Abstract
By laying their eggs in the nests of other birds, avian brood parasites impose the cost of rearing young upon their hosts. The recognition and rejection of foreign eggs are primary host defenses against costly brood parasitism. Hosts of parasitic brown-headed cowbirds (Molothrus ater) challenge coevolutionary theory because most cowbird hosts accept parasitic eggs despite their drastically different appearance from the hosts’ own eggs. American robins (Turdus migratorius) are one of only 10 % of the over 200 potential cowbird host species to robustly reject parasitic eggs, but the mechanisms driving the sensory bases of foreign egg rejection remain elusive. Our research combined avian visual perceptual modeling and behavioral experimentation to investigate chromatic cues eliciting parasitic egg rejection in American robins. We assessed the effects of perceivable background color differences between real host and model parasite eggs, across all four avian photoreceptors, on rates of rejection of model eggs spanning in color across the entire avian spectral sensitivity range, and including immaculate model eggs matching the natural colors of robin and cowbird eggs. The results suggest that egg rejection in robins is driven by the overall perceivable difference in color between own and artificial eggs, and input from all four single-cone avian photoreceptors affects the rejection decision. The results, however, also reveal that when viewed by the avian eye, natural cowbird eggs appear more similar in background color to robin eggs than predicted by the high rejection rate of these parasitic eggs. This suggests that robins respond specifically to parasitism by cowbirds, despite an apparent lack of sensory tuning toward the detection of the background color of cowbird eggs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 1 % of all avian species are obligate brood parasites (Payne 1977), which lay their eggs in the nest of other bird species (the hosts), thereby releasing themselves from the temporal and energetic costs of rearing their own offspring (Davies 2000). Hosts of brood parasites experience depressed reproductive success (Røskaft et al. 1990; Payne and Payne 1997; Øien et al. 1998; Lorenzana and Sealy 2001; Hauber 2003a, Hauber 2003b). The recognition and removal of parasitic eggs is a primary and effective host defense against brood parasitism (Rothstein 1975; Rothstein and Robinson 1998; Grim et al. 2011; Kilner and Langmore 2011), which in turn exerts selective pressure on the parasites to lay eggs that mimic host eggs in appearance (Davies and Brooke 1989; Moksnes and Røskaft 1995; Stoddard and Stevens 2011). The result is a reciprocal evolutionary pressure on hosts to fine-tune their sensory, discriminatory, and rejection abilities in response to increasingly more mimetic parasites (Anderson et al. 2009).
The degree of host–parasite egg mimicry and concurrent host specialization varies dramatically among parasitic lineages and their hosts (Rothstein 1990). Brown-headed cowbirds (Molothrus ater; hereafter, cowbirds) are generalist obligate brood parasites, and lay eggs in the nests of 245 North American passerine species (Friedmann 1929, 1971; Lowther 2013). Cowbird nestlings, while less virulent than those of many other obligate brood parasite lineages (Hauber 2003a; Kilner 2003; Kilner et al. 2004), are typically larger and more competitive than their hosts’ nestlings, and able to monopolize hosts’ parental care leading to reduced growth of nest mates, especially in small-bodied host species (Payne 1977; Rothstein 1990; Slagsvold 1998; Kilpatrick 2002; Hauber 2003b). Unlike the historically well-studied common cuckoos (Cuculus canorus; Davies and Brooke 1988, 1989; Moksnes and Røskaft 1995; Avilés 2008; Stoddard and Stevens 2010, 2011; Igic et al. 2012; Stoddard and Kilner 2013), cowbird eggs do not appear to closely mimic those of their hosts, when assessed by human vision (Friedmann 1929; Rothstein 1982; Klippenstine and Sealy 2010). Given the many costs of providing care for unrelated offspring, the prevalence of egg rejection among hosts of egg-mimetic brood parasites (Davies 2000), and the wide range of cowbird hosts with highly variable egg appearances, it is paradoxical that few cowbird host species eject the parasite’s eggs (Hosoi and Rothstein 2000).
The removal of parasitic eggs and other foreign objects may be driven mechanistically by differences in shape (Moskát et al. 2003a; Guigueno and Sealy 2012), size (Marchetti 2000), maculation (Lahti and Lahti 2002; López-de-Hierro and Moreno-Rueda 2010; Moskát et al. 2010), ultraviolet (UV) reflectance (Honza et al. 2007; Honza and Polačiková 2008), overall brightness (Lahti 2006), color difference in a particular part of the egg shell (e.g., blunt pole; Polačiková and Grim 2010), or inherent aspects of eggshell background coloration (Moskát et al. 2008; Ban et al. 2013). Hosts may also respond to overall differences by integrating several different visual and tactile characteristics (Rothstein 1982; Spottiswoode and Stevens 2010; de la Colina et al. 2012). In many earlier studies of brood parasitic egg rejection, the artificial egg stimulus and resulting analyses relied on either human assessment of egg colors or comparison based on spectrophotometric reflectance measures of host and parasite eggs (Croston and Hauber 2010). As such analyses do not fully or specifically account for differences between human and avian vision, including avian UV sensitivity (Cuthill et al. 2000), it is necessary to use UV-inclusive reflectance spectrophotometric data (Cherry and Bennett 2001; Honza et al. 2007; Cherry et al. 2007a, b) in conjunction with the known spectral sensitivities of focal host bird species (Hart et al. 2000) to establish the perceptual thresholds of own-foreign egg color discrimination (e.g., Avilés 2008; Cassey et al. 2008; Langmore et al. 2009; Igic et al. 2010, 2012; Stoddard and Stevens 2010). In doing so, we can test the role of avian-perceived color differences in eliciting egg rejection decisions.
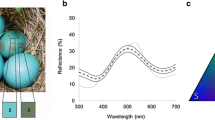
American robins (Turdus migratorius; hereafter, robins) are one of only ca. 26 cowbird host species which grasp and eject cowbird eggs (Peer and Sealy 2004; Rasmussen et al. 2009) in ca. 100 % of trials, where nests are experimentally parasitized with real or model parasite eggs, in areas of sympatry with cowbirds (Rothstein 1982; Briskie et al. 1992). To the human observer, cowbird and robin eggs differ markedly in both background coloration (i.e., external ground color of the eggshell) and maculation (Friedmann 1929) (Fig. 1). Physical reflectance spectra also show differences in background coloration in both the UV- (UV–vis) and human-visible parts of the light spectrum (Underwood and Sealy 2008) (Fig. 1). Previous work only assessed discontinuous, two-character state differences between cowbird and robin eggs in size (robin vs. cowbird sized), background color (robin vs. cowbird colored), maculation (presence vs. absence; Friedmann 1929; Rothstein 1982), and shape (host vs. cowbird shaped; egg vs. non-egg shaped; Underwood and Sealy 2006) and neither quantified physical color traits nor modeled avian perception of the appearance of natural or experimental eggs. Therefore, it remains unexplored whether robins’ cues for identifying and rejecting foreign cowbird eggs lie in finer-scale continuous differences in background color perception between own and foreign eggs, across any part of the avian-visible light spectrum.
Here, we focus on the role of egg background coloration in eliciting egg ejection in robins by manipulating it semi-continuously using artificial eggs ranging in color across the avian-visible light-reflectance spectrum. We first evaluate the hypothesis that (1) similarity between own and foreign egg background color is an important cue for egg rejection (Davies and Brooke 1988, 1989; Moskát et al. 2003a; Stevens et al. 2013). To this aim, we test the prediction that eggs of different colors, ranging across the entire spectral sensitivity range of songbirds (Aidala et al. 2012a), will be rejected at predictably different rates such that artificial eggs with colors more perceivably different from the hosts’ own egg colors will be more likely to be rejected (Cassey et al. 2008; Avilés et al. 2010; Stoddard and Stevens 2010). We then investigate the alternative hypotheses that (2) robins specifically reject cowbird-egg-colored foreign eggs or (3) robins specifically reject foreign eggs based on perceivable color differences in the UV part of the avian visible spectrum, as this has received particular attention in brood parasite literature (e.g., UV-matching hypothesis, Cherry and Bennett 2001), and specific response to differences in UV reflectance has been demonstrated in a congener of the American robin, the song thrush T. philomelos Turdus merula and song thrush Turdus philomelos (Honza et al. 2007). We set out to model effects of avian-perceived differences in color on rejection rates of model parasitic eggs following prior perceptual modeling work on foreign egg ejection in the song thrush (Cassey et al. 2008). We evaluate the effects of differences in egg color across the sensitivity ranges of each photoreceptor, including areas of overlapping sensitivities, by using avian visual modeling to evaluate effects of relative photoreceptor catches and their interactions on rejection rates across model parasitic eggs (hypothesis 1). This allows us to assess which of the four avian photoreceptors, including the UV-sensitive (UVS) cone (hypothesis 3), contribute consistently to the decision to reject foreign eggs. Finally, we investigate the predictive value of the relationship between overall perceivable color differences and experimentally elicited egg rejection in our experiment on the perceivable difference in background color and the published likelihood of egg rejection by robins in response to natural conspecific and parasite eggs (hypothesis 2).
Methods
Study site and nests
In May–July 2010 and 2011, we monitored nesting activities of American robins in and around Ithaca, Tompkins County, NY, USA. Cowbird eggs are rarely found in robin nests, and during the course of our study (N = 64 nesting attempts included in this study), we only detected a single cowbird egg laid in an abandoned robin nest (Fig. 1). Nests were located by searching in and around natural and human-made structures, as robins show high nesting densities near human settlements (Sallabanks and James 1999; RC, personal observation). Additional nests were located with the help of local residents recruited using various signboards, local internet list-serves, and internet advertisements (following Hauber 2003b; Wagner et al. 2013).
Upon finding a nest containing two or more host eggs, we numbered all existing eggs with a nontoxic felt-tip pen (Sharpie™ brand, black), and artificially parasitized the nest by adding one plaster-of-Paris cowbird-sized egg (see below for details on artificial eggs) to the clutch. Nests were parasitized throughout both laying and incubation as available, and timing of parasitism (day in the nesting cycle) was included as a potential predictor of response to parasitism in our analysis (see Data Analysis, below). We removed no host eggs during this experiment, following prior work on this cowbird host (Briskie et al. 1992), and instead mirrored cases of natural parasitism without egg-removal by the female cowbird (reviewed in Sealy 1992). In other European Turdus species (Moskát et al. 2003b; Honza et al. 2005, 2007), egg rejection rates were not dependent on replacing or adding artificial eggs (Grim et al. 2011). We monitored the nest by returning daily, checking on previously marked eggs, marking any additional eggs, and determining the status of the artificial egg.
As robins reject model cowbird-sized eggs by grasping and ejecting these from the nest (Rothstein 1975, 1982), eggs were considered ejected if they were not present in the nest the following day, except when hatching or full predation (indicated by the absence of all eggs from the nest) occurred. No nests were abandoned as a result of experimental manipulation. Eggs were considered accepted if they remained in the nest for 6 consecutive days, after which the artificial egg was removed (following Grim et al. 2011 for other Turdus species). Each nest was parasitized multiple times to test for possible effects of presentation order on rejection rate; a single model egg was added whenever one had previously been ejected or accepted. Prior to any statistical tests, the data were randomized to avoid pseudoreplication, such that only one presentation per nest is included in the analysis, and each nest is included only once. We also avoided including data replicated within nesting pairs and across two consecutive nesting attempts by conservatively assuming that nest ownership was shared between any two nests located within ∼10 m of each other throughout the season. For each nest, we recorded the site location, parasitic model egg color, timing of artificial parasitism (day in the incubation cycle), presentation number, and clutch size.
All nests were monitored until hatching to assess timing of the onset of each treatment relative to the laying and incubation cycle (typical robin incubation period: 12–14 days; Sallabanks and James 1999). This study was conducted on private properties with the express consent of the landowners and followed the protocol approved by the Institutional Animal Care and Use Committed of Hunter College (# MH 2/13-T3).
Artificial eggs
Artificial eggs were molded from plaster-of-Paris, following the dimensions of brown-headed cowbird eggs. All eggs weighed between 2.6–3.4 g, and measured 21 × 16 mm, based on the documented average dimensions (21.4 × 16.4 mm, 3.03 g; Lowther 1993) of cowbird eggs near Ithaca. The eggs were then painted with either nontoxic acrylic or latex house paint (Behr PREMIUM PLUS™ Interior Paint), using colors with reflectance peaking at wavelength intervals spanning the avian visual sensitivity range. Colors were chosen by inspecting the characteristic shape of their reflectance spectra, as determined using avian-visible range spectrophotometric measurements (described below; Fig. 2), based on both the wavelengths of peak quantum receptor catches, as “color” is determined by the relative rather than absolute receptor catches (Endler and Mielke 2005), and the wavelength at peak reflectance, which influences hue (Endler 1990). Red, yellow, and blue model egg colors peak at even intervals across the avian visual range (wavelength of 650, 550, and 450 nm, respectively). Additional experimental eggs were dyed to resemble (mimic) the background color of otherwise maculated, real (natural) cowbird eggs (“BHCO ground”), or the “background” color of immaculate, real (natural) robin eggs (“AMRO ground”; Figs. 1 and 2 see below for measurement methods). As the importance of ultraviolet reflectance has also been demonstrated for some avian host–parasite systems to establish mimicry and to mediate hosts’ egg rejection responses to some brood parasitic species’ eggs (Cherry and Bennett 2001; Honza et al. 2007), we also included “UV-blocked” model eggs. These were painted the same color as AMRO ground model eggs, and then coated with unscented SPF 50 lotion sunscreen to cut out reflectance specifically in the ultraviolet part of the avian visual spectrum (sensu Avilés et al. 2005; Honza and Polačiková 2008). While all model eggs differ slightly in texture from real, unmanipulated robin eggs, the presence of dried sunblock lotion did not affect the texture of the model egg surface (RC, personal observation). That our uncoated mimetic model eggs were never rejected, and only one UV-blocked egg was ever rejected, suggests that any unquantified difference in texture between real and model eggs also did not affect rates of model egg rejection.
Spectral measurements
We characterized model and natural egg color across the entire avian visual spectrum by measuring spectral reflectance using a high resolution spectrometer with deuterium tungsten halogen light source and 455 μm solarization-resistant shielded cable (Ocean Optics Jaz spectrometer with UV–vis light source, Ocean Optics Inc., Dunedin, FL, USA). Measurements were taken using a fiber optic probe held perpendicular to the egg surface for each individual measurement. The spectrometer was calibrated and spectra expressed relative to a Spectralon reflectance standard (WS-1, Ocean Optics, Inc.), which reflects >95 % of UV–vis light, and a fully dark standard (a paper-box with black felt entry hole to block all light from entering; Igic et al. 2010), to account for baseline noise in the spectrophotometer. The percent reflectance at each wavelength was calculated automatically with reference to the light and dark standards. To minimize measurement error, the spectrometer was calibrated repeatedly throughout sampling.
Nine measurements were taken for each model egg, three measurements each at the blunt pole, middle, and sharp pole. These measurements were averaged for each model and natural egg, yielding the average spectral reflectance curve for each. For natural robin eggs, spectral measures were taken on the day after clutch completion. Representative spectra for natural and model eggs are shown in Figs. 1 and 2.
Data analysis
Describing model egg color
First, we quantified color variation between natural robin eggs and each model egg color for each photoreceptor. We divided the spectral sensitivity range into four regions based on the maximal sensitivities of each of the four photoreceptors of the congeneric European blackbird T. merula as described in Hart et al. 2000. Values of reflectance ratios (R 300–400/300–700, R 400–475/300–700, R 475–550/300–700, and R 550–700) were used as estimates of UVS photon catch, short-wavelength sensitive (SWS) photon catch, medium-wavelength sensitive (MWS) photon catch, and long-wavelength sensitive (LWS) photon catch, respectively (Sheldon et al. 1999). Quantitative descriptions of all model egg colors are listed in Tables 1 and 2 with photos and reflectance spectra shown in Figs. 1 and 2.
Comparing egg rejection responses between model eggs
Prior to any analysis and to avoid pseudoreplication, data were randomized such that only one presentation at each nest was included in the analyses and each nest was included in an analysis only once. To test for independence of frequency of rejection (i.e., ejection) among different colored model eggs, we conducted a χ 2 test for a multi-way contingency table of acceptance and rejection (egg color × outcome (accept or reject)). When expected cell counts were less than 5, we analyzed differences among ranked rejection rates across colors using Kruskal–Wallis tests. Post hoc t tests were then used to evaluate differences among rejection rates for specific color pairs. As each nest was artificially parasitized multiple times, we applied additional Kruskal–Wallis tests to assess the effect of presentation order as a potential confound of egg rejection rates in response to sequential parasitism (e.g., Hauber et al. 2006; Samaš et al. 2011). The timing of experimental parasitism (day in the laying cycle that parasitism took place; day 1 = day of 1st egg laid) was also included as a possible predictor, as this is known to effect rejection rate (Welbergen et al. 2001; Moskát and Hauber 2007). Again, χ 2 tests were used to examine effects of study year on acceptance/rejection, and Kruskal–Wallis tests were used to examine potential effects of egg color on the latency to rejection, defined as the number of days lapsing between experimental parasitism and egg rejection.
Avian visual modeling effects of egg color on rejection
To estimate differences between colors with respect to the spectral sensitivities of avian photoreceptors (Bennet and Théry 2007), we used the Vorobyev and Osorio (1998) model for tetrachromatic vision in AVICOL v5 software (Gomez 2010). American robins are known to be UVS (Chen et al. 1984; Chen and Goldsmith 1986; Aidala et al. 2012a), but physiological data for detailed spectral sensitivity of each photoreceptor were not available for our focal species. Therefore, we extracted spectral sensitivity data for the congeneric European blackbird, T. merula, from data published in Hart et al. 2000 using Vistametrix software (Vista Metrix 1.3, SkillCrest LLC, www.skillcrest.com) and ranging from 330 to 700 nm. As AVICOL requires sensitivity data ranging from 300 to 700 nm, we set photoreceptor absorbance for 300–330 nm to 0 (sensu Igic et al. 2010, 2012). Relative cone densities were set to UVS, 1; SWS, 2; MWS, 2; LWS, 2 (as listed for T. merula; Hart et al. 2000); and Weber fraction was set to 0.1 (Vorobyev et al. 1998). As the ability to discriminate different colors is influenced by environmental light (Langmore et al. 2005; Munoz et al. 2007; Avilés 2008; Honza et al. 2011), we used published ambient light irradiance data for broken canopy forest (Vorobyev and Osorio 1998), which may most closely simulate the variable forest-edge light environments in which many American robins nest, even when breeding in sub/urban sites (Sallabanks and James 1999; RC, personal observation).
AVICOL extracts quantum receptor catches for each single-cone receptor type, and combines these with the birds’ spectral sensitivities to quantify the birds’ ability to distinguish between any two colors as the perceptual distance between spectra (ΔS) or as JNDs (“just noticeable differences”); JNDs exceeding 1.0 indicate a chromatic difference that is discriminable based on our estimates of avian spectral sensitivities (Osorio and Vorobyev 1996). AVICOL also extracts discriminability based on achromatic contrasts using the sum of the sensitivities of MWS and LWS cones, as these are similar to the sensitivities of rods and principal double-cone cells in the avian retina (Hart et al. 2000).
For the subsequent analyses, reflectance data for each natural robin egg was paired randomly with another (either wild or model) egg. We first evaluated the quality of our ‘mimetic’ model egg colors (i.e., AMRO ground and BHCO ground), designed to resemble natural eggs by comparing these to the natural eggs (cowbird or robin). We calculated mean JNDs distinguishing each natural egg type from its respective model, and compared these between groups using Welch’s two-sample t tests, to evaluate whether one model egg better mimicked its natural counterpart. We also compared our model mimetic robin eggs with our UV-blocked model eggs to ensure that these were perceptually discriminable based on avian visual sensitivities and therefore suitable for use in our analyses. We calculated mean JNDs differentiating these model egg types, and tested whether this differed statistically from 1 (as 1 JND signifies discriminable difference) using a one-sample t test. As our UV-blocked and robin mimetic eggs differ only in the UV part of the spectrum and are discriminable based on avian vision (μ = 3.83, t = 3.17, df = 6, p = 0.019; Fig. 2), we examined the UV-blocked egg treatment as a test of differences specifically in the ultraviolet part of the avian visual spectrum.
We then calculated the difference between quantum receptor catches for real and model eggs across each of the four single-cone receptors. We summed these values within each pair, and then calculated the proportion of the total difference between eggs in each pair attributable to differences in each photoreceptor sensitivity region (normalizing photoreceptor catches across all four photoreceptors to equal 1 for each egg pair). Using these data, we tested the effect of proportionate differences in receptor catches on rejection rate across wild and model egg colors. To determine which avian photoreceptors contribute predictably to the rejection of foreign eggs, we used the Akaike Information Criterion (AIC) model selection approach (Burnham and Anderson 2002) to choose the best fit from among candidate photoreceptor models. Candidate models were derived by stepwise removal from an initial global logistic regression including proportionate differences in photoreceptor catches across each egg pair (as described above) and all possible interactions, with percent rejection (of eggs of a given model egg color) as dependent variable. The model with the lowest value of AIC provides the best balance between loss of precision due to overfitting and bias due to underfitting, and is therefore the best fit model. Given the relatively small values of N and large values of K, we here report AICc values, AIC values corrected for finite sample sizes (N = 4 − 5 eggs’ reflectance spectra measured per color type in this analysis). The Akaike weights give the relative support for a given model compared with the other models in the set (Burnham and Anderson 2002).
Finally, for each model egg, we extracted the JND value differentiating model egg color spectra from natural robin egg spectra, as well as the pairwise achromatic contrast values. To test for effects of JNDs and achromatic contrasts on rate of rejection of foreign eggs, we fitted separate logistic regressions describing percent of model eggs rejected as a function of JNDs or achromatic contrast difference from natural robin eggs, across all model egg colors. When this regression analysis was significant (i.e., for JNDs but not for achromatic contrasts; see “Results”), we then calculated 95 % confidence intervals (Mermoz and Ornelas 2004) around the rejection rates for each model egg type as predicted by JNDs differentiating that model egg color from the natural robin eggs. We plotted the positions (JND X Rejection %) of our artificial eggs, as well as the positions of natural (robin and cowbird) eggs (with experimental rejection data taken from Briskie et al. 1992), and examined whether these positions fell within the predicted 95 % confidence interval. Analyses were conducted in R version 2.12.1.
Results
Egg rejection rates among model eggs
Model egg color significantly predicted rejection rate (Kruskal–Wallis χ 2 6 = 25, p < 0.001). Eggs dyed to resemble the ground color of cowbird eggs (“BHCO ground,” N = 10) were rejected in 100 % of trials. Yellow eggs (N = 13) were rejected in 70 % of trials. Red eggs (N = 14) were rejected in 64 % of trials. Blue eggs (N = 15) were rejected in 58 % of trials. UV-blocked eggs (N = 5) were rejected in 20 % of trials. Eggs dyed to resemble the background color of robin eggs (“AMRO ground,” N = 7) were never rejected. Of the six model egg types presented, all were rejected at statistically similar rates except “AMRO ground” and “UV-blocked” eggs, which were rejected at significantly lower rates than all other model egg types (see Table 3 for pairwise comparisons; Fig. 2 shows 95 % Wilson binomial confidence intervals for rejection rates).
Egg color did not significantly predict latency to rejection (Kruskal–Wallis χ 2 3 = 2.98, p = 0.394; Fig. 3a). Presentation order and study year also did not statistically covary with rejection rates (presentation order, Kruskal–Wallis χ 2 4 = 4, p = 0.406; year, χ 2 1 = 0.32, p = 0.569; Fig. 3b, c). Likewise, the timing of parasitism within the incubation cycle was not significantly related to rejection rates (Kruskal–Wallis χ 2 8 = 8, p = 0.434; Fig. 3d).
Non-significant relationships among (a) model egg color and latency to rejection (days), (b) presentation order and rejection (percent) across all model egg types, (3) study year and percent rejection (percent) across all model egg types, and (4) timing of parasitism (day in the incubation cycle, day 1 = 1st egg laid) and rejection (percent) across all model egg types
Avian visual modeling
Individual natural American robin eggs were, on average, discriminable from all model egg colors (for JNDs across all model egg colors, p < 0.05 relative to 1.0; one sample t tests; Fig. 4). In particular, model eggs mimicking the ground color of natural robin eggs differed from natural robin eggs by a mean of 4.6 JNDs. Surprisingly, however, both model cowbird and, especially, natural cowbird eggs showed relatively low JND values against natural robin eggs (mean JNDs difference for model cowbird eggs = 5.51 JNDs; mean JNDs difference for natural cowbird eggs = 13.25), implying more avian-perceivable similarity between host and parasite eggs than previously appreciated (Friedmann 1929; Rothstein 1982). Chromatic and achromatic contrasts are summarized in Fig. 4.
By contrast, our model eggs mimicking the background color of natural cowbird eggs differed from natural cowbird eggs by 26.7 JNDs. This was likely because natural cowbird eggs reflect strongly in the UV part of the spectrum (Fig. 1) yet methodological constraints prevented us from mimicking reflectance in UV. When we also calculated JNDs between natural and model cowbird eggs after setting reflectance values between 300 and 400 nm to 0, JND values differentiating these eggs were reduced dramatically, to 5.6 JNDs. JNDs differentiating natural robin eggs from their model counterparts were significantly higher than JNDs differentiating natural and model cowbird eggs (Welch’s two-sample t test; t 52 = −3.53, p < 0.001). This indicates that across the SWS, MWS, and LWS parts of the avian visual spectrum, our model cowbird eggs were closer in appearance to natural cowbird egg ground color than our model robin eggs were to natural model robin egg color. These calculations illustrate that using human-based (400–700 nm) wavelength sensitivity to design or assess color-similarity between host and parasite (including experimental) eggs is likely to result in misleading levels of avian-perceivable similarity.
The model best predicting rejection rate for model eggs supports the consistent role of differential photoreceptor catch across the entire avian spectral sensitivity range in eliciting egg rejection. This model included terms for UVS, SWS, MWS, and LWS photoreceptor catches, and their interaction terms (Table 4).
Finally, avian visual modeling also revealed that JND values significantly predicted rejection rates across model egg colors (logistic regression, t 28 = 2.12, p = 0.044; Fig. 5). By contrast, achromatic contrast values did not significantly predict rejection rates (logistic regression, t 28 = 1.09, p = 0.287, bivariate plot not shown). The 95 % confidence interval surrounding rejection rates, as predicted by JNDs includes rejection rates for yellow, red, and blue model egg colors. By contrast, rejection rates for robin ground color model eggs and UV-blocked eggs fall below the 95 % confidence interval threshold, as these eggs are rarely rejected (robin ground = 0 % rejection, UV-blocked = 20 % rejection), despite their appreciable avian-perceivable discriminability from natural robin eggs (Fig. 4). Natural robin eggs fall well below the 95 % confidence interval, and these are never rejected (Briskie et al. 1992). Model eggs mimicking the background color of cowbird eggs fall well above the 95 % confidence interval, as they are rejected in 100 % of experimental trials (Fig. 5). In contrast, natural cowbird eggs fall well above the 95 % confidence interval and are always rejected (Briskie et al. 1992).
Bivariate scatterplot of mean JNDs difference between natural robin eggs and each egg type plotted against the rejection (percent) for that respective egg type. Shaded area represents 95 % confidence interval based on the best fit logistic regression (fit line) for model eggs used in this experiment. Egg rejection rates of experimentally introduced, natural robin or cowbird, eggs were taken from Briskie et al. (1992)
Discussion
In American robins, the likelihood of rejection of model eggs dyed with various artificial colors, spanning the full range of avian-visible light, is best predicted by a model containing quantum photoreceptor catches of all four avian photoreceptors. Likewise, overall avian-perceivable chromatic difference between natural and model eggs (JNDs) significantly predicts rates of rejection. In support of hypothesis (1), these results imply that model egg colors perceived as more different from the robins’ own eggs will be rejected at higher rates.
More critically, visual modeling revealed that our mimetic experimental robin eggs were predicted to be perceptually discriminable from natural robin eggs (JNDs > 1.0 threshold for our mimetic model eggs) yet these were never rejected (Figs. 4 and 5). This may reflect a caveat in our perceptual modeling methodology, as we modeled avian vision using the photoreceptor sensitivities of congeneric European blackbirds, rather than of American robins per se, which are not yet available. In turn, cowbird eggs, whether model or natural (Rothstein 1982; Briskie et al. 1992; this study) are typically always rejected, despite the relatively low overall avian-perceived discriminability from natural robin eggs (Figs. 4 and 5). The latter pattern of unpredictably high rejection rates of cowbird eggs is in support of our hypothesis (2). Coevolution with cuckoos and cowbirds, then, may have shaped the robins’ visual system in ways which cannot be predicted from our visual modeling approach and/or the use of known visual physiology of T. merula. Statistical techniques now exist to detect sensory coevolution between single pairs of host–parasites within a set of multispecies comparative analyses (Anderson et al. 2009), but these would require detailed new anatomical and physiological studies of the robin’s actual visual system, perhaps at the level of individual variation (Fernández-Juricic et al. 2013). Further research should also address potential differences in photoreceptor evolution between hosts of mimetic versus nonmimetic brood parasites by modeling spectral sensitivities of American robins, and comparing these to those of species within the species rich lineage of the closely related European Turdus thrush clade (Voelker et al. 2007).
That mimetic model robin eggs were discriminable from natural eggs and yet were never rejected may demonstrate that robins tolerate some degree of color difference within their clutch. Behaviors otherwise indicating the detection of a parasitic egg, which are not then followed by egg rejection, have been documented in song thrush (Honza et al. 2007), yellow warblers (Setophaga petechia; Guigueno and Sealy 2012), great reed warblers (Acrocephalus arundinaceous; Moskát and Hauber 2007), and eastern olivaceous warblers (Hippolais pallida; Antonov et al. 2009). This is consistent with the suggestion that there exists a plastic, perceptual, and/or cognitive threshold for rejection that is separate from that of visual discrimination; alternatively, this may result from plasticity in host acceptance threshold in response to context of parasitism (Hauber et al. 2006; but see Vikan et al. 2009). Either case implies that egg rejection decisions are not wholly governed by limitations of the sensory and perceptual systems (de la Colina et al. 2012). Future work should also reconcile the differences between the sensory models and the observed behavioral thresholds of egg discrimination vs. rejection, including the possibility that robins perceivably discriminate between more eggs than they reject (see Moskát and Hauber 2007; Antonov et al. 2009).
Using a combination of artificial egg colors spanning the full range of the avian-visible spectrum, and the specific UV-blocking treatment in our experiments, these data also allow us to assess the role of UV-matching (Cherry and Bennett 2001) in eliciting egg rejection. In contrast to hypothesis (3), we suggest that egg rejection in robins is not driven specifically by differences in the ultraviolet part of the avian visual spectrum, because UV-blocked model eggs were rejected at low rates, which were statistically similar to rejection rates for our model robin mimetic eggs and lower than all other colors of model eggs. Similarly, rejection rates for both fell below the 95 % confidence interval for predicted rejection based on their respective JNDs (Table 3; Fig. 5). Cherry and Bennett (2001) posited that eggs appearing dissimilar to humans may actually appear similar in the ultraviolet part of the spectrum not visible to humans. For song thrush, congeneric with the robin, egg rejection is elicited by differences in photoreceptor catches for the UVS and SWS photoreceptors (Cassey et al. 2008). Considering that song thrush are parasitized, if rarely, by common cuckoos (Grim 2006; Grim et al. 2011), an egg-removing parasite and a member of a violet- (not UV) sensitive parasitic lineage (Mullen and Pohland 2008; Aidala et al. 2012b), differences in UV reflectance may benefit hosts by allowing for discrimination between their own and parasitic eggs without conferring that same advantage on the parasite (but see Avilés et al. 2005). As both robins and cowbirds are predicted to be UVS (Parrish et al. 1984; Aidala et al. 2012a), this selective advantage would not exist in the latter host–parasite system. UV reflectance varies widely among cowbird host species’ eggs (Underwood and Sealy 2008), yet there is very little variation in host responses to cowbird parasitism, in that most hosts are either strong rejecters or acceptors of natural or artificial cowbird eggs (Takasu 1998). Thus, UV chroma is unlikely to act as a particular cue for egg rejection by cowbird hosts, including robins (Underwood and Sealy 2008); this suggestion is here supported not only by our rejection data from the experiments with robin ground and UV-blocked eggs, but also by the detailed visual analyses which did not highlight a disproportionate role for UVS and SWS receptors in predicting egg rejection rates across different model egg colors (Table 4).
The results of this study indicate that egg rejection in robins occurs in response to overall differences in color across the entire avian visual spectrum, including quantum receptor catches from all four avian single-cone photoreceptors, and not limited to input from UVS photoreceptors. However, all cowbird eggs are rejected by this host, despite relatively high overall avian-perceived similarity to the robins’ own egg color. This suggests that robins respond specifically to parasitism by cowbirds, despite an apparent lack of sensory tuning toward detection of cowbird eggs. Further study should investigate both the nature and extent of selective pressures on the sensory, cognitive, and behavioral mechanisms of egg-rejection by American robins in response to parasitism by brown-headed cowbirds.
References
Aidala Z, Huynen L, Brennan P, Musser J, Fidler A, Chong N, Machovsky Capuska G, Anderson M, Talaba A, Lambert D, Hauber ME (2012a) Ultraviolet visual sensitivity in three avian lineages; paleognaths, parrots, and passerines. J Comp Physiol A 198:495–510
Aidala Z, Chong N, Anderson MG, Hauber ME (2012b) Predicted visual sensitivity for short-wavelength light in the brood parasitic cuckoos of New Zealand. Chinese Birds 3:295–301
Anderson MG, Ross HA, Brunton DH, Hauber ME (2009) Begging call matching between a specialist brood parasite and its host: a comparative approach to detect coevolution. Biol J Linn Soc 98:208–216
Antonov A, Stokke BG, Moksnes A, Røskaft E (2009) Evidence for egg discrimination preceding failed rejection attempts in a small cuckoo host. Biol Lett 5:169–171
Avilés JM (2008) Egg color mimicry in the common cuckoo Cuculus canorus as revealed by modelling host retinal function. Proc R Soc Lond B275:2345–2352
Avilés JM, Soler JJ, Perez-Contreras T, Soler M, Møller AP (2005) Ultraviolet reflectance of great spotted cuckoo eggs and egg discrimination by magpies. Behav Ecol 17:310–314
Avilés JM, Vikan JR, Fossøy F, Antonov A, Moksnes A, Røskaft E, Stokke BG (2010) Avian color perception predicts behavioral responses to experimental brood parasitism in chaffinches. J Evol Biol 23:293–301
Ban M, Moskát C, Barta Z, Hauber ME (2013) Simultaneous viewing of own and parasitic eggs is not required for egg rejection by a cuckoo host. Behav Ecol 24:1014–1021
Bennett ATD, Théry M (2007) Avian color vision and coloration: Multidisciplinary evolutionary biology. The American Naturalist 169:S1–S6
Briskie JV, Sealy SG, Hobson KA (1992) Behavioral defenses against avian brood parasitism in sympatric and allopatric host populations. Evolution 46:334–340
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information–theoretic approach. Springer, New York
Cassey P, Honza M, Grim T, Hauber ME (2008) The modelling of avian visual perception predicts behavioral rejection responses to foreign egg colors. Biol Lett 4:515–517
Chen D-M, Goldsmith TH (1986) Four spectral classes of cone in the retinas of birds. J Comp Physiol A 159:473–479
Chen D-M, Collins JS, Goldsmith TH (1984) The ultraviolet receptor of bird retinas. Science 225:337–340
Cherry MI, Bennett ATD (2001) Egg color matching in an African cuckoo, as revealed by ultraviolet-visible reflectance spectrophotometry. Proc R Soc Lond B 268:565–571
Cherry MI, Bennett ATD, Moskat C (2007a) Host intra-clutch variation, cuckoo egg matching and egg rejection by great reed warblers. Naturwissenschaften 94:441–447
Cherry MI, Bennett ATD, Moskat C (2007b) Do cuckoos choose nests of great reed warblers on the basis of host egg appearance? J Evol Biol 20:1218–1222
Croston R, Hauber ME (2010) The Ecology of Avian Brood Parasitism. Nat Educ Knowledge 1:3
Cuthill IC, Partridge JC, Bennett ATD, Church SC, Hart NS, Hunt S (2000) Ultraviolet vision in birds. Adv Study Behav 29:159–213
Davies NB (2000) Cuckoos, cowbirds and other cheats. Poyser, London
Davies NB, Brooke MDL (1988) Cuckoos versus reed warblers: adaptations and counteradaptations. Anim Behav 36:262–284
Davies NB, Brooke MDL (1989) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its hosts. I. Host egg discrimination. J Anim Ecol 58:207–224
de la Colina MA, Pompillo L, Hauber ME, Reboreda JC, Mahler B (2012) Different recognition cues reveal the decision rules used for egg rejection by hosts of a variably mimetic avian brood parasite. Anim Cogn 15:881–889
Endler JA (1990) On the measurement and classification of color in studies of animal color patterns. Biol J Linn Soc 41:315–352
Endler JA, Mielke PW (2005) Comparing entire color patterns as birds see them. Biol J Linn Soc 86:405–431
Fernández-Juricic E, Ojeda A, Deisher M, Burry B, Baumhardt P, Stark A, Elmore AG, Ensminger AL (2013) Do male and female cowbirds see their world differently? Implications for sex differences in the sensory system of an avian brood parasite. PLOS One 8:e58985
Friedmann H (1929) The cowbirds: a study in the biology of social parasitism. Charles C. Thomas, Springfield, IL
Friedmann H (1971) Further information of the host relations of the parasitic cowbirds. Auk 88:239–255
Gomez D (2010) AVICOL v.5, a program to analyse spectrometric data. Available from the author at dodogomez@yahoo.fr or by download from http://sites.google.com/site/avicolprogram/
Grim T (2006) Cuckoo growth performance in parasitized and unused hosts: not only host size matters. Behav Ecol Sociobiol 60:716–723
Grim T, Samaš P, Moskát C, Kleven O, Honza M, Moksnes A, Røskaft E, Stokke BG (2011) Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? J Anim Ecol 80:508–518
Guigueno MF, Sealy SG (2012) Increased investigation of manipulated clutches suggests egg recognition without rejection in a Brown-headed Cowbird (Molothrus ater) host, the Yellow Warbler (Setophaga petechia). Auk 129:17–25
Hart NS, Partridge JC, Cuthill IC, Bennett ATD (2000) Visual pigments, oil droplets, ocular media and cone photoreceptor distribution in two species of passerine bird: the blue tit (Parus caeruleus L.) and the blackbird (Turdus merula L.). J Comp Physiol A 186:375–387
Hauber ME (2003a) Interspecific brood parasitism and the evolution of host clutch sizes. Evol Ecol Res 5:559–570
Hauber ME (2003b) Hatching asynchrony, nestling competition, and the cost of interspecific brood parasitism. Behav Ecol 14:227–235
Hauber ME, Moskát C, Bán M (2006) Experimental shift in hosts’ acceptance threshold of inaccurate-mimic brood parasite eggs. Biol Lett 2:177–180
Honza M, Polačiková L (2008) Experimental reduction of ultraviolet wavelengths reflected from parasitic eggs affects rejection behavior in the blackcap Sylvia atricapilla. J Exp Biol 211:2519–2523
Honza M, Kuiper SM, Cherry MI (2005) Behavior of African turdid hosts towards experimental parasitism with artificial red-chested cuckoo Cuculus solitarius eggs. J Avian Biol 6:517–522
Honza M, Polačiková L, Procházka P (2007) Ultraviolet and green parts of the color spectrum affect egg rejection in the song thrush (Turdus philomelos). Biol J Linn Soc 92:269–276
Honza M, Procházka P, Morongová K, Čapek M, Jelínek V (2011) Do nest light conditions affect rejection of parasitic eggs? A test of the light environment hypothesis. Ethology 117:539–546
Hosoi S, Rothstein S (2000) Nest desertion and cowbird parasitism: evidence for evolved responses and evolutionary lag. Anim Behav 59:823–840
Igic B, Leuschner N, Parker KA, Ismar SMH, Gill BJ, Lovegrove TG, Millar CD, Hauber ME (2010) Size dimorphism and avian-perceived sexual dichromatism in a New Zealand endemic bird, the whitehead Mohoua albicilla. J Morphol 271:697–704
Igic B, Cassey P, Grim T, Greenwood DR, Moskát C, Rutila J, Hauber ME (2012) A shared chemical basis of avian host–parasite egg color mimicry. Proc R Soc Lond B 279:1068–1076
Kilner RM (2003) How selfish is a cowbird nestling? Anim Behav 66:569–576
Kilner RM, Langmore NE (2011) Cuckoos versus hosts in insects and birds: adaptations, counter-adaptations and outcomes. Biol Rev 86:836–852
Kilner RM, Madden JR, Hauber ME (2004) Nestlings use host young to procure resources. Science 305:877–879
Kilpatrick AM (2002) Variation in growth of brown-headed cowbird (Molothrus ater) nestlings and energetic impacts on their host parents. Can J Zool 80:145–153
Klippenstine DR, Sealy SG (2010) Assessing generalized egg mimicry: a quantitative comparison of eggs of brown-headed cowbirds and grassland passerines. Wilson J Ornithol 122:346–353
Lahti DC (2006) Persistence of egg recognition in the absence of cuckoo brood parasitism: pattern and mechanism. Evolution 60:157–168
Lahti DC, Lahti AR (2002) How precise is egg discrimination in weaverbirds? Anim Behav 63:1135–1142
Langmore NE, Kilner RM, Butchart SHM, Maurer G, Davies NB, Cockburn A, Macgregor NA, Peters A, Magrath MJL, Dowling DK (2005) The evolution of egg rejection by cuckoo hosts in Australia and Europe. Behav Ecol 16:686–692
Langmore NE, Stevens M, Maurer G, Kilner RM (2009) Are dark cuckoo eggs cryptic in host nests? Anim Behav 78:461–468
López-de-Hierro MDG, Moreno-Rueda G (2010) Egg-spot pattern rather than egg color affects conspecific egg rejection in the house sparrow (Passer domesticus). Behav Ecol Sociobiol 64:317–324
Lorenzana JC, Sealy SG (2001) Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav Ecol 12:325–329
Lowther PE (1993) Brown-headed cowbird (Molothrus ater). In: Poole (ed) The Birds of North America Online. Cornell Lab of Ornithology, Ithaca. Birds of North America Online. Available from http://bna.birds.cornell.edu
Lowther PE (2013) Lists of victims and hots of the parasitic cowbirds (Molothrus). Available from http://www.fieldmuseum.org/sites/default/files/Molothrus_hosts-26aug2013.pdf. Accessed 27 October 2013
Marchetti K (2000) Egg rejection in a passerine bird: size does matter. Anim Behav 59:877–883
Mermoz ME, Ornelas JF (2004) Phylogenetic analysis of life-history adaptations in parasitic cowbirds. Behav Ecol 15:109–119
Moksnes A, Røskaft E (1995) Egg-morphs and host preference in the common cuckoo (Cuculus canorus): an analysis of cuckoo and host eggs from European museum collections. J Zool 236:625–648
Moskát C, Hauber ME (2007) Conflict between egg recognition and egg rejection decisions in common cuckoo (Cuculus canorus) hosts. Anim Cogn 10:377–386
Moskát C, Sze'kely T, Kisbenedek T, Karcza Z, Ba'rtol I (2003a) The importance of nest cleaning in egg rejection behavior of great reed warblers Acrocephalus arundinaceus. J Avian Biol 34:16–19
Moskát C, Karcza Z, Csörgö T (2003b) Egg rejection in European Blackbirds (Turdus merula): the effect of mimicry. Ornis Fennica 80:86–91
Moskát C, Szekely T, Cuthill IC, Kisbenedek T (2008) Hosts’ responses to parasitic eggs: which cues elicit hosts’ egg discrimination? Ethology 114:186–194
Moskát C, Bán M, Szekely T, Komdeur J, Lucassen RWG, Van Boheemen LA, Hauber ME (2010) Discordancy or template-based recognition? Dissecting the cognitive basis of the rejection of foreign eggs in hosts of avian brood parasites. J Exp Biol 213:1976–1983
Mullen P, Pohland G (2008) Studies on UV reflection in feathers of some 1000 bird species: are UV peaks in feathers correlated with violet-sensitive and ultraviolet-sensitive cones? Ibis 150:59–68
Munoz AR, Altamirano M, Takasu F, Nakamura H (2007) Nest light environment and the potential risk of Common Cuckoo (Cuculus canorus) parasitism. Auk 124:619–627
Øien JI, Moksnes A, Røskaft E, Honza M (1998) Costs of Cuckoo Cuculus canorus parasitism to Reed Warblers Acrocephalus scirpaceus. J Avian Biol 29:209–215
Osorio D, Vorobyev M (1996) Color vision as an adaptation to frugivory in primates. Proc R Soc Lond B 263:593–599
Parrish JW, Ptacek JA, Will KL (1984) The detection of near-ultraviolet light by nonmigratory and migratory birds. Auk 101:53–58
Payne RB (1977) The ecology of brood parasitism in birds. Annu Rev Ecol Syst 8:1–28
Payne RB, Payne LL (1997) Brood parasitism by cowbirds : risks and effects on reproductive success and survival in indigo buntings. Behav Ecol 9:64–73
Peer BD, Sealy SG (2004) Correlates of egg rejection in hosts of the Brown-headed Cowbird. Condor 106:580–599
Polačiková L, Grim T (2010) Blunt egg pole holds cues for alien egg discrimination: experimental evidence. J Avian Biol 41:111–116
Rasmussen JL, Sealy SG, Underwood TJ (2009) Video recording reveals the method of ejection of brown-headed cowbird eggs and no cost in American Robins and Gray Catbirds. Condor 11:570–574
Røskaft E, Orians GH, Beletsky LD (1990) Why do red-winged blackbirds accept the eggs of brown-headed cowbirds? Evol Ecol 4:35–42
Rothstein SI (1975) An experimental and teleonomic investigation of avian brood parasitism. Condor 77:250–271
Rothstein SI (1982) Mechanisms of avian egg recognition: which egg parameters elicit responses by rejector species? Behav Ecol Sociobiol 11:229–239
Rothstein SI (1990) A model system for coevolution: avian brood parasitism. Annu Rev Ecol Syst 21:481–508
Rothstein SI, Robinson SK (1998) The evolution and ecology of avian brood parasitism. In: Rothstein SI, Robinson SK (eds) Parasitic birds and their hosts: studies in coevolution. Oxford University Press, New York, pp 3–56
Sallabanks R, James FC (1999) American Robin (Turdus migratorius). In: Poole (ed) The Birds of North America Online. Cornell Lab of Ornithology, Ithaca. Birds of North America Online. Available from http://bna.birds.cornell.edu/bna/species/462
Samaš P, Hauber ME, Cassey P, Grim T (2011) Repeatability of foreign egg rejection: Testing the assumptions of co-evolutionary theory. Ethology 117:606–619
Sealy SG (1992) Removal of Yellow Warbler eggs in association with cowbird parasitism. Condor 94:40–54
Sheldon BC, Andersson S, Griffith SC, et al. (1999) Ultraviolet colour variation influences blue tit sex ratios. Nature 402:874–877
Slagsvold T (1998) On the origin and rarity of interspecific nest parasitism in birds. Am Nat 152:264–272
Spottiswoode CN, Stevens M (2010) How to evade a coevolving brood parasite: egg discrimination versus egg variability as host defences. Proc R Soc Lond B 278:3566–3573
Stevens M, Troscianko J, Spottiswoode CN (2013) Repeated targeting of the same hosts be a brood parasite compromises host egg rejection. Nature Comm 4:2745
Stoddard MC, Kilner RM (2013) The past, present and future of ‘cuckoos versus reed warblers’. Anim Behav 85:693–699
Stoddard MC, Stevens M (2010) Pattern mimicry of host eggs by the common cuckoo, as seen through a bird’s eye. Proc R Soc Lond B 277:1387–1393
Stoddard MC, Stevens M (2011) Avian vision and the evolution of egg color mimicry in the common cuckoo. Evolution 65:2004–2013
Takasu F (1998) Why do all host species not show defense against avian brood parasitism: evolutionary lag or equilibrium? Am Nat 151:193–205
Underwood TJ, Sealy SG (2006) Influence of shape on egg discrimination in American robins and grey catbirds. Ethology 122:164–173
Underwood TJ, Sealy SG (2008) UV reflectance of eggs of Brown-headed Cowbird (Molothrus ater) and accepter and rejecter hosts. J Ornithol 149:313–321
Vikan JR, Stokke BG, Fossøy F, Jackson C, Huhta E, Rutila J, Moksnes A, Røskaft E (2009) Fixed rejection responses to single and multiple experimental parasitism in two Fringilla hosts of the common cuckoo. Ethology 115:840–850
Voelker GS, Rohwer R, Bowie K, Outlaw DC (2007) Molecular systematics of a speciose, cosmopolitan songbird genus: defining the limits of, and relationships among, the Turdus thrushes. Mol Phylogenet Evol 42:422–434
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of color thresholds. Proc R Soc Lond B Bio 265:351–358
Vorobyev M, Osorio D, Bennett ATD, Marshall NJ, Cuthill IC (1998) Tetrachromacy, oil droplets and bird plumage colors. J Comp Physiol A 183:621–633
Wagner GF, Aidala Z, Croston R, Hauber ME (2013) Repeated brood parasitism by brown-headed cowbirds (Molothrus ater) at Eastern Phoebe (Sayornis phoebe) nesting sites across non-consecutive years. Wilson J Ornithol 125:389–394
Welbergen J, Komdeur J, Kats R, Berg M (2001) Egg discrimination in the Australian reed warbler (Acrocephalus australis): rejection response toward model and conspecific eggs depending on timing and mode of artificial parasitism. Behav Ecol 12:8–15
Acknowledgments
For financial support, the authors thank the CUNY Graduate Center, American Ornithologists’ Union, Animal Behavior Society, the PSC-CUNY grant scheme, and the Human Frontier Science Program. For discussions and assistance, the authors also thank Zachary Aidala, Jennifer Basil, Phill Cassey, Tomas Grim, Brani Igic, David Lahti, Lisa Manne, Csaba Moskát, Lainga Tong, Michael Webster, and Sarah Woolley.
Ethical standards
This study was conducted on private land with the express permission of landowners and following the protocols and permissions of institutional and governmental agencies. The protocol was approved by the Institutional Animal Care and Use Committed of Hunter College (# MH 2/13-T3).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Soler
Rights and permissions
About this article
Cite this article
Croston, R., Hauber, M.E. Spectral tuning and perceptual differences do not explain the rejection of brood parasitic eggs by American robins (Turdus migratorius). Behav Ecol Sociobiol 68, 351–362 (2014). https://doi.org/10.1007/s00265-013-1649-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1649-8