs are able to self-feed. While nutritionally independent juveniles are not doomed if parents die young, the time period before they contribute to the gene pool is extended. The longer developmental time of nymphs may additionally result in diminished survivorship. There is substantial early mortality in field-collected young families, as indicated by a decline in family size with the age of the offspring (Seelinger and Seelinger 1983; Nalepa 1984; Park et al. 2002; Nalepa and Mullins 2009). The survivorship of older nymphs and subadults is unknown, but likely continues to decrease because of risks associated with their exodus from the protective environment of the natal nest.
Proctodeal trophallaxis in Cryptocercus is suggested to affect post-hatch nymphal development via its complicated impact on the social, nutritional, and microbial environments of early instars (Nalepa 2015); the present study and that of Park and Choe (2003) demonstrate that adults also influence offspring development independently of trophallactic behavior. Two aspects of the parental environment may be implicated. First, parents may improve the nutritional status of offspring by means other than proctodeal trophallaxis, and second, adults may be sources of pheromonal and tactile stimuli that directly influence offspring growth rates.
Adult wood-feeding cockroaches produce numerous large fecal pellets as they feed on wood; these are potential sources of protein, semiochemicals, and metabolites originating with the insect excretor as well as its complex of gut symbionts (Nalepa et al. 2001). Parents also sporadically produce unique fecal pellets that are avidly and competitively eaten by juveniles (Park et al. 2002, Fig. 3c; Bell et al. 2007, Fig 5.4). Another nutritional influence of adults is rooted in the nature of their primary food source. The ingestion of wood is a physically difficult task, and nymphs have been observed feeding on slivers of wood pulled free by adults (Park and Choe 2003, Fig. 3). Cryptocercus juveniles groom their parents (Seelingers and Seelinger 1983; Park and Choe 2003); consequently, groomers may ingest cuticular secretions (Farine et al. 1989; Park et al. 2006) and externally attached debris as well as give and receive tactile stimulation. Physical contact such as that which occurs during grooming and antennation is known to significantly affect cockroach development (Chauvin 1952; Pettit 1940; Izutsu et al. 1970; Lihoreau and Rivault 2008). It may be that the absence of the large-bodied parents in this study substantially decreased the tactile and chemical stimulation experienced by nymphs, influencing their development independently of any direct form of parental care. The tight clustering of nymphs around adults is common, particularly in young families (Seelinger and Seelinger 1983, Fig. 4; Park et al. 2002, Fig. 3a).
In cockroaches, a wide variety of traits are shaped by their social partners (e.g., Holbrook and Schal 1998; Moore et al. 2002; Bell et al. 2007, Table 8.3; Lihoreau and Rivault 2008; Uzsak et al. 2014). These ‘group effects’ have been studied in cockroaches for more than a half century, with early studies characteristically comparing grouped to isolated individuals in gregarious species (e.g., Willis et al. 1958; Izutsu et al. 1970). The outcome of these studies was typically most conspicuous in the youngest cockroaches, where the social environment modified behavior, synchronized molting, and altered both developmental rates and adult body size (Nalepa and Bandi 2000, Fig. 4; Bell et al. 2007). The current study (Table 2) is consistent with those results, in that the youngest nutritionally independent nymphs were most significantly affected by the absence of their parents.
Results indicate that the parental environment provides optimum conditions for progressive development in Cryptocercus for at least the first 27 months of life. Further exploration of developmental effects arising from the family social environment in the genus will be pivotal in analyzing termite eusocial origins. Termites are best characterized as hemimetabous, phenotypically plastic, juvenilized cockroaches that arose from a subsocial ancestor. As such, the transition from subsocial to eusocial in the termite ancestor was based on lineage specific family dynamics and best framed not as the suppression of reproduction in adult offspring, but as the evolutionary remodeling of development in juveniles (Nalepa 1994, 2011, 2015).
References
Bell WJ, Roth LM, Nalepa CA (2007) Cockroaches: behavior, ecology and evolution. The Johns Hopkins University Press, Baltimore, MD
Chauvin R (1952) L’effet de groupe. Coll Int CNRS Struct Physiol Soc Anim 34:81–90
Cleveland LR, Hall SR, Sanders EP, Collier J (1934) The wood feeding roach Cryptocercus, its protozoa, and the symbiosis between protozoa and roach. Mem Amer Acad Arts Sci 17:185–342
Farine JP, Brossut R, Nalepa CA (1989) Morphology of the male and female tergal glands of the woodroach Cryptocercus punctulatus (Insecta, Dictyoptera). Zoomorphology 109:153–164
Holbrook GL, Schal C (1998) Social influences on nymphal development in the cockroach Diploptera punctata. Physiol Entomol 23:121–130
Izutsu M, Veda S, Ishii S (1970) Aggregation effects on the growth of the German cockroach Blattella germanica (L.) (Blattaria: Blattellidae). Appl Entomol Zool 5:159–171
Lihoreau M, Rivault C (2008) Tactile stimuli trigger group effects in cockroach aggregations. Anim Behav 75:1965–1972
Maekawa K, Matsumoto T, Nalepa CA (2008) Social biology of the wood-feeding cockroach genus Salganea (Dictyoptera, Blaberidae, Panesthiinae): did ovoviviparity prevent the evolution of eusociality in the lineage? Ins Soc 55:107–114
Moore AJ, Haynes KF, Preziosi RF, Moore PJ (2002) The evolution of interacting phenotypes: genetics and evolution of social dominance. Amer Nat 160:S186–S197
Nalepa CA (1984) Colony composition, protozoan transfer and some life history characteristics of the woodroach Cryptocercus punctulatus Scudder. Behav Ecol Sociobiol 14:273–279
Nalepa CA (1988a) Reproduction in the woodroach Cryptocercus punctulatus Scudder (Dictyoptera: Cryptocercidae): mating, oviposition and hatch. Ann Entomol Soc Amer 81:637–641
Nalepa CA (1988b) Cost of parental care in Cryptocercus punctulatus Scudder (Dictyoptera: Cryptocercidae). Behav Ecol Sociobiol 23:135–140
Nalepa CA (1990) Early development of nymphs and establishment of hindgut symbiosis in Cryptocercus punctulatus (Dictyoptera: Cryptocercidae). Ann Entomol Soc Amer 83:786–789
Nalepa CA (1994) Nourishment and the evolution of termite eusociality. In: Hunt JH, Nalepa CA (eds) Nourishment and evolution in insect societies. Westview Press, Boulder, pp 57–104
Nalepa CA (2011) Altricial development in wood-feeding cockroaches: the key antecedent of termite eusociality. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, NY, pp 69–95
Nalepa CA (2015) Origin of termite eusociality: trophallaxis integrates the social, nutritional, and microbial environments. Ecol Entomol 40:323–335
Nalepa CA, Bandi C (2000) Characterizing the ancestors: paedomorphosis and termite evolution. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwar Academic, Dordrecht, the Netherlands, pp 53–75
Nalepa CA, Bell WJ (1997) Postovulation parental investment and parental care in cockroaches. In: Choe JC, Crespi BJ (eds) Social behavior in insects and arachnids. Cambridge University Press, Cambridge, pp 26–51
Nalepa CA, Bignell DE, Bandi C (2001) Detritivory, coprophagy, and the evolution of digestive mutualisms in Dictyoptera. Ins Soc 48:194–201
Nalepa CA, Byers GW, Bandi C, Sironi M (1997) Description of Cryptocercus clevelandi (Dictyoptera: Cryptocercidae) from the northwestern United States, molecular analysis of bacterial symbionts in its fat body, and notes on biology, distribution and biogeography. Ann Entomol Soc Amer 90:416–424
Nalepa CA, Grayson KL (2011) Surface activity of the xylophagous cockroach Cryptocercus punctulatus (Dictyoptera: Cryptocercidae) based on collections from pitfall traps. Ann Entomol Soc Amer 104:364–368
Nalepa CA, Maekawa K, Shimada K, Saito Y, Arellano C, Matsumoto T (2008) Altricial development in subsocial wood-feeding cockroaches. Zool Sci 25:1190–1198
Nalepa CA, Mullins DE (2009) Hatching asynchrony, survivorship, and development in young colonies of the subsocial cockroach Cryptocercus punctulatus (Dictyoptera: Cryptocercidae). Sociobiology 54:489–508
Nalepa CA, Mullins DE (2011) Repeated copulation in the wood-feeding cockroach Cryptocercus punctulatus does not influence number or development of offspring. J Ins Behav 24:44–54
Park Y-C, Choe JC (2003) Effects of parental care on offspring growth in the Korean wood-feeding cockroach, Cryptocercus kyebangensis. J Ethol 21:71–77
Park Y-C, Grandcolas P, Choe JC (2002) Colony composition, social behavior and some ecological characteristics of the Korean wood-feeding cockroach (Cryptocercus kyebangensis). Zool Sci 19:1133–1139
Park Y-C, Kim J-P, Choe JC (2006) Grooming behavior and a possible morphological structure for secretions from abdominal glands of a Korean wood-eating cockroach, Cryptocercus kyebangensis (Insecta: Blattodea). Anim Syst Evol Divers 22:17–22
Pettit LC (1940) The effect of isolation on growth in the cockroach Blattella germanica (L.) (Orthoptera: Blattidae). Entomol News 51:293
SAS Institute, Inc (2015) Cary, North Carolina
Seelinger G, Seelinger U (1983) On the social organization, alarm and fighting in the primitive cockroach Cryptocercus punctulatus. Z Tierpsychol 61:315–333
Uzsak A, Dieffenderfer J, Bozkurt A, Schal C (2014) Social facilitation of insect reproduction with motor-driven tactile stimuli. Proc Roy Soc B-Biol Sci 281(1783):20140325
Willis ER, Riser GR, Roth LM (1958) Observations on reproduction and development in cockroaches. Ann Entomol Soc Amer 51:53–69
Yaguchi H, Hayashi Y, Tohoku T, Nalepa CA, Maekawa K (2016) Genetic studies indicate that most field-collected woodroach pairs are unrelated. Insect Sci (submitted)
Acknowledgements
We are grateful to the director and staff of Mountain Lake Biological Station for their support of the field work and to Jim Hunt for astute comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were performed in accordance with the ethical standards of the institution at which the studies were conducted.
Additional information
Communicated by J. C. Choe
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
Supplementary Material (DOCX 15 kb)
Rights and permissions
About this article
Cite this article
Nalepa, C.A., Arellano, C. Parental social environment alters development of nutritionally independent nymphs in Cryptocercus punctulatus (Dictyoptera: Cryptocercidae). Behav Ecol Sociobiol 70, 881–887 (2016). https://doi.org/10.1007/s00265-016-2110-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2110-6