Abstract
Predation exerts tremendous selection pressure on all organisms. In this study, we exposed embryos of convict cichlids (Amatitlania siquia) twice daily to one of the following: (1) chemical alarm cues of damaged conspecifics + odour of a novel predator (Polypterus endlicheri), (2) chemical alarm cues of damaged conspecifics + water or (3) blank water. No chemical cues were presented after the eggs hatched. When the larvae were 9 days old (mean total length = 5.7 mm), they were exposed to either predator odour or water. Those larvae that had been conditioned as embryos on alarm cues + predator odour showed a significant reduction in activity (i.e. anti-predator behavioural response) to predator odour relative to the other treatments. This is the first demonstration of acquired predator recognition by fish embryos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In aquatic habitats, assessment of predation risk is often mediated by chemical cues passively released by predators or by chemicals released from prey tissues damaged during predator attack (Wisenden and Chivers 2006; Ferrari et al. 2010a). Moreover, when damage-released alarm cues co-occur with a predator's odour, prey learn to associate predator odour with predation risk. This form of associative learning has been well studied in amphibians and littoral fishes (Ferrari et al. 2010a). Behavioural responses to these cues significantly reduce the probability of predation (Hews 1988; Mathis and Smith 1993; Wisenden et al. 1999; Mirza and Chivers 2000). Selection pressure to acquire predator recognition is highest when the rate of predation mortality is greatest, which is during early life stages. In this study, we aimed to test if acquisition of predator recognition can occur at the embryonic interval of development.
Embryonic responses to ambient information are known for food preferences [cuttlefish (Darmaillacq et al. 2008), fish (Brannon 1972), frogs (Hepper and Waldman 1992), chickens (Sneddon et al. 1998)] and predation risk [salamanders (Sih and Moore 1993)]. Recently, Mathis et al. (2008) showed that wood frogs (Rana sylvatica) acquire recognition of the odour of a novel predator when the embryos were exposed to a combination of damage-released alarm cues from conspecifics simultaneously with the odour of a novel predator. When retested at the age of 2 weeks post-hatch, the conditioned tadpoles responded with anti-predator behaviour to predator odour.
We chose convict cichlids as our study organism because they respond to injury-released alarm cues as adults (Wisenden and Sargent 1997), juveniles (Foam et al. 2005) and larvae (Alemadi and Wisenden 2002). Convict cichlids are serially monogamous and practise biparental brood defence of their embryos and larvae for 4 to 6 weeks (Wisenden 1994, 1995). If selection for parental care is driven by anti-predator incompetence of the young, then the ability of embryos to detect and acquire recognition of predators has broad implications for the ecology of species with parental care.
Materials and methods
Test subjects
Convict cichlids used in this study were from lab stock derived from wild-caught adults from the Río Cabuyo watershed in Guanacaste, Costa Rica. Breeding aquaria were 75 l in volume, filled with 26 °C dechlorinated water and set up with a 3-cm layer of naturally coloured gravel, a heater and a sponge filter. Terra cotta clay pots (diam. = 11 cm) lined with an acetate sheet were placed into the tanks to serve as spawning shelters.
Cue preparation
The Minnesota State University Moorhead Institutional Animal Care and Use Committee protocol 10-R/T-BIOL-010-N-Y-C was used to conduct this study. Alarm cue was made from convict cichlid larvae [mean ± 1 standard error (SE), total length (TL) = 6.369 ± 0.034 mm, n = 650]. To make alarm cue, we placed 650 larvae divided among seven 473-ml containers and then placed the containers into a −20 °C freezer for 30 min to render the larvae unconscious. A pestle was used to pulverise the young into a fine paste. The paste was suspended in 60 ml of dechlorinated water and filtered through a loose wad of polyester fibre, diluted to a final volume of 3,250 ml (1 larva per 5 ml), aliquotted into 650 5-ml doses and frozen at −20 °C until needed. Predator odour was derived from two African saddled bichirs (Polypterus endlicheri) (TL = 20, 31 cm) that were acquired from a local commercial fish dealer and maintained in a 190-l aquarium in the lab on a diet of commercially prepared dry pellets. The bichirs were placed individually into 19-l aquaria for 24 h. The stimulus collection tanks were aerated but not filtered, and the bichirs were not fed during this time. The fish were then returned to their holding tank. We stirred the water with a glass rod and then combined 1 l from each tank, poured it through a loose wad of polyester fibre and aliquotted it into 5-ml doses, and these were frozen at −20 °C until needed. Blank dechlorinated water was used as the control cue. Dechlorinated water was passed through a loose wad of polyester fibre and aliquotted into 5 ml doses and stored at −20 °C until needed.
Egg treatment protocol
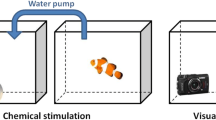
Egg from five different breeding pairs of convict cichlids were used for this experiment. For spawning substrate we provided terracotta pots lined with a removable sheet of acetate. Within 12 h of spawning, the acetate sheet containing the adhesive eggs was removed from the clay pot and cut into small pieces such that each piece contained between two and five eggs. The transparency film was inserted into a 1-cm slit cut into a plastic post cemented to a glass slide with silicone adhesive (Fig. 1). This allowed the eggs to remain elevated from the substrate and inverted as they had been in the spawning pot in the breeding tank. The egg holder fitted neatly into an egg incubator (small circular plastic tubs with opening diam. = 10.7 cm and filled with 450 ml of dechlorinated water) and could be easily transferred from one incubator to another (see below). Egg containers were placed on a large heating pad set at 25 °C. An airline provided aeration and promoted water circulation.
Cue treatments were started as soon as the eggs had been transferred to the egg incubator. Each container received one of the following: (1) one 5-ml dose of alarm cue mixed with one 5-ml dose of predator odour, (2) one 5-ml dose of alarm cue mixed with one 5-ml dose of water control or (3) one 5-ml dose of water control. Thawed cue was gently released into the treatment containers using a 10-ml syringe. After 2 h of cue exposure, each egg holder was transferred to a new egg incubator containing fresh, preheated dechlorinated water. Treatments were applied twice daily between 0800 to 1000 h and 2000 to 2300 h. By transferring embryos to a fresh egg incubator after 2 h, we ensured that cue conditioning treatments were applied only to embryos. The conditioning treatments were discontinued when the eggs hatched (between exposure treatments). The hatchlings (wrigglers) were then allowed to grow and develop into free-swimming larvae in cue-free dechlorinated water.
Testing protocol
Cichlid larvae (mean ± SE, total length = 5.73 ± 0.06 mm, age = 9.16 ± 0.22 days, n = 60) were tested in a cylindrical plastic vessel (vol. = 532 ml) filled with 150 ml of dechlorinated water. Two perpendicular lines (when viewed from above) were formed by black monofilament line fed through four small holes in the sides of the cylinder above the water line. Individual larvae were transferred to the test cup and left for 30 min to acclimate. Each fish was observed for a 5-min pre-stimulus period. Activity was recorded as the number of line crosses. Immediately following the pre-stimulus observation, either 5 ml of predator odour or 5 ml of water control was gently released along the side of the test arena using a 10-ml syringe. A 5-min post-stimulus observation immediately followed in which the number of line crosses (activity) was recorded.
Statistical analysis
Activity data were log transformed (Ln +1) to reduce extremes in variation. Effect of the test cue was determined by the change in the number of lines crossed from the post-stimulus period to the pre-stimulus period (post-stimulus minus pre-stimulus). Data were analysed with a one-way ANOVA followed by Duncan's post hoc pairwise comparisons among treatment combinations.
Results
There was a significant effect of treatment on the change in activity in the post-hatch test (F 5, 54 = 3.739, P = 0.006). Duncan's post hoc pairwise comparisons revealed that relative to other treatment combinations, only those young convict cichlids that were exposed to alarm cue + predator odour as embryos significantly reduced activity when re-exposed to predator odour as larvae (Fig. 2). No other treatment combinations resulted in a behavioural response in the post-hatch test.
Mean (±1 SE) change in activity scored as the number of lines crossed before minus the number of lines crossed after the addition of the test cue. W water, P predator odour, A alarm cues. Treatment codes on the x-axis indicate the chemical cue(s) used to condition the embryos shown in parentheses followed by the chemical cue used to test the larvae. For example, (A + P)→P indicates larvae that were exposed to a combination of alarm cues and predator odour as embryos and later tested with predator odour as larvae. Letters below the change bars are the result of Duncan's post hoc pairwise comparisons. Shared letters indicate no difference (P > 0.05)
Discussion
Convict cichlid embryos do indeed learn to recognise odour cues to which they are exposed and later respond to these cues as larvae with anti-predator behaviour. This is the first demonstration of embryonic learning of predator odour by a fish. The potential survival benefit of information about predator identity is greatest during the larval stage when predation risk is greatest.
Recent work on wood frog embryos showed that exposure to predator odour and chemical cues from damaged conspecifics results in acquired predator recognition (Mathis et al. 2008). Subsequent experiments revealed sophisticated use of this information. For example, tadpole wood frogs (Rana sylvatica) adjust the intensity of their behavioural responses to the concentration of cue to which they were exposed as embryos and generalise this information to predators that are phylogenetically related to the predators to which they were conditioned as embryos (Ferrari and Chivers 2009a). Wood frog embryos exhibit a latent inhibition of inadvertently learning to associate risk with omnipresent odours of non-predators to which they are exposed as embryos (Ferrari and Chivers 2009b) and even fine-tune responses to predator odour to specific times of day that match the temporal pattern of the conditioning regime experienced as embryos (Ferrari and Chivers 2010; Ferrari et al. 2010b). Given the ubiquity of chemically mediated anti-predator responses in aquatic habitats, we anticipate that many, perhaps most, fish may be capable of similarly adaptive use of chemical information about predation while at the embryonic stage of development. Because embryonic learning has also been observed in some invertebrates (Darmaillacq et al. 2008), the findings reported here may apply across many aquatic taxa.
Novel odorants, such as predator odour, become indicators of predation risk when they are presented simultaneously with damage-released chemical cues from injured conspecifics (Suboski 1990; Magurran 1989; Chivers and Smith 1994; see Ferrari et al. 2010a for review). This is a form of associative learning known as releaser-induced recognition learning (Suboski 1990). The unconditional stimulus (alarm cue) becomes associated with the conditional stimulus (predator odour) such that the conditional stimulus elicits the unconditional response (reduction in activity). Associative learning is distinct from sensitisation in that sensitisation is non-associative learning that occurs when repeated presentations of a stimulus (novel odour) result in increased response intensity to the stimulus. For example, sensitisation would be the conclusion if embryos conditioned with bichir odour + water later responded as larvae to the odour of bichir odour. We did not include this treatment in the experimental design to economise on the scale of the experiment because releaser-induced recognition learning is so deeply established in the literature (e.g. reviews of Chivers and Smith 1998; Wisenden 2000; Brown 2003; Wisenden and Chivers 2006; Ferrari et al. 2010a). The possibility of sensitisation explaining the response to alarm cue + predator odour conditioning was not likely because response to predator odor was negligible for larvae conditioned with water or alarm cue as embryos, i.e. there was no initial response to amplify through sensitisation because the response on first exposure to bichir odour did not differ from the water–water group. Moreover, if embryos were sensitised to respond to any novel odorant, then embryos that were conditioned with alarm cue only and later tested with bichir odour as larvae would have responded with an anti-predator response. We, therefore, conclude that our data demonstrate associative learning rather than sensitisation.
The anatomical and physiological proximate mechanisms by which associative learning occurs in embryos are a ripe area for future research. The molecular components of alarm cues are still in the first stages of chemical characterisation (Mathuru et al. 2012) as are knowledge of olfactory receptors and the neural wiring of cognitive processes involved in associative learning (Døving and Lastein 2009).
Implications of these results take on special significance because the convict cichlid is a model organism for the study of biparental care. Parental care in fishes is mainly in the form of brood defence because fish generally do not provide direct nourishment to their young. Parental care in this species is a problem of anti-predator behaviour that is met by a combination of parental defence behaviour and the ability of the young to detect and evade attacks by brood predators (BDW et al., unpublished data). As such, the capacity of embryos to acquire recognition of predators has potential implications for the study of the evolution of parental care. Anti-predator competence of the young therefore shapes the selection gradient promoting care behaviour in terms of the duration of care (energetic costs and cost of missed mating opportunities while engaged in prolonged care of a brood) and, ultimately, the allocation of resources in the trade-off between egg size and egg number (Sargent et al. 1987; Kolm et al. 2006a, b).
References
Alemadi SD, Wisenden BD (2002) Antipredator response to injury-released chemical alarm cues by convict cichlid young before and after independence from parental care. Behaviour 139:603–611
Brannon EL (1972) Mechanisms controlling migration of sockeye salmon fry. Intl Pac Salmon Fish Comm Bull 21:1–86
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234
Chivers DP, Smith RJF (1994) The role of experience and chemical alarm signalling in predator recognition by fathead minnows, Pimephales promelas. J Fish Biol 44:273–285
Chivers DP, Smith RJF (1998) Chemical alarm signalling in aquatic predator–prey systems: a review and prospectus. Ecoscience 5:338–352
Darmaillacq A-S, Lesimple C, Dickel L (2008) Embryonic visual learning in the cuttlefish, Sepia officinalis. Anim Behav 76:131–134
Døving KB, Lastein S (2009) The alarm reaction in fishes—odorants, modulations of responses, neural pathways. Int Symp OlfactionTaste: Ann NY Acad Sci 1170:413–423
Ferrari MCO, Chivers DP (2009a) Sophisticated early life lessons: generalization of predator recognition by frog embryos. Behav Ecol 20:1295–1298
Ferrari MCO, Chivers DP (2009b) Latent inhibition of predator recognition by embryonic amphibians. Biol Lett 5:160–162
Ferrari MCO, Chivers DP (2010) The ghost of predation future: threat-sensitive and temporal assessment of risk by embryonic woodfrogs. Behav Ecol Sociobiol 64:549–555
Ferrari MCO, Wisenden BD, Chivers DP (2010a) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Ferrari MCO, Manek AK, Chivers DP (2010b) Temporal learning of predation risk by embryonic amphibians. Biol Lett 6:308–310
Foam PE, Mirza RS, Chivers DP, Brown GE (2005) Juvenile convict cichlids (Archocentrus nigrofasciatus) allocate foraging in response to temporal variation in predation risk. Behaviour 142:129–144
Hepper PG, Waldman B (1992) Embryonic olfactory learning in frogs. Q J Exp Psychol B 44:179–197
Hews DK (1988) Alarm response in larval western toads, Bufo boreas: release of larval chemicals by a natural predator and its effect on predator capture efficiency. Anim Behav 36:125–133
Kolm N, Goodwin NB, Balshine S, Reynolds JD (2006a) Life history evolution in cichlids 1: revisiting the evolution of life histories in relation to parental care. J Evol Biol 19:66–75
Kolm N, Goodwin NB, Balshine S, Reynolds JD (2006b) Life history evolution in cichlids 2: directional evolution of the trade-off between egg number and egg size. J Evol Biol 19:76–84
Magurran AE (1989) Acquired recognition of predator odor in the European minnow (Phoxinus phoxinus). Ethology 82:216–223
Mathis A, Smith RJF (1993) Chemical alarm signals increase survival time of fathead minnows (Pimephales promelas) during encounters with northern pike (Esox lucius). Behav Ecol 4:260–265
Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP (2008) Learning by embryos and the ghost of predation future. Proc R Soc Lond B 275:2603–2607
Mathuru AS, Kibat C, Cheong WF, Shui G, Wenk MR, Friedrich RW, Jesuthasan S (2012) Chondroitin fragments are odorants that trigger fear behavior in fish. Curr Biol 22:1–7
Mirza RS, Chivers DP (2000) Predator-recognition training enhances survival of brook trout: evidence from laboratory and field-enclosure studies. Can J Zool 78:2198–2208
Sargent RC, Taylor PD, Gross MR (1987) Parental care and the evolution of egg size in fishes. Am Nat 129:32–46
Sih A, Moore RD (1993) Delayed hatching of salamander eggs in response to enhanced larval predation risk. Am Nat 142:947–960
Sneddon H, Hadden R, Hepper PG (1998) Chemosensory learning in the chicken embryo. Physiol Behav 64:133–139
Suboski MD (1990) Releaser-induced recognition learning. Psychol Rev 97:271–284
Wisenden BD (1994) Factors affecting reproductive success of convict cichlids in Costa Rican streams. Can J Zool 72:2177–2185
Wisenden BD (1995) Reproductive behaviour in free-ranging convict cichlids. Environ Biol Fish 43:121–134
Wisenden BD (2000) Olfactory assessment of predation risk. Philos T Roy Soc B 355:1205–1208
Wisenden BD, Chivers DP (2006) The role of public chemical information in antipredator behaviour. In: Ladich F, Collins SP, Moller P, Kapoor BG (eds) Fish communication. Science, New Hamphire, pp 259–278
Wisenden BD, Sargent RC (1997) Antipredator behavior and suppressed aggression by convict cichlids in response to injury-released chemical cues of conspecifics but not to those of an allopatric heterospecific. Ethology 103:283–291
Wisenden BD, Cline A, Sparkes TC (1999) Survival benefit to antipredator behavior in the amphipod Gammarus minus in response to injury-released chemical cues from conspecifics and heterospecifics. Ethology 105:407–414
Acknowledgments
Funding was provided by faculty research grants to BDW from the College of Social and Natural Sciences, MSUM, and the Dille Fund for Excellence, MSUM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Buston
Ethical standards
The experiments described herein comply with the current laws of the USA and were reviewed and approved by the Minnesota State University Moorhead Institutional Animal Care and Use Committee in protocol number 10-R/T-BIOL-010-N-Y-C.
Rights and permissions
About this article
Cite this article
Nelson, A.B., Alemadi, S.D. & Wisenden, B.D. Learned recognition of novel predator odour by convict cichlid embryos. Behav Ecol Sociobiol 67, 1269–1273 (2013). https://doi.org/10.1007/s00265-013-1554-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1554-1