Abstract
In cuckoo-host coevolution, rejection of parasite eggs based on visual discrimination is the key host-defensive mechanism reducing the costs of parasitism. Although host discriminatory tasks often occur in variable environmental conditions, the influence of nest light variation on the perceptual processes involved in egg discrimination has been seldom considered. Here, we combine visual modeling, experimental manipulation of nest ambient light, and egg recognition experiments with model eggs differing in background color (cream vs. blue) to explore the possibility that variation in ambient light in magpie (Pica pica) domed nests may affect the perceptual process involved in discrimination of foreign eggs. We found that the architecture of magpie nests affects the quality of ambient light for egg recognition and that changes in luminosity did not differently affect rejection of blue and cream models. However, we found that ejection of model eggs declined throughout the season in nests in which luminosity remained unmodified, but that magpies rejected eggs at a similar rate over the season in nests in which luminosity was increased. These results therefore suggest that variation in ambient light at the nests might potentially influence the perceptual processes involved in visual detection of parasite eggs by cuckoo hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Discriminating parasite eggs from their own ones is critical for hosts of avian brood parasites. Brood parasites lay their eggs in the nests of host species and leave them to care for their offspring (Davies 2000). Brood parasitism is harmful to hosts since once the parasite egg hatches the young parasite can readily evict all host eggs and chicks and/or monopolize most of parental care, which causes a drastic deterioration of host reproductive outcome (Wyllie 1981; Anderson et al. 2009). Brood parasitism has therefore selected for effective defensive mechanisms in hosts, which at the same time has selected for further counter defenses in the parasite (Brooke and Davies 1988; Davies et al. 1988). In this evolutionary battle, many host species have evolved finely tuned discrimination abilities allowing them to discard odd-looking eggs added to their clutches (Rothstein 1982; Moksnes et al. 1991), thereby avoiding costly parasitism.
Despite the fact that host discrimination of parasite eggs often occur in a huge range of environmental conditions, the influence of this variation on the perceptual process involved in egg discrimination has received surprisingly little attention. Cherry and Bennett (2001) suggested the theoretical possibility that given the known effects of variation in ambient light on perception of bird color patterns (Endler 1993), nest light environments could constrain egg discrimination and, thus, under some circumstances, lead to acceptance of non-mimetic eggs. Up to now, three studies have tried to establish a link between ambient light and host perception of egg matching. A phylogenetically controlled study showed low support for a role of ambient light at the nest on egg discrimination of different cuckoo hosts (Langmore et al. 2005). Also, perceptual visual model calculations suggested a negligible effect of ambient light variation on redstart Phoenicurus phoenicurus and wagtail Motacilla alba perception of cuckoo egg mimesis (Avilés 2008). Finally, Honza et al. (2011) did not find a relationship between ambient light at the nest and rejection of real cuckoo eggs in great reed warbler Acrocephalus arundinaceus. However, although valuable, these studies did not relate ambient light manipulations at the nest with cuckoo egg discrimination experiments (Langmore et al. 2005; Honza et al. 2011).
Here, we combine intensive sampling of nest ambient light, visual modeling, and field experiments to investigate egg rejection in relation to nest light conditions in the magpie Pica pica. The magpie is the most common host of the great spotted cuckoo Clamator glandarius in Spain (Soler 1990), and evidence suggests that these two species are involved in a coevolutionary arms race (Soler and Soler 2000) in which great spotted cuckoo parasitism has selected for egg recognition and rejection in magpies (Soler and Møller 1990). Magpies build characteristic large and conspicuous domed nests (Cramp 1998; Fig. 1). The dome is an open work incomplete-looking layer of twigs with one or more main openings which is expected to filter the quantity and quality of light for egg discrimination (Endler 1993). The huge variation in the density of twigs composing the roof of magpie nests (Quesada 2007) makes this system ideal for studying the role of ambient light on egg discrimination.
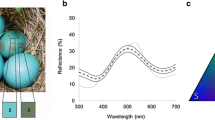
a Representative appearance of magpie, great spotted cuckoo and model (blue and cream) eggs. In this image, great spotted cuckoo eggs do not necessarily come from the same parasitized clutch. b Average reflectance spectra of natural magpie and great spotted cuckoo eggs and blue and cream models. Sample sizes for each egg type are blue models, N = 29 sampled eggs; cream models, N = 10 sampled eggs; magpie eggs, N = 507 eggs measured in 84 nests; and great spotted cuckoo eggs, N = 118 eggs measured in 51 naturally parasitized nests. c Representative appearance of magpie nests in Guadix
The aim of this study is threefold. We first examine the quantity and quality of ambient light variation in relation to characteristics of magpie nests. In a second stage, we experimentally manipulate ambient light in magpie nests to test whether variation in nest light environments could constrain egg discrimination. Specifically, the light environment hypothesis (sensu Cherry and Bennett 2001) would predict higher acceptance of non-mimetic eggs at low-light levels. Finally, given that we contrasted the effect of ambient light on rejection of two model eggs differing in the predominant color of the background: a color background rich in long-wavelength shades (cream models hereafter, Fig. 1) versus model eggs with a background rich in short-wavelength shades (blue models hereafter, Fig. 1), in the third stage, we perform the first experimental test of Cherry and Bennett (2001) suggesting that acceptance of cuckoo eggs could be performed predominantly at certain combinations of wavelengths.
Methods
Study area and field work
The magpie population of Guadix (37° 18′ N, 3° 11′ W, southern Spain) is situated in a high-altitude plateau at an altitude of 1000 m. The vegetation is sparse, including cultivated cereals (especially barley) and many groves of almond trees (Prunus dulcis) and oaks (Quercus rotundifolia) in which magpies preferentially build their nests (Soler 1990).
Fieldwork was conducted during the breeding season of 2009. At the beginning of spring (mid March–early April), we intensely searched the study area for newly built magpie nests.
Experimental design
In order to modify the light conditions within the nest environment, we performed a nest dome manipulation consisting of the removal (reduced, N = 53 nests) of a bag (80 × 80 × 50 cm) of twigs from the external layer of the dome, which was then added to the dome of a second group of nests (enlarged, N = 53 nests). A third group of nests (N = 55 nests) was left as a control (i.e., some of the nest roof sticks were removed and included again in the nest structure without affecting its density and/or position) and were visited at the same rate as the enlarged and reduced ones. Nests that were randomly assigned to one of the three treatments and manipulations were always performed when nests were in their final stage of building (i.e., when magpies are starting to line the bowl with fibrous roots, hairs, and grass (Birkhead 1991), which occurred on average 16.5 days (SE = 0.76) before magpies started to lay eggs. The date of nest dome manipulation did not differ between enlarged, reduced, and control nests suggesting a proper randomization of treatments over time in our experiment (one-way ANOVA, F 2,158 = 0.23, P = 0.79). Seventeen out of 53 reduced nests (i.e., 32.07 %) and 11 out of 53 enlarged nests (i.e., 20.75 %) were abandoned after being manipulated. These proportions of nest abandonment did not significantly differ from that of control nests (13 out of 55 control nests (i.e., 24.52 %) (test for comparing two proportions: reduced vs. control, χ 2 1 = 0.96, P = 0.33; enlarged vs. control, χ 2 1 = 0.13, P = 0.64)), suggesting that our experiment had a negligible effect on nest abandon. Thus, it is unlikely that a bias occurred in which magpies that are more prone to reject were inadvertently weeded out in one or some of the experimental group(s) thereby causing the resulting patterns of rejection.
When magpies started to lay eggs and always before they started incubation (i.e., four or five eggs in the nest), we carried out egg recognition experiments with artificial model eggs in all experimental nests. Therefore, latency between branch removal/addition and the introduction of the model egg ranged between 17 and 22 days. We introduced one model egg made of plaster of paris in each nest. Model eggs mimicked shape and pattern of spottiness of natural great spotted cuckoo eggs and had blue or cream backgrounds (Fig. 1). Background of blue (ARTECOLOR Code E12-22) and cream (ARTECOLOR Code E04-22) models were deliberately painted to have similar total brightness and to differ in the relative importance of long (yellow-red) versus short (blue-green) wavelength with magpie eggs (Fig. 1). Blue and cream models have identical negligible reflectance in the UV part of the spectrum (Fig. 1), and previous experimental work demonstrated a minor influence of ultraviolet reflectance of cuckoo eggs in magpie rejection behavior (Avilés et al. 2006), thus differences in rejection between the two models are unlikely due to UV spectra.
Using real great spotted cuckoo eggs or more realistic model eggs would, in principle, help avoiding pitfalls of artificially built eggs (Martin-Vivaldi et al. 2002) or of presenting non-realistic visual stimuli at the nest. However, this was not possible in our system. On the one hand, swapping real cuckoo eggs might be problematic because besides background color, real great spotted cuckoo eggs may greatly differ in size, distribution and pattern of spottiness (Cramp 1998; see Fig. 1), which would make it difficult in isolating the effect of egg background color and its interaction with nest light luminosity on magpie discrimination. Moreover, real great spotted cuckoo eggs are only very rarely rejected in Guadix (about 5 %, Soler et al. 1995b), so that gathering enough sample size to isolate background color effects in egg discrimination of real great spotted cuckoo eggs would be exceptionally difficult. Finally, by using models, we avoid affecting the reproductive success of cuckoos during the study, and we are not limited by the number of available real cuckoo eggs. On the other hand, using more realistic models may also be problematic as they do not have a perfect matching with real eggs neither in short- nor in long-wavelengths (see Molina-Morales et al. 2014), thus being unsuitable for the specific issue of determining the relative importance of long- versus short-wavelength shades in determining rejection.
Blue and cream model eggs were randomly introduced into magpie nests, and the response was regarded as ejection if the model egg disappeared from the nest or as acceptance if the model egg was incubated with the host’s clutch after 6–7 days.
Quantifying architecture and concealment of magpie nests
Light in magpie nests might be affected by the architecture (i.e., size and materials used to build the nest) and/or the location of the nest (i.e., degree of concealment of the nest into vegetation). Nest volume (Soler et al. 1995a; Molina-Morales et al. 2013) and density of twigs in the dome (Quesada 2007) are features known to vary between different magpie nests. Also, some magpie pairs build very conspicuous nests while others do conceal them into tree vegetation (Birkhead 1991). Here, we estimated nest volume, density of twigs, and degree of nest concealment in the subset of control nests aiming to differentiate whether light in magpie nests was affected by nest architecture and/or by concealment. We measured the size of nests the day of the nest dome manipulation using a flexible steel tape (precision ± 1 cm) and calculated nest volume as 4/3 (πab 2)/1000 (in liters), where a is the largest radius of the ellipsoid nest and b is half of the nest width (Soler et al. 1995b). In addition, we estimated the level of twig density in the dome in each nest using a six-degree ordinal scale ranging from 0 to 5 corresponding to very low and high densities of twigs, respectively. Finally, we estimated the degree of nest concealment into vegetation using a six-degree scale ranging from 0, when the nest was not visible for human observers standing at a distance of 20 m from the nest at any angle, to 5, which corresponds to a highly conspicuous nest which could be observed entirely at whatever angle. Correlation analyses revealed that the density of twigs in the dome of magpie nests was positively and significantly correlated with the volume of the nest (r = 0.38, P = 0.021, N = 36), so we discarded nest volume in subsequent analyses because the aim was to assess the effect of nest architecture on light conditions inside magpie nests.
Quantifying nest luminosity
Given that our main aim was reporting nest luminosity variation between—rather than within—nests, we measured irradiance (ambient light) in 129 magpie nests during the morning (9:00–11.00 a.m.) of sunny days aiming to minimize the possible influence of weather conditions on light measures. Measures were taken using an Ocean Optics USB2000 spectrometer supplied with batteries with a cosine-corrected fiber-optic probe (P400-1-UV-VIS, Ocean Optics) with a 180° angle of acceptance and a measurement surface of 6 mm in diameter (CC-3-UV, Ocean Optics). The spectrometer was calibrated with light source of known color temperature (LS-1-CAL; Ocean Optics) and was connected to the laptop with a 5-meter USB cable allowing to collect irradiance data on top of magpie nests. To get representative measures of ambient light in the nest, we gently introduced the probe through the main opening and collected three irradiance readings in every nest (i.e., about 10–15 cm over the bottom of the nest) and pointing vertically toward the dome. In addition, we collected three readings on top of the nest dome pointing to the sky, allowing to calculate the amount and quality of ambient light filtered by the dome. The mean of the three readings was used in subsequent analyses.
We summarized nest irradiance data by calculating summed irradiance in the range between 300 and 700 nm wavelengths as an estimate of the amount of ambient light in magpie nests. In addition, because we were interested in describing variation in richness of light in particular wavelengths, we also summarized irradiance spectra by using principal component analyses (PCA) on irradiance data (Montgomerie 2006; Fig. 2). We performed a PCA on irradiance spectra collected within the nest cup and on top of the nest cup of magpie nests before (N = 129 nests) and after the nest cup manipulation (N = 95 nests) on the same day and on the day of experimental parasitism with parasite model eggs (N = 109 nests). The graphical representation of factor loadings in relation to wavelength reveals that PC1 is spectrally flat and describes achromatic variation and explains 92.7 % of overall variation in nest luminosity (Fig. 2). Principal component 2 (PC2) was not spectrally flat and explained 57.3 % of chromatic variation in nest luminosity and had high and negative loadings at short wavelengths and high positive ones approximately at the green (475–550 nm) wavelengths (Fig. 2) and could therefore classify the sampled nest environments along a gradient of ultraviolet versus green rich luminosities. PC3 only explained 9.5 % of chromatic variation in nest luminosity (i.e., 0.7 % of variation in nest luminosity) and was thus disregarded for subsequent analyses. Analyses on variation in level and quality of ambient light were therefore based on summed irradiance and PC scores arising from the PCA on irradiance data.
a Average (SE) irradiance spectra of the nest light in magpie nests (N = 129 nests). Curves are the mean of individual means of three measurements taken at every nest and position (within the nest cup (red line) versus on the top of the nest cup pointing to the sky (blue line)) before performing any nest cup manipulation. b Factor loadings in relation to wavelength, derived from irradiance spectra collected within the nest cup and on the top of the nest cup of magpie nests before (N = 129 nests) and after nest cup manipulation (N = 95 nests) on the same day and on the day of experimental parasitism with parasite model eggs (N = 109 nests) (see “Methods”). PC1 indicates principal component 1, PC2 principal component 2, and PC3 principal component 3. c Average (SE) characteristics (PC1, PC2 scores on irradiance spectra) of ambient light in magpie nests (N = 129 nests). Values are the mean of individual means of three PC scores derived from three measurements taken at every nest and position (within the nest cup (red bar) versus on the top of the nest cup pointing to the sky (blue bar)) before performing any nest cup manipulation
Effects of the nest dome manipulation on nest irradiance
To assess the effect of the nest dome manipulation on ambient light within the nests, we measured irradiance at three different times in most sampled magpie nests (Fig. 3). Nest luminosity did not differ between enlarged, reduced, and control nests before nest cup manipulation suggesting a proper randomization of treatments in relation to nest luminosity in our experiment (one-way ANOVA, summed irradiance: F 2,124 = 0.94, P = 0.39; PC1 irradiance: F 2,124 = 0.92, P = 0.39; PC2 irradiance: F 2,124 = 0.54, P = 0.58; Fig. 3).
Luminosity in magpie nests in relation to manipulation of the nest cup. a Before the nest cup manipulation (sample sizes are 39, 40, and 48 nests for reduced, enlarged, and control group of nests, respectively). b Immediately after the nest cup manipulation (sample sizes are 45 and 47 nests for reduced and enlarged group of nests, respectively). c After the nest cup manipulation on the day of introducing experimental model eggs (i.e., nest size manipulations were performed 16–20 days before insertion of model eggs, see “Methods”) (sample sizes are 29, 39, and 39 nests for reduced, enlarged, and control group of nests, respectively). Sample sizes in the three groups of experimental nests changed between the three periods (i.e., panels) due to (i) logistic problems that impeded collecting luminosity measures in some instances, (ii) due to non-collection of luminosity measures in control nests after the nest cup manipulation, and (iii) because some nests were abandoned after the nest cup manipulation (see “Methods”) and thus were not further considered. d Specific effect of the removal of branches from the nest cup on nest light characteristics (average (SE) summed luminosity and PC scores of the PCA on irradiance measures)
Removal of branches from the nest cup increased light levels within magpie nests by 2.64 orders of magnitude (paired t test, summed irradiance: t 38 = 4.85, P < 0.001; PC1 irradiance: t 38 = 4.94, P < 0.001) and affected the chromatic composition of light creating relatively rich blue-green light (PC2 irradiance: t 38 = 2.59, P = 0.01; Fig. 3). The effect of branch removal on nest luminosity lasted until the introduction of the model egg (paired t test, summed irradiance: t 23 = 2.17, P = 0.040; PC1 irradiance: t 23 = 2.20, P = 0.03; PC2 irradiance: t 23 = 0.63, P = 0.53; Fig. 3).
The addition of branches, however, did not influence significantly the luminosity levels within the nests either immediately after the nest cup manipulation (t test for dependent samples, summed irradiance: t 38 = 1.41, P = 0.16; PC1 irradiance: t 38 = 1.42, P = 0.16; PC2 irradiance: t 38 = 1.34, P = 0.18; Fig. 3) or at the time of the introduction of the model egg (t test for dependent samples, summed irradiance: t 29 = 0.47, P = 0.63; PC1 irradiance: t 29 = 0.51, P = 0.61; PC2 irradiance: t 29 = 1.53, P = 0.13; Fig. 3). Furthermore, Levene’s tests of homogeneity of variance suggested that our nest dome manipulation did not obviously affect variance in quantity and quality of light in magpie nests. Before, nest dome manipulation control and experimental nests did not differ in variance in irradiance (F 1,125 < 1.99, P > 0.16 in the three cases, Fig. 3). Also, variance in irradiance did not differ between experimental and control nests at the day of model introduction (F 1125 < 2.79, P > 0.98 in the three cases, Fig. 3).
Finally, resampling of luminosity in control nests in different days allowed testing for consistency in light condition in magpie nests. Interestingly, luminosity in control nests did not change between the time of the nest cup manipulation and the day of introducing the experimental model eggs (i.e., nest size manipulations were performed 16–20 days before insertion of model eggs) (t test for dependent samples, summed irradiance: t 34 = 1.21, P = 0.23; PC1 irradiance: t 34 = 1.22, P = 0.22; PC2 irradiance: t 34 = 0.86, P = 0.39; Fig. 3), suggesting consistency in luminosity levels in the same nest.
Quantification of egg color
We estimated egg coloration (i.e., spectral reflectance at the 300–700 nm waveband) of magpie and cuckoo eggs after magpie clutch completion with Ocean Optics spectrometer equipment [S2000 spectrometer connected to a deuterium-halogen light (D2-W, mini) by a coaxial reflectance probe (QR-400-7-UV–vis) and the OOIBase32™ operating software (Ocean Optics, Inc. Dunedin, FL, USA)]. Reflectance was measured with the probe placed at a constant distance and a 45° angle. Measurements were relative to a standard white (WS-2) and to a dark, which was calibrated before measurement of each clutch. All measurements were performed in a dark hand bag fixed to the probe to avoid an effect of ambient light on color measurements. Model eggs were measured using the same methods, before being introduced into magpie nests. Sample sizes for each egg type are displayed in Fig. 1. Spots are so small and widely spread on the shell of magpie and great spotted cuckoo eggs (Fig. 1) that they cannot be deliberately avoided, so we calculated the mean of five measures taken at random throughout the long axes of each egg and averaged all values per egg in a clutch to get a reflectance spectrum of magpie eggs per sampled nest (e.g., Avilés et al. 2012).
Modeling avian color perception of matching
Differences in the reflectance of model and host eggs do not directly equate to how matching would be processed by a receiver host as they do not account for what the host eyes actually perceive and disregard the luminal environment where the eggs should be discriminated (Avilés 2008). Here, we ran a log form of a discriminability model of avian visual processing developed by Vorobyev and Osorio (1998) with Avicol software v3 (Gomez 2006) that accounts for nest luminosity and bird sensitivity for estimating chromatic and achromatic matching of model eggs from the perspective of a magpie host (see Avilés and Soler 2009 for further details and equations).
Spectral sensitivity has not been measured in the magpie. However, genetic evidence suggests that magpies are violet sensitive (Odeen et al. 2011); therefore, we modeled perceived matching by magpie hosts by using spectral sensitivity data for the peafowl Pavo cristatus as representative of a violet sensitive (VS) (Hart 2002). The model outcome is in JNDs (just noticeable differences) and following previous empirical work about color discriminability by birds (Siddiqi et al. 2004; Cassey et al. 2009; Avilés et al. 2010, 2011), we assumed that contrast values below 1 JND are impossible to discriminate by birds, and those with values between 1 and 3 JND would be difficult to distinguish even under favorable light conditions. The lack of information on the sensory physiology of magpies forces us to make several sensory assumptions about its visual system that may not be necessarily true. This caveat constraints our ability to make any species-specific generalization based on model calculations in this study.
To assess nest light effects due to our dome manipulation on host perception of experimental blue and cream model eggs, we calculated contrasts between average reflectance values of experimental model eggs and average reflectance values of magpie eggs in each nest. Calculations were thus done separately for enlarged, reduced, and control nests after the nest dome manipulation at the time of egg recognition experiments and using in each case the average values of luminosity reported for that nest group (Fig. 3).
Statistical methods
We tested for changes in ambient light quantity (i.e., summed luminosity and PC1 irradiance scores) and chromatic characteristics (i.e., PC2 irradiance scores) in a given nest due to filtering of the nest dome by comparing light characteristics under and above the dome with t tests for dependent samples. We used Pearson correlations to assess which factors (i.e., nest concealment, building date (i.e., day when magpies are starting to line the bowl (see above) and density of twigs in the dome)) covaried with ambient light levels (log transformed summed luminosity and PC1 irradiance scores) and chromatic characteristics (PC2 irradiance scores) in control magpie nests.
We used general linear models (GLM procedure in SAS, SAS Institute, 1996) for testing the effect of the color of the model egg (i.e., blue versus cream) and nest cup treatment (i.e., enlarged, reduced, and control) as fixed effects on the degree of chromatic and achromatic contrast between magpie host and parasite model eggs.
Finally, we used a logistic regression (GENMOD procedure in SAS) to test whether rejection of model eggs in magpies (i.e., rejection vs. acceptance modeled as a binomial response variable using a logistic link function) was explained by model egg coloration (i.e., blue versus cream) and/or nest dome treatment (i.e., dome-enlarged, dome-reduced or control). Because probability of rejecting cuckoo eggs might vary during the season due to differences in magpie quality (e.g., Lotem et al. 1992), magpie laying date was included as a covariate in the model in addition to all possible interactions.
Results
Nest luminosity in control nests
The dome filters and affects the amount and quality of ambient light into magpie nests: we found that the amount of light inside a magpie nest is almost eight times lower than that above of the same nest (paired t test, summed irradiance: t 129 = 18.41, P < 0.001; PC1 irradiance: t 129 = 18.66, P < 0.001; Fig. 2). Second, nest light environment is relatively poorer in blue and green and richer in ultraviolet light than that of ambient light (PC2 irradiance: t 129 = 5.95, P < 0.001; Fig. 2).
We found remarkable consistency between magpie nests in the level of ambient light (Fig. 4a): at 81.3 % of the 48 control magpie nests, there were luminosity levels below 2 μmol s−1 m−2 nm−1, and 97.9 % of nests had luminosity levels below 6 μmol s−1 m−2 nm−1. Correlation analyses revealed that the amount and quality of light in these nests were related to the density of twigs in the dome. Luminosity within magpie nests decreased with density of the dome (summed luminosity: r = −0.34, P = 0.038, N = 36; PC1 scores irradiance: r = 0.38, P = 0.020, N = 36, Fig. 4B,C). Furthermore, nests with denser domes had light poorer in blue and green and richer in ultraviolet light (PC2 scores irradiance: r = −0.35, P = 0.036, N = 36, Fig. 4d) than those with less dense domes. Amount and quality of light in magpie nests were not related with the nest building date (r < −0.14, P > 0.315, N = 48 in the three cases) or the degree of nest concealment into vegetation (r < −0.05, P > 0.776, N = 27 in the three cases).
Nest luminosity variation between magpie nests in Guadix (N = 48 control nests). a Histogram of the number of magpie nests in relation to luminosity. b Summed luminosity in magpie nests (log scale) in relation to density of twigs in the dome (log scale). c Achromatic variation in luminosity (i.e., PC1 irradiance scores) in magpie nests in relation to density of twigs in the dome (log scale). d Ultraviolet versus green rich luminosity (i.e., PC2 irradiance scores) in magpie nests in relation to density of twigs in the dome (log scale). See “Methods” for interpretation of the PC values. The gression lines are derived from the univariate regressions of the respective color feature and density of the dome
Nest dome manipulation and model magpie egg matching
Color matching of blue and cream eggs with magpie eggs were differently affected by the nest dome manipulation (egg color*nest dome manipulation effect: F 2,75 = 3.44, P = 0.03; nest dome manipulation effect: F 2,75 = 3.15, P = 0.04; egg color effect: F 1,75 = 316.87, P < 0.001, Fig. 5). Blue model eggs showed a general poor color matching with magpie eggs that was minimally affected by nest dome treatment (post hoc Tukey’s HSD tests: P > 0.97 for the three comparisons, Fig. 5). Cream models eggs, however, showed poorer matching with magpie eggs in dome-enlarged nests than in control ones (post hoc Tukey’s HSD test: P = 0.013). However, no significant differences in chromatic matching of cream models were found between nests with enlarged and reduced domes (post hoc Tukey’s HSD test: P = 0.85) or between nest with reduced domes and control nests (post hoc Tukey’s HSD test: P = 0.28).
a Average (SE) perceptual color and b achromatic contrasts (in “just noticeable differences” [JNDs]) between magpie host and parasite model eggs (i.e., blue- and cream-colored eggs) in relation to nest dome manipulation (i.e., enlarged, reduced, and control). Color of bars corresponds to color of experimental models and sample sizes are shown on bars
Regarding the achromatic matching, cream models showed a general poorer matching with magpie eggs than blue models (egg color effect: F 1,75 = 26.92, P < 0.001, Fig. 5) irrespective of the nest dome treatment applied in the nest (egg color*nest dome manipulation effect: F 2,75 = 1.39, P = 0.25; nest dome manipulation effect: F 2,75 = 0.072, P = 0.93, Fig. 5).
Egg rejection in relation to model egg color and nest dome manipulation
Differences in rejection of blue and cream models changed seasonally (egg color*laying date effect: χ 2 1 = 4.37, P = 0.036; egg color effect: χ 2 1 = 3.90, P = 0.048), so that cream models were more markedly discarded by magpie hosts than blue models late in the breeding season (Fig. 6), when more than 80 % of blue eggs were accepted (Fig. 6). Differences in rejection of cream and blue model eggs, however, were unaffected by the performed nest dome manipulation (egg color*nest dome manipulation effect: χ 2 2 = 1.19, P = 0.55), and laying date did not affect this pattern (laying date*egg color*nest dome manipulation effect: χ 2 2 = 1.12, P = 0.57).
We found that seasonal changes in rejection of model eggs were dependent on the nest dome manipulation (laying date*nest dome manipulation effect: χ 2 2 = 6.02, P = 0.048; nest dome manipulation effect: χ 2 2 = 6.03, P = 0.049; laying date effect: χ 2 1 = 0.44, P = 0.51). Specifically, rejection of model eggs declined with season in dome-enlarged and control nests (i.e., nests in which luminosity remained unchanged (see “Methods”)), but not in magpie nests in which luminosity was increased through the reduction of the dome (Fig. 7).
Discussion
We found that the dome of magpie nests consistently filtered the amount and quality of light creating a distinct light environment inside the nest. In addition, we found that ambient light for cuckoo egg discrimination in magpie nests was affected by allocation and/or arrangement of nest material into the nest dome. The higher the density of twigs in the dome, the lower the amount of light for cuckoo egg discrimination, and the poorer it was in blue and green wavelengths. Previous studies revealed that magpie nest dome characteristics could be related to aerial predation risk (Baeyens 1981; Quesada 2007; Soler et al. 2014). Hence, magpies might modify aspects of their nest architecture in order to limit nest predation. Given that the density of potential corvid predators for magpie nests is high in Guadix (i.e., (Soler 1990)), this raises the challenging possibility that selection for dense domes reducing predation might also have induced changes in the nest light environments which may affect cuckoo egg discrimination.
Egg discrimination in relation to experimental egg coloration
We found that magpies were more prone to reject cream compared to blue background-colored eggs late in the breeding season. Evidence suggests that achromatic cues are probably more important for discrimination tasks than chromatic cues at low-light levels (Vorobyev and Osorio 1998; Lind and Kelber 2009). In this study, blue eggs showed a better achromatic match than cream eggs with magpie eggs, suggesting that they should be more difficult to discriminate than cream eggs inside dark magpie nests. Thus, visual constraints for detection of chromatic cues in magpie nests might be a likely explanation for the observed patterns.
Alternatively, acceptance of the blue models might be explained if magpie egg coloration played a role as a post-mating sexual signal, and based on this, magpies exhibited a preference for blue colors. Evidence suggests that blue-green coloration of eggs might be related to female and/or egg quality and may affect parental effort (Moreno and Osorno 2003; Soler et al. 2008; see however Reynolds et al. 2009). A recent comparative study has shown that hosts of the European cuckoo that have been parasitized for a long time with natural blue cuckoo eggs were more prone to accept non-mimetic model eggs in their nests (Soler et al. 2012), suggesting some evidence of sensory bias toward blue-green colored eggs among host species. Unlike common cuckoos, great spotted cuckoos have not evolved host-specific races in egg appearance and great spotted cuckoo eggs found in magpie nests are not characteristically blue (Soler et al. 2003). Further work is needed to clarify whether blueness of magpie eggs is related to female and/or egg quality and might influence parental investment and parasite egg discrimination.
Why late-breeding magpies accept more blue eggs is intriguing? Laying date is related to individual quality and/or age in magpies with late breeders being more often low in quality and/or young individuals (Birkhead 1991). Also, a recent study suggests that the probability of egg rejection increases with the relative age of the female in magpies (Molina-Morales et al. 2014). Thus, perhaps discrimination based on blue-green colors is quality- and/or age-dependent in magpies. Experiments with banded individuals of known age and quality are needed to further explore this suggestion. Irrespective of the mechanism behind, however, our results agree with the previous findings suggesting that host selection on particular parasite egg phenotypes may change over the source of a breeding season (Lotem et al. 1992).
At this point, it must be highlighted that predictions about egg discrimination based on visual model calculations in this study have been based on general assumptions about a visual system (VS) and not on the sensory physiology of the magpies. This caveat limits our ability to make species-specific generalization on discrimination ability and raises the possibility that our visual contrast calculations may have under- or over-estimated actual magpie discrimination capacity.
Egg discrimination in relation to nest ambient light
Cherry and Bennett (2001) suggested that low-light conditions might hinder cuckoo egg detection, potentially leading to acceptance of cuckoo parasitism. We found some evidence that egg discrimination in magpies is affected by ambient light at their nest. The manipulation of the characteristic dome of magpie nests effectively modified ambient light for egg discrimination, as nests with a reduced dome had increased levels of light compared to control or dome-enlarged nests. Interestingly, we found that rejection of model eggs decreased seasonally in magpie nests in which ambient light for discrimination was unchanged, whereas in nests with increased levels of light magpies rejected eggs at a similar rate over the season. These results are consistent with the previous findings suggesting a key role of ambient light on visual detection of colored sexual signals (Gamble et al. 2003) but are not in agreement with expectation from the nest light environment hypothesis proposed by Cherry and Bennett (2001), as this would predict higher acceptance of non-mimetic eggs at lower nest light levels.
Why increased ambient light induced model acceptance in early breeding magpie hosts remains a conundrum given that increased luminosity does not lead to significant changes in the degree of chromatic and achromatic mismatching of cream and blue models (Fig. 5)? One possible explanation is that the reduced and control group of nests included individuals differing in age and quality. Even though our study was based on non-banded birds, we think this possibility is unlikely because groups were established at random throughout the breeding season (see above). A second possibility is that ambient light in magpie nests changed throughout the breeding season due to changes in coverage of leaves. Early in the breeding season, most of the almond trees are leafless and, as the season progresses, magpie nests become more likely hidden by the growing leaves (Molina-Morales et al. 2013), which may induce lower luminosity into magpie nests and thus that the effect of removing a portion of the dome on ambient light was lower. However, we found that ambient light inside the nests did not differ between early- and late-breeding magpies and that ambient light inside the nests did not change once magpies had completed the dome, at least until exposure to the egg discrimination experiment (Fig. 3). Moreover, this possibility would predict higher rejection early in the season in nests with increased luminosity, and we found the opposite pattern. Finally, it is possible that early magpies breeding in a nest with high-light levels did perceive egg models as an odd object rather than as a parasite threat, and thus were more prone to accept it. At low-light levels, however, perception of egg artificiality might be visually constrained. However, this possibility seems unlikely as nest sanitation experiments have revealed that odd objects are mostly removed from avian nests (Moskat et al. 2003), including magpies (Álvarez et al. 1976).
Conclusion
A large number of studies have provided a strong support for a role of visual cues of cuckoo and host eggs in the sensory–perceptual processes involved in egg discrimination by cuckoo hosts. Surprisingly, despite long-term knowledge that propagation of colored signals is affected by environmental conditions, the potential role of ambient light on the perceptual processes implicated in egg discrimination has been rarely considered. Here, we have found that experimental manipulation of ambient light in magpie nests influenced detection of model eggs irrespective of their coloration. Our results thus suggest that environmental conditions could potentially affect the perceptual processes involved in visual detection of parasite eggs.
References
Anderson MG, Moskat C, Ban M, Grim T, Cassey P, Hauber ME (2009) Egg eviction imposes a recoverable cost of virulence in chicks of a brood parasite. PLoS One 4:A67–A73
Alvarez F, Arias de Reyna L, Segura M (1976) Experimental brood parasitism of the magpie (Pica pica). Anim Behav 24:907–916
Avilés JM (2008) Egg colour mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc R Soc Lond B 275:2345–2352
Avilés JM, Soler JJ (2009) Nestling colouration is adjusted to parent visual performance in altricial birds. J Evol Biol 22:376–386
Avilés JM, Soler JJ, Hart NS (2011) Sexual selection based on egg colour: physiological models and egg discrimination experiments in a cavity-nesting bird. Behav Ecol Sociobiol 65:1721–1730
Avilés JM, Soler JJ, Pérez-Contreras T, Soler M, Møller AP (2006) Ultraviolet reflectance of great spotted cuckoo eggs and egg discrimination by magpies. Behav Ecol 17:310–314
Avilés JM, Vikan JR, Fossoy F, Antonov A, Moksnes A, Røskaft E, Shykoff JA, Møller AP, Stokke BG (2012) Egg phenotype matching by cuckoos in relation to discrimination by hosts and climatic conditions. Proc R Soc Lond B 279:1967–1976
Avilés JM, Vikan JR, Fossoy F, Antonov A, Moksnes A, Røskaft E, Stokke BG (2010) Avian color perception predicts behavioral responses to experimental brood parasitism in chaffinches. J Evol Biol 23:293–301
Baeyens G (1981) Magpie breeding success and carrion crow interference. Ardea 69:125–139
Birkhead TR (1991) The magpies: the ecology and behaviour of black-billed and yellow-billed magpies. T & A D Poyser, London
Brooke MD, Davies NB (1988) Egg mimicry by cuckoos Cuculus canorus in relation to discrimination by hosts. Nature 335:630–632
Cassey P, Grim T, Honza M, Hauber ME (2009) The modeling of avian visual perception predicts behavioural rejection responses to foreign egg colours. Biol Lett 4:515–517
Cherry MI, Bennett ATD (2001) Egg colour matching in an African cuckoo, as revealed by ultraviolet—visible reflectance spectrophotometry. Proc R Soc Lond B 268:565–571
Cramp S (1998) The complete birds of the Western Palearctic. Oxford University Press, Oxford
Davies NB (2000) Cuckoos, cowbirds and other cheats. T & A D Poyser, London
Davies NB, de Brooke M (1988) Cuckoos versus reed warblers—adaptations and counteradaptations. Anim Behav 36:262–284
Endler JA (1993) The color of light in forests and its implications. Ecol Monogr 63:1–27
Gamble S, Lindholm AK, Endler JA, Brooks R (2003) Environmental variation and the maintenance of polymorphism: the effect of ambient light spectrum on mating behaviour and sexual selection in guppies. Ecol Lett 6:463–472
Gomez D (2006) AVICOL. A program to analyse spectrometric data
Hart NS (2002) Vision in the peafowl (Aves: Pavo cristatus). J Exp Biol 205:3925–3935
Honza M, Prochazka P, Morongova K, CapekM JV (2011) Do nest light conditions affect rejection of parasitic eggs? A test of the light environment hypothesis. Ethology 117:539–546
Langmore NE, Kilner RM, Butchart SHM, Maurer G, Davies NB, Cockburn A, Macgregor NA, Peters A, Magrath MJL, Dowling DK (2005) The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav Ecol 16:686–692
Lind O, Kelber A (2009) The intensity threshold of colour vision in two species of parrot. J Exp Biol 212:3693–3699
Lotem A, Nakamura H, Zahavi A (1992) Rejection of cuckoo eggs in relation to host age: a possible evolutionary equilibrium. Behav Ecol 3:128–132
Martin-Vivaldi M, Soler M, Møller AP (2002) Unrealistically high costs of rejecting artificial model eggs in cuckoo Cuculus canorus hosts. J Avian Biol 33:295–301
Moksnes A, Røskaft E, Braa AT (1991) Rejection behavior by common cuckoo hosts towards artificial brood parasite eggs. Auk 108:348–354
Molina-Morales M, Martínez JG, Martin-Galvez D, Dawson D, Rodriguez J, Burke T, Avilés JM (2013) Evidence of long-term structured cuckoo parasitism on individual magpie hosts. J Anim Ecol 82:389–398
Molina-Morales M, Martínez JG, Martin-Galvez D, Dawson D, Burke T, Avilés JM (2014) Cuckoo hosts shift from accepting to rejecting parasitic eggs across their lifetime. Evolution 68:3020–3029
Montgomerie R (2006) Analyzing colors. In: Hill GE, McGraw KJ (eds) Bird coloration, vol. 1: mechanism and measurements. Harvard University Press, Harvard, pp. 90–147
Moreno J, Osorno JL (2003) Avian egg colour and sexual selection: does eggshell pigmentation reflect female condition and genetic quality? Ecol Lett 6:803–806
Moskat C, Szekely T, Kisbenedek T, Karcza Z, Bartol I (2003) The importance of nest cleaning in egg rejection behaviour of great reed warblers Acrocephalus arundinaceus. J Avian Biol 34:16–19
Odeen A, Hastad O, Alstrom P (2011) Evolution of ultraviolet vision in the largest avian radiation—the passerines. BMC Evol Biol 11:313
Quesada J (2007) The different roles of the roof density and nest size in the Iberian magpie nest. Acta Ethol 10:41–45
Reynolds SJ, Martin GR, Cassey P (2009) Is sexual selection blurring the functional significance of eggshell coloration hypotheses? Anim Behav 78:209–215
Rothstein SI (1982) Mechanisms of avian egg recognition—which egg parameters elicit responses by rejecter species. Behav Ecol Sociobiol 11:229–239
Siddiqi A, CroninTW LER, Vorobyev M, Summers K (2004) Interspecific and intraspecific views of color signals in the strawberry poison frog Dendrobates pumilio. J Exp Biol 207:2471–2485
Soler JJ, Avilés JM, Martín-Gálvez D, de Neve L, Soler M (2014) Eavesdropping cuckoos: further insights on great spotted cuckoo preference by magpie nests and egg colour. Oecologia 175:105–115
Soler JJ, Avilés JM, Møller AP, Moreno J (2012) Attractive blue-green egg coloration and cuckoo-host coevolution. Biol J Linn Soc 106:154–168
Soler JJ, Avilés JM, Soler M, Møller AP (2003) Evolution of host egg mimicry in a brood parasite, the great spotted cuckoo. Biol J Linn Soc 79:551–563
Soler JJ, Navarro C, Pérez-Contreras T, Avilés JM, Cuervo JJ (2008) Sexually selected egg coloration in spotless starlings. Am Nat 171:183–194
Soler JJ, Soler M (2000) Brood-parasite interactions between great spotted cuckoos and magpies: a model system for studying coevolutionary relationships. Oecologia 125:309–320
Soler JJ, Soler M, Møller AP, Martínez JG (1995a) Does the great spotted cuckoo choose magpie host according to their parenting ability? Behav Ecol Sociobiol 36:201–206
Soler M (1990) Relationships between the great spotted cuckoo Clamator glandarius and its corvid hosts in a recently colonized area. Ornis Scand 21:212–223
Soler M, Møller AP (1990) Duration of sympatry and coevolution between the great spotted cuckoo and its magpie host. Nature 343:748–750
Soler M, Soler JJ, Martínez JG, Møller AP (1995b) Magpie host manipulation by great spotted cuckoos: evidence for an avian mafia. Evolution 49:770–775
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B 265:351–358
Wyllie I (1981) The cuckoo. BT Batsford, London
Acknowledgments
Funding was provided by Spanish Ministerio de Educación y Ciencia and European funds (FEDER) (CGL2008-00718 and CGL2011-27561 to JMA, CGL2010-19233-C03-01 to JJS, and CGL2007-61940/BOS to MS) and by JAE and “Juan de la Cierva” postdoctoral Grants from the CSIC and the Spanish Ministry of Education and Science, respectively, to DMG and LDN.
Ethical standards
Permission for nest visits and nests and egg color experiments was granted by the Consejería de Agricultura, Pesca y Medio Ambiente from the Junta de Andalucía (Spanish Regional Government).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by E. Fernandez-Juricic
Rights and permissions
About this article
Cite this article
Avilés, J.M., Martín-Gálvez, D., De Neve, L. et al. Ambient light in domed nests and discrimination of foreign egg colors. Behav Ecol Sociobiol 69, 425–435 (2015). https://doi.org/10.1007/s00265-014-1855-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-014-1855-z