Abstract
One of the important aspects of the animal social behavior is the laterality in perception of conspecifics. Spatial laterality in adult–infant interactions is usually revealed in primates as a cradling/holding bias in adults or nipple preference in infants. The origin and function of such biases, however, remain unclear. Here, we investigated spatial laterality in adult–infant pairs in beluga whales from two geographically distinct locations using aerial photography analysis. In addition, behavioral observations on individually identified mother–infant pairs at a belugas’ breeding aggregation were conducted to assess the infants’ age influence on the lateralization in pairs. A general preference of the calves to position themselves to the right of the accompanied adult was found. We failed to reveal any influence of geographical location, presence or relative position of other individuals escorting the adult–infant pair, and position of the calf along the body of the escorting adult. A significant right-sided bias in infants’ position was present in all age classes, but 2–6 months-old belugas were found to be stronger lateralized, than the newborns and 7–18 months-old calves. That may reflect age-related changes in infants’ motor and social behavior. We argue that the revealed laterality is associated with the calves’ left eye–right hemisphere preference in perceiving social stimuli, and we then discuss its possible advantages. Pronounced adult–infant spatial laterality in condition (unlike that seen in primates) when forelimbs do not directly determine subjects’ relative positioning suggests sensory lateralization alone to be the determining factor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the aspects of social behavior, which recently has received a considered attention of researchers, is its laterality (Rosa Salva et al. 2012). Behavioral laterality―one-sided bias in any behavioral response―usually originates from general differences in information processing styles between the two brain hemispheres (Vallortigara and Rogers 2005; Vallortigara et al. 2011). While acknowledging species’ differences, the general rule is that left eye–right hemisphere system is specialized for recognition and discrimination of social partners in vertebrates that has been argued to derive from a more general ability of the right brain to form a detailed and contextual representation of the observed objects. A preference for left visual hemifield has been found in various manifestations of sociality ranging from sexual and aggressive interactions to schooling and face recognition (Robins et al. 1998; Peirce et al. 2000; Sovrano et al. 2001; Ventolini et al. 2005; Karenina et al. 2013). The right eye–left hemisphere system may also play predominant role in some aspects of social behavior, for instance, in categorization of a stimulus as a conspecific (Rosa Salva et al. 2012).
Several studies clearly demonstrate that social laterality may take place early in the ontogenesis. For instance, anuran tadpoles and fish fry prefer to keep shoal mates in the visual field of the left eye (Dadda et al. 2003; Sovrano and Andrew 2006; Karenina et al. 2013, but see Bisazza and Brown 2011 for interspecific and population differences). One of the earliest and strongest social bonds in mammals exists between infants and parents. It is reasonable to suppose that like many other aspects of sociality, the parent–infant interactions are lateralized. In fact, it is now well established that in primates a pronounced spatial lateralization takes place in relative positioning of adults and offspring (Damerose and Vauclair 2002). The left-sided bias in infant holding has been found in humans of various cultures and in different periods of history (e.g., Salk 1960; Saling and Tyson 1981; Saling and Cooke 1984; Harris 2010). Intriguingly, human left-side cradling bias expresses in different categories of holders: mothers (Salk 1960), never-pregnant adult females (Saling and Tyson 1981), fathers (Harris et al. 2007; Scola and Vauclair 2009), children (de Château and Andersson 1976), and in different circumstances, such as holding a real baby (Salk 1960), a doll (de Château and Andersson 1976; Vauclair and Donnot 2005), and even a dog (Abel 2010), or just during imagining of holding a baby (Harris et al. 2000). The observational studies on non-human primates (reviewed in Hopkins 2004) clearly showed that the cradling laterality emerged definitely before Homo sapiens. For instance, captive chimpanzees, Pan troglodytes, and gorillas, Gorilla gorilla, have population-level preferences to cradle infants on the left (Manning and Chamberlain 1990; Manning et al. 1994). Several primate species have been studied in terms of infants’ preferences for one of the mother’s nipples (reviewed in Hopkins 2004). A left-nipple preference was reported for infants of wild chimpanzees (Nishida 1993), captive rhesus macaques, Macaca mulatta (Tomaszycki et al. 1998) and bonobos, Pan paniscus (Hopkins and De Lathouwers 2006); however, only individual nipple preferences were found in captive common marmosets, Callithrix jacchus jacchus (Kaplan and Rogers 1998) and wild snub-nosed monkeys, Rhinopithecus roxellana (Zhao et al. 2008).
Despite the growing body of evidence showing asymmetry in parent–offspring positioning, the determining factors and mechanisms of this phenomenon remain not fully understood. A variety of hypotheses have been proposed to explain cradling and nipple preferences (reviewed in Sieratzki and Woll 1996; Harris 2010). Some of them, currently discussed in the literature, could be combined into two bunches: “handedness” hypotheses and “sensory” hypotheses. The former are based on the suggested link between parents’ or infants’ manual preferences and positional asymmetry in a dyad (e.g., Hopkins 2004; Negayama et al. 2010; Scola and Vauclair 2010). For instance, Hopkins (2004) proposed that in primates a greater strength of the right limb may be a reason for infants’ positioning themselves preferably on the mother’s left side. However, many empirical studies revealed the parent–infant laterality to be unrelated to handedness (e.g., Salk 1960; Bogren 1984; Reissland 2000; Bourne and Todd 2004; Reissland et al. 2009). The “sensory” hypotheses rely on the known dominate role of the left eye (ear)–right brain hemisphere system in social responses like processing of emotions and facial expressions (Sieratzki and Woll 1996). It has been suggested that left-side cradling derives from the preference of a parent to monitor the emotional state of the infant with the left eye and ear (Manning and Chamberlain 1990; Manning et al. 1997; Bourne and Todd 2004). Further, it has been proposed that the cradling bias may have great significance for the infant itself (Hendriks et al. 2011; Vervloed et al. 2011). Left-sided positioning of the infant on the mother’s body is argued to direct more maternal communication to the infant’s right hemisphere, which is better in emotion recognition (Sieratzki and Woll 1996). A more recent study showed that visibility of the mother’s face from the infant’s point of view is reduced when the infant is held on the right arm (Hendriks et al. 2011). Thus, left-side cradling seems to provide optimal flow of sensory information between the mother’s and the infant’s right hemispheres what may provide significant benefits to both and explain the emergence of such laterality in the course of primate evolution. However, some studies do not support the relationship between brain specialization and cradling bias (e.g., Donnot and Vauclair 2007; Scola and Vauclair 2010), and after more than five decades of intensive research (reviewed in Harris 2010), the issue about the nature of parent–infant laterality remains open.
Primates are characterized by complex parent–infant spatial interactions, partially due to a valuable involvement of the forelimbs in the maintaining of proximity between infants and adults. In many non-primate mammals, infants are significantly more spatially independent from their parents, and forelimbs are rarely or never used directly in parental care. Investigation of such species can provide important insight into the role of sensory perception in parent–infant laterality. In a recent study (Karenina et al. 2010a), we collected evidence of spatial asymmetry in mother–calf pairs of wild beluga whales, Delphinapterus leucas. Observations on one of the belugas’ breeding aggregation in the White Sea revealed that the majority of calves spent significantly more time on mother’s right side during both surface swimming and diving. We argued that this bias was a result of the calves’ preference to keep their mothers in the visual field of the left eye caused by the right hemisphere dominance in social information processing. Notably, during the last two decades, a number of visually guided behaviors were found to be highly lateralized in cetaceans including common bottlenose dolphins, Tursiops truncatus (von Fersen et al. 2000; Kilian et al. 2000; Yaman et al. 2003; Kilian et al. 2005; Delfour and Marten 2006; Thieltges et al. 2011; Blois-Heulin et al. 2012), Indo-Pacific bottlenose dolphins, Tursiops aduncus (Sakai et al. 2006), striped dolphins, Stenella coeruleoalba (Siniscalchi et al. 2012), and beluga whales (Karenina et al. 2010b). For instance, Kilian et al. (2000) and Yaman et al. (2003) showed the right eye (left hemisphere) prevalence for visual discrimination task, respectively, in two and three captive individuals of common bottlenose dolphins. Laterally placed eyes and the general tendency to use monocular vision spontaneously during object examination (Delfour and Marten 2006) underline naturalness and relevance of the eye preferences for cetacean behavior.
Studies in the wild may help to understand how laterality is affected by natural environmental conditions, and to shed some light on its nature and evolution. Therefore, in the present study we aimed to investigate the plasticity of adult–infant laterality in wild beluga whales. Since our previous study (Karenina et al. 2010a) was conducted only on the animals in special and unique conditions of a single breeding aggregation, here we studied social laterality in belugas in the White Sea beyond the aggregation. We further tested geographical variability comparing the expression of laterality in adult–infant pairs of belugas from the White Sea and the Sea of Okhotsk, which assumed to belong to different populations (IWC 2000; Jefferson et al. 2012). Also, we explored the influence of pair’s escort conditions (presence and position of other individuals near a pair), calf’s age and position as regards to the adult on adult–infant laterality.
Methods
Two methods of data collecting were used in this study: analysis of aerial photographs and direct visual observations on the belugas’ breeding aggregation.
Analysis of aerial photographs
Photographs were obtained during aerial surveys of the distribution and abundance of beluga whales in the White Sea (years 2007, 2008, 2010, and 2011) and the Sea of Okhotsk (years 2009 and 2010) (Glazov et al. 2010; Glazov et al. 2012; Solovyev et al. 2012). All aerial surveys were conducted with the aircraft specially equipped for the instrumental aerial surveys of marine mammals (see Chernook et al. 2008 for details). Sampling was made using a method of serial standard line transects, which was widely applied in animal density studies (Buckland et al. 1993). Transects covered the water area of the White Sea and the shore line of the Sea of Okhotsk. Aerial surveys were carried out mainly in good weather at altitudes of about 300 m in the case of the White Sea and about 400 m in the case of the Sea of Okhotsk. The mean flight lengths for all years of the survey were 3,356 ± 153 km in the White Sea and 4,784 ± 320 km in the Sea of Okhotsk. The minimum estimated population size of Beluga whales in years of our study was about 4,500–7,500 individuals in the White Sea (Solovyev et al. 2012) and about 4,500 individuals in the Sea of Okhotsk (Glazov et al. 2012).
The analysis of the entire set of photographs started from determining the presence/absence of adult–infant pair. An adult–infant pair was defined as an adult (white colored normal size beluga whale) with a calf (an individual visually estimated to be less than 2/3 of the length of the accompanying adult and from grey to black in color) (Doidge 1990). Beluga calves are known to form pairs not only with their mothers, but also with other female relatives, “aunts” (Krasnova et al. 2009), therefore, we use the term “adult–infant pair” instead of “mother–infant pair”. We included in the analysis only the images, on which the calf was situated in close proximity to the adult (at the distance less than one whale length separating the animals in the pair) and laterally with regards to the adult. The relative positions of the calves were classified as “to the left” or “to the right” of the adult. In addition, we recorded positions of calves along the longitudinal axis of the adults’ body: (1) ahead of the adult, i.e., the position when the calf’s head was situated at the front of the adult’s head (Fig. 1a), (2) side by side, i.e., the position when the calf’s head was situated between the adult’s tip of the head and the tail (Fig. 1b), and (3) echelon, i.e., the position when the calf’s head was situated at the level of the adult’s tail region (Fig. 1c). If there were two or more adults at the distance less than one whale length from a calf, such images were discarded. The position of other belugas accompanying the pair (located at the distance less than five whale length from the adult–infant pair; Fig. 1d), in terms of possible influence on laterality manifestation, were classified in four categories: to the left of the pair, to the right of the pair, around the pair (when other individuals situated both to the right and to the left of the pair), and other positions (i.e., other rarely observed positions: behind and in front of the pair). When a group of whales was detected during the aerial survey, the long series of subsequent images were usually made. The detailed analysis of such image series allowed revealing all the individuals accompanying each adult–infant pair with a great degree of confidence. If an adult–infant pair was depicted several times on a series of images, the data on this pair were scored only once (image series were recognized by timing and whales’ relative positions). The procedure of the aerial survey was aimed at avoidance of any repeated recordings of whales during one season (except the series of subsequent images as described above), thus the probability that the same adult–infant pair was included into our analysis repeatedly was kept to the absolute minimum. We could not completely exclude, though, the possibility that the same pairs were present on the photographs made in different years. Many more belugas inhabited both study areas than our sample size and the great majority of data was surely independent.
The photographs, on which a clear determination of the calf’s position or the position of individuals accompanying the pair was not possible (about 10 % from the White Sea and about 17 % from the Sea of Okhotsk), were not included into the analysis. The percentage of photos discarded from the Sea of Okhotsk was slightly higher possibly because of higher water turbidity in the region.
In order to assess positional laterality in adult–infant pairs in the aerial survey, the number of pairs with the left positioned and the number of pairs with the right positioned calf were compared using binomial test. Two-proportion Z test was conducted to compare proportion of pairs with the calf on the left and with the calf on the right side between the White Sea and the Sea of Okhotsk, as well as between pairs accompanied by other individuals and pairs traveling alone. Chi-square contingency table tests were used to determine influence of the calf position, as well as the position of other individuals accompanying the pair, on laterality expression in the pair (2 × 3 table in the case of calf position and 2 × 4 table in the case of companions’ position).
Behavioral observations
To investigate the effect of calves’ age on the laterality, direct observations and video recording of adult–infant pairs were carried out on belugas’ breeding aggregation near the Beluzhiy Cape (65.07N 35.52E) of the Bolshoy Solovetsky Island (Onega Bay, the White Sea, Russia). This aggregation is situated uniquely close to the shore and is formed mainly by females, calves, and juveniles that visit this area as stable “family” groups (Chernetsky et al. 2011). Behavioral observations on adult–infant pairs were conducted during summer expeditions in July–August 2009 and 2010. Full details of the methods have been published elsewhere (Karenina et al. 2010a). We continually video recorded the behavior of adult–infant pairs directly from the shore. The individual identification of belugas was carried out using natural markers on the whales’ bodies (Chernook et al. 2008; Karenina et al. 2010a). Calves’ age was estimated by coloration characteristics, body size in relation to the adult beluga, and other age-related features, e.g., behavioral patterns (Kingsley 1996; Litzky 2001; Krasnova et al. 2006).
From the total time of observations, only the first minute of video recording from each pair was included into analysis. The data from the pairs video recorded for less than 1 min were discarded. The calves were classified into three age classes, distinguished visually with great confidence on the basis of skin coloration, breath pause time, and body size relative to mother’s body size. Age classes included: newborns (0–1 month old; Fig. 2a), 2–6-month-old calves (Fig. 2b), and 7–18-month-old calves (Fig. 2c). The time spent by a calf to the left and to the right side of an adult was scored. According to Kolmogorov–Smirnov test, the data were not normally distributed (P < 0.05). Therefore, we used nonparametric tests (two-tailed) for further analysis. The Kruskal–Wallis test of independent samples (with post hoc Dunn’s tests for between-pair comparisons) was carried out to estimate the effect of calves’ age on the laterality expression. One-sample Wilcoxon signed–rank tests were performed to explore group-level preference separately in three age categories.
Results
Analysis of aerial photographs
A total of 279 adult–infant pairs (on 242 aerial photographs) were included into analysis: 105 pairs from the White Sea and 174 pairs from the Sea of Okhotsk. Two raters (KK and AG) analyzed independently all aerial photographs selected for this study. The degree of agreement between the two raters was quantified by kappa. In determining the calf position, the inter-rater agreement was 97.70 % (kappa = 0.960, SE of kappa = 0.028) for the pairs with the left situated calf and 97.92 % (Kappa = 0.959, SE of kappa = 0.020) for the pairs with the right situated calf. In determining the position of other individuals accompanied, the pair on the photo the inter-rater agreement was 97.14 % (Kappa = 0.960, SE of kappa = 0.039) for the pairs with the left situated calf and 95.45 % (Kappa = 0.939, SE of kappa = 0.030) for the pairs with the right situated calf. Since the agreement between the two raters was extremely high, we took a data set obtained by only one of the raters (KK) for further analysis.
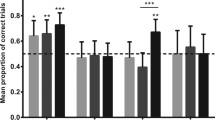
No significant difference in proportion of left and right positioned calves was found between beluga pairs in two geographical locations (two-proportion Z test: Z = 1.24, P = 0.214) with the prevalence of the pairs with the calf situated to the right of the adult in both locations (77 out of 105, 73 %; binomial test: Z = 4.68, P < 0.001, in the White Sea, and 115 out of 174, 66 %; Z = 4.17, P < 0.001, in the Sea of Okhotsk; Fig. 3). Since no difference had been found between the two geographical locations, further analysis was conducted on combined set of photos from both locations. Overall, the number of pairs with the right positioned calf (192 out of 279, 69 %) was significantly higher than the number of pairs, where the calf was to the left of the adult individual (87 out of 279, 31 %; binomial test: Z = 6.23, P < 0.001).
Analysis failed to reveal any difference when compared to laterality in calves in different positions (“ahead of the adult”, “side by side”, or “echelon”) near to the adult beluga (chi square test: χ 2 = 1.09, df = 2, P = 0.578). Differences in the proportions of the left situated vs. the right situated calves between pairs accompanied by other individuals and traveling alone were also not significant (two-proportion Z test: Z = 0.90, P = 0.366). The position of these companions (to the left of a pair, to the right of a pair, around a pair, and other positions) had also no influence on proportion of left situated and right situated calves (chi square test: χ2 = 1.87, df = 3, P = 0.599). Thus, the position of companions did not affect the laterality within adult–infant pairs.
Behavioral observations
In total, we obtained 1 min-video clips from 47 individually identified adult–infant pairs: 8 with newborns (0–1 month old; Fig. 2a), 19 with 2–6-month-old calves (Fig. 2b), and 20 with 7–18-month-old calves (Fig. 2c). Analysis revealed that the age of calves significantly affected the laterality expression (Kruskal–Wallis test: χ 2 = 10.95, df = 2, P = 0.004). Dunn’s post hoc tests showed that strength of laterality in 2–6-month-old calves was higher than in newborns (0–1 month old) and 7–18-month-old calves (P < 0.05; Fig. 4). However, group-level preference of calves to swim to the right of an adult was found in all three age classes, if taken separately (one-sample Wilcoxon signed–rank test: Z = 34, P = 0.021, n = 8 for newborns; Z = 169, P < 0.001, n = 19 for 2–6-month-old calves; Z = 124, P = 0.021, n = 20 for 7–18-month-old calves). In newborns, 7 out of 8 individuals were right-biased (spent ≥ 70 % of time to the right of the adult), among 19 calves aging 2–6 months, 17 had a right bias, and among 20 calves aging 7–18 months, 13 were right-biased.
Discussion
The results of the present study showed the spatial laterality in adult–infant pairs in beluga whales from two geographical locations. Both in belugas from the White Sea and from the Sea of Okhotsk, calves were found to situate on the right side of adults significantly more often than on the left side. We failed to reveal any difference between the populations, and the combined data set confirmed the general right-sided bias. Since the same directional bias was found in two geographically distinct populations, it is suggestive that the right-side preference in calves’ positioning is species-typical characteristic of belugas. During aerial photography analysis used in this study, only one observation from each pair was taken into account. Continuous observations on the individually recognized beluga mother–calf pairs on the Solovetsky breeding aggregation in our previous work (Karenina et al. 2010a) showed that in the great majority of pairs, the individual calves spent significantly more time on a particular side of the mother, and in 93 % of pairs, the right-side preference was found. Thus, regardless of the method used and the region studied, the pronounced spatial laterality was found in beluga whale adult–calf pairs.
The revealed laterality appeared to be not affected by the position of the calf in respect to the adult beluga in the pair (ahead, side-by-side, echelon; Fig.1a–c). Taking into account a close bond between beluga mothers and infants (Krasnova et al. 2006) and the fact that on the most of the aerial photographs (56 %) used in the study, adult–infant pairs were depicted traveling alone (i.e., no other belugas were visible nearby the pair), we assumed that in most of the cases, the observed adult animal in the pair was the mother accompanied by its calf. In cetaceans, mother–calf joint swimming is typically characterized by a high synchrony and plays a critical role for calves’ survival (Mann and Smuts 1999; Hill 2009). The positions “at the mother’s side” and “at the mother’s tail” similar to the “echelon” and “side-by-side” positions in the present study, respectively, have been shown to prevail in the beluga calves of various age, when accompanying their mothers (Krasnova et al. 2006, 2009). The same is true for bottlenose dolphin calves during the first year of life (Gubbins et al. 1999). These positions, especially the echelon one, have been argued to be beneficial for a calf, because the pressure wave created by its mother’s body facilitates calf’s swimming and consequently reduces the cost of transport (Williams et al. 1992; Krasnova et al. 2006). Despite the possible differences in the relative significance for the calf between taken positions near to the mother, they did not affect the lateral (right-sided) preference. More specifically, no significant difference was found between the “echelon” position, which is beneficial for the calf, and “ahead of the mother” position, in which the calf apparently had no hydrodynamic benefit from the mother astir. This result confirms that the revealed laterality is not associated with motor or morphological asymmetries of the mothers that potentially could make the right-sided positioning preferable for the calves.
In our previous work (Karenina et al. 2010a), we proposed that the spatial mother–calf bias in beluga whales is caused by lateralization in sensory perception of social stimuli in the species; and most likely, visual modality is predominantly involved in the interactions between whale mothers and calves at such close distances (less than 4 m). The significance of visual inspection of the mother for the calf is illustrated by the evidence that during monocular sleep the dolphin calves preferably direct the open eye to their mothers and the closed eye to the opposite side (Lyamin et al. 2007). Notably, dolphin mothers do not display such a preference as regards to their calves. In case of beluga whales, behavioral analysis showed that calves usually initiate changes in the position in relation to mothers and are responsible for maintenance of the spatial asymmetry in pairs, e.g., after a rapid direction change by their mothers (Karenina et al. 2010a). This is in line with recent data on captive belugas showing that calves of this species exhibit independence and control proximity to their mothers at very early ages (Hill 2009). From the first weeks of life, beluga calves are mainly responsible for initiating separations and reunions with mothers, and, thus, are much more independent than infants of many other mammalian species.
In the present study, we failed to reveal any influence of external social context on the laterality in adult–infant pairs. No differences were found between two situations: when a pair traveled alone or when it was accompanied by other individuals. Furthermore, the specific position of accompanying whales in relation to the pair (to the left, to the right, around, other) did not affect the within-pair laterality. These results can be interpreted in favor of intra-pair origin of the revealed laterality, because they contradict to the possibility that calves prefer to swim on the right side of mothers (adults) just to leave the right visual field free to monitor other conspecifics. They also show that the mother is the object preferable for monitoring, for which the “social” left eye is predominantly used. Thus, taking all these into account, we can conclude that revealed spatial laterality in beluga adult–infant pairs is caused by calves’ preference to keep the mother in the visual field of the left eye. Intriguingly, the pivotal role of the left eye–right hemisphere system in social behavior can be traced already in basal aquatic vertebrates―fish (reviewed in Bisazza and Brown 2011). The preference to use the monocular field of their left eye when viewing a conspecific has been found in adults of eight teleost species (Sovrano et al. 1999, 2001, but see Bisazza and Brown 2011 for inter- and intraspecific differences). This lateralization may be triggered in fish by the key element of the social stimulus (Karenina et al. 2013). Much like in beluga whales, social lateralization was showed to be expressed in fish already in infancy: zebrafish fry demonstrated left-eye preference for observing a group of conspecific fry (Sovrano and Andrew 2006).
Recent studies on captive (Blois-Heulin et al. 2012) and wild (Siniscalchi et al. 2012) delphinids clearly confirmed that in these animals, lateralized visual inspection originates from the functional specialization of the brain hemispheres for processing of different types of information. In both common bottlenose dolphins, T. truncatus (Blois-Heulin et al. 2012), and striped dolphins, S. coeruleoalba (Siniscalchi et al. 2012), stimuli of different degrees of familiarity evoked different patterns of eye use: unfamiliar objects were inspected preferentially with the right eye (left hemisphere), while very familiar previously manipulated objects facilitated the use of the left eye (right hemisphere). In the context of our study, the mother (or another accompanied adult) apparently can be categorized as a familiar social stimulus for the calf. Social stimulus familiarity has been demonstrated to influence significantly on the expression of lateralization (Vallortigara and Andrew 1991; Deng and Rogers 2002; Zucca and Sovrano 2008; Lemasson et al. 2010). For instance, quails, Coturnix japonica, display the preference to use their left eye to monitor well-familiar companions, while when approaching unfamiliar conspecifics, they prefer to use the right eye (Zucca and Sovrano 2008). Exactly the same pattern of visual preferences in response to familiar/unfamiliar stimuli has been shown in fish (Sovrano 2004). Thus, beluga whales reveal a similar visual preference in response to the familiar stimuli with vertebrates studied to date. However, in terms of lateralized reaction to novelty, there is some inconsistency between the results on beluga whales and other Odontoceti species. If two delphinid species inspect unfamiliar objects predominantly with the right eye (Blois-Heulin et al. 2012; Siniscalchi et al. 2012), beluga whales prefer to use their left eye when viewing a novel unanimated object (Karenina et al. 2010b). Notably, it is the left visual preference that is typical for perceiving novel, unexpected stimuli in vertebrates in general (MacNeilage et al. 2009).
Being able to recognize familiar conspecifics could be especially important for species having a fission–fusion social system (Blois-Heulin et al. 2012) such as beluga whales. Spatial separations between mothers and calves occur in belugas from very early ages (Hill 2009), and rapid recognition of the mother or another familiar individual could be crucial for infant belugas in complex underwater environment. As we found the left eye preference in interactions between infants and familiar elder individuals (Karenina et al. 2010a), it is legitimate to hypothesize that in beluga whales, like in many other vertebrates (reviewed in Rosa Salva et al. 2012), the left eye–right hemisphere system plays predominant role in individual recognition.
The present study provides evidence that laterality in beluga adult–infant pairs on the breeding aggregation is influenced by the calf’s age. The significant right-sided bias in infants’ position was present in all three age classes (newborns (0–1 month old), 2–6- and 7–18-month-old calves), if taken separately. However, the calves in the middle age class (2–6 months old) showed stronger laterality, than the newborns or 7–18-month-old calves. We propose that this difference is linked with general developmental changes in infants’ behavior. Studies on the development of beluga calves in the wild showed that the locomotion of newborn belugas is not well coordinated, movements are imperfect and inaccurate (Krasnova et al. 2006, 2009). Insufficient development of motor skills can result in some difficulties for a newborn calf in reaching the desirable position near to the mother, and, thus, explains a lower degree of laterality expression as compared with elder calves. At the age of 1 month, the calves noticeably improve and enrich their locomotor and behavioral repertoire (Krasnova et al. 2006). In 1-month-old calves, interactions with their mothers became more complex with prolific play elements and imitation of mother’s behavior. Enhanced social activity in the middle-aged calves increases the significance of a mother as a social object. Thus, more developed motor abilities together with increased social attention to the mother may result in more pronounced laterality in positioning of infants relative their mothers. With further growth and development, beluga calves became more independent from mothers. From the second half of the first year of life (third class of calves in the present study), the swims and interactions with other conspecifics, besides the mothers, steadily increase in beluga calves, whereas the frequency of mother–calf joint swims decreases over time (Hill 2009). The slightly reduced laterality can be possibly associated with decreased attention of 6–18-month-old calves to their mothers as to the social partners.
Spatial asymmetries in adult–infant interactions have been repeatedly shown in primate taxa (e.g., Salk 1960; Saling and Tyson 1981; Saling and Cooke 1984; Damerose and Vauclair 2002; Harris 2010). To the best of our knowledge, only one study has showed group-level laterality in social responses of non-primate mammalian infants, besides our own study on belugas (Karenina et al. 2010a). When facing an obstacle, sheep lambs, Ovis aries, preferred to detour it to rejoin their mothers while keeping them in the visual field of the left eye (Versace et al. 2007). This bias has been argued to be caused by the left visual field preference in recognition of familiar faces typical for this species (Peirce et al. 2000). These reports, together with the present study, demonstrate a pronounced laterality in infants’ visual perception of their mothers and confirm that social lateralization occurs in mammals already in early ontogenesis. Our data indicate further that population-level one-sided bias in adult–infant interactions can occur in a situation when only sensory modalities are involved, and, in contrast to primates, when limb preferences cannot determine the subjects’ relative positioning. This finding underlines the role of contralateral eye-hemisphere specializations in the origination of adult–infant spatial laterality in mammals, first hypothesized for asymmetric infant cradling in primates (reviewed in Sieratzki and Woll 1996; Harris 2010). Lateralized perception and processing of social information, which can be traced in vertebrate evolution from fish to mammals (e.g., MacNeilage et al. 2009; Bisazza and Brown 2011; Ocklenburg and Güntürkün 2012; Ocklenburg et al. 2013) together with the fact that mother–infant laterality is found in such ecologically diverse groups as primates and cetaceans, strongly favors the hypothesis that biased infant positioning is related to lateralization in social cognition.
Laterality in infant cradling and holding is suggested to be beneficial for both mother and infant (Sieratzki and Woll 1996). From the infant’s point of view, the left-side cradling favors the visibility of the mother’s face (Hendriks et al. 2011) and receiving of more maternal communication to the preferable right hemisphere (Sieratzki and Woll 1996). We hypothesize that in beluga infants, similarly lateralized positioning as regards to adult companions may bear some benefits and have an important impact in its welfare. Keeping conspecifics in the left visual field may help whale infants’ survival (Karenina et al. 2010a) because the right hemisphere has been repeatedly shown to be more effective than the left in governing of some social behaviors, especially those requiring an immediate reaction (MacNeilage et al. 2009). One notable example illustrates the significance of perceptual laterality in conditions most similar to those of the present study. Like beluga infant in complex aquatic environment, pigeons in aerial medium prefer to follow their partners on the right side, viewing them with the left eye (Nagy et al. 2010). Analysis of flight trajectories revealed that while keeping leaders in the left visual field, the following pigeons adjust their behavior accordingly to their movements more quickly and/or strongly. As a result, pigeons flying at the partner’s right side better respond to the changes in a flock and achieve coordination with conspecifics. In a similar way, swimming on the right side of the mother or another accompanied conspecific may allow beluga calves to be more responsive to its movements and thus to benefit in any rapidly changing situations.
References
Abel EL (2010) Human left-sided cradling preferences for dogs. Psychol Rep 107:336–338
Bisazza A, Brown C (2011) Lateralization of cognitive functions in fish. In: Brown C, Laland K, Krause J (eds) Fish cognition and behavior. Wiley–Blackwell, Oxford, pp 298–324
Blois-Heulin C, Crevel M, Boye M, Lemasson A (2012) Visual laterality in dolphins: importance of the familiarity of stimuli. BMC Neurosci 13:9
Bogren LY (1984) Side preference in women and men when holding their newborn child: psychological background. Acta Psychol Scand 69:13–23
Bourne VJ, Todd BK (2004) When left means right: an explanation of the left cradling bias in terms of right hemisphere specializations. Developmental Sci 7:19–24
Buckland ST, Anderson DR, Burnham KP, Laake JL (1993) Distance sampling: estimating abundance of biological populations. Chapman & Hall, London
Chernetsky AD, Krasnova VV, Bel’kovich VM (2011) Studies of the structure of the Solovetsky reproductive gathering of beluga whales (Delphinapterus leucas) in the White Sea using the photo identification method. Oceanology 51:275–280
Chernook VI, Vasilev AN, Melentyev VV, Glazov DM (2008) Experience of using specially equipped L-410 airplane for instrumental survey of marine mammals. Marine Mammals of the Holarctic. Conference papers, Odessa, , Ukraine, pp 132–137
Dadda M, Sovrano VA, Bisazza A (2003) Temporal pattern of social aggregation in tadpoles and its influence on the measurement of lateralised response to social stimuli. Physiol Behav 78:337–341
Damerose E, Vauclair J (2002) Posture and laterality in human and non-human primates: asymmetries in maternal handling and the infant’s early motor asymmetries. In: Andrew RJ (ed) Comparative Vertebrate Lateralization. Cambridge University Press, Cambridge, pp 306–362
de Château P, Andersson Y (1976) Left-side preference for holding and carrying newborn infants. II: Doll-holding and carrying from 2 to 16 years. Dev Med Child Neurol 18:738–744
Delfour F, Marten K (2006) Lateralized visual behavior in bottlenose dolphins (Tursiops truncatus) performing audio–visual tasks: the right visual field advantage. Behav Process 71:41–50
Deng C, Rogers LJ (2002) Prehatching visual experience and lateralization in the visual Wulst of the chick. Behav Brain Res 134:375–385
Doidge DW (1990) Age–length and length–weight comparisons in the beluga, Delphinapterus leucas. In: Smith TG, St. Aubin DJ, Geraci JR (eds) Advances in Research on the Beluga Whale, Delphinapterus leucas. Can B Fish Aquat Sci 224:59–68
Donnot J, Vauclair J (2007) Infant holding preferences in maternity hospitals: testing the hypothesis of the lateralized perception of emotions. Dev Neuropsychol 32:881–890
Glazov DM, Chernook VI, Nazarenko EA, Zharikov KA, Shpak OV, Mukhametov LM (2010) Summer distribution and abundance of belugas in the White Sea based on aerial survey data 2005–2008. Marine Mammals of the Holarctic. Conference papers, Kaliningrad, , Russia, pp 134–140
Glazov DM, Chernook VI, Shpak OV, Solovyev BA, Nazarenko EA, Vasilev AN, Chelintsev NG, Kuznetsova DM, Mukhametov LM, Rozhnov VV (2012) The results of beluga whale (Delphinapterus leucas) aerial surveys in the Okhotsk Sea in 2009 and 2010. In: Marine Mammals of the Holarctic. Conference papers, Suzdal, Russia, pp 167–172
Gubbins C, McOwan B, Lynn SK, Hooper S, Reiss D (1999) Mother–infant spatial relations in captive bottlenose dolphins, Tursiops truncatus. Mar Mamm Sci 15:751–765
Harris LJ (2010) Side biases for holding and carrying infants: reports from the past and possible lessons for today. Laterality 15:56–135
Harris LJ, Almerigi JB, Kirsch EA (2000) Side preference in adults for holding infants: contributions of sex and handedness in a test of imagination. Brain Cognition 43:246–252
Harris LJ, Spradlin MP, Almerigi JB (2007) Mothers’ and fathers’ lateral biases for holding their newborn infants: a study of images from the World Wide Web. Laterality 12:64–86
Hendriks AW, van Rijswijk M, Omtzigt D (2011) Holding-side influences on infant’s view of mother’s face. Laterality 16:641–655
Hill HM (2009) The behavioral development of two beluga calves during the first year of life. Int J Comp Psychol 22:234–253
Hopkins WD (2004) Laterality in maternal cradling and infant positional biases: implications for the development and evolution of hand preferences in nonhuman primates. Int J Primatol 25:1243–1265
Hopkins WD, De Lathouwers M (2006) Left nipple preferences in infant Pan paniscus and P. troglodytes. Int J Primatol 27:1653–1662
IWC (2000) Report of the sub-committee on small cetaceans. J Cetac Res Manage 2:235–264
Jefferson TA, Karczmarski L, Laidre K, O’Corry-Crowe G, Reeves RR, Rojas-Bracho L, Secchi ER, Slooten E, Smith BD, Wang JY, Zhou K (2012) Delphinapterus leucas. http://www.iucnredlist.org/details/summary/6335/0
Kaplan G, Rogers LJ (1998) Teat preference for suckling in common marmosets: relationship to side of being carried and hand preference. Laterality 3:269–281
Karenina K, Giljov A, Baranov V, Osipova L, Krasnova V, Malashichev Y (2010a) Visual laterality of calf-mother interactions in wild whales. PLoS One 5:e13787
Karenina KA, Giljov AN, Malashichev YB, Baranov VS, Bel’kovich VM (2010b) Visual lateralization in the wild: perceiving of novel object in beluga whale (Delphinapterus leucas). Asymmetry Journal 4:3–12
Karenina KA, Giljov AN, Malashichev YB (2013) Eye as a key element of conspecific image eliciting lateralized response in fish. Anim Cogn 16:287–300
Kilian A, von Fersen L, Güntürkün O (2000) Lateralization of visuospatial processing in the bottlenose dolphin (Tursiops truncatus). Behav Brain Res 116:211–215
Kilian A, von Fersen L, Gunturkun O (2005) Left hemispheric advantage for numerical abilities in the bottlenose dolphin. Behav Process 68:179–184
Kingsley MCS (1996) Population index estimate for the belugas of the St. Lawrence in 1995. Can Tech Rep Fish Aquat Sci 2117:1–38
Krasnova VV, Bel’kovich VM, Chernetsky AD (2006) Mother–infant spatial relations in wild beluga (Delphinapterus leucas) during postnatal development under natural conditions. Biol Bull Russ Acad Sci 33:53–58
Krasnova VV, Bel’kovich VM, Chernetskii AD (2009) Formation of behavior in the White Sea beluga calf, Delphinapterus leucas, during early postnatal ontogenesis. Russ J Mar Biol 35:53–59
Lemasson A, Koda H, Kato A, Oyakawa C, Blois-Heulin C, Masataka N (2010) Influence of sound specificity and familiarity on Japanese macaques’ (Macaca fuscata) auditory laterality. Behav Brain Res 208:286–289
Litzky LK (2001) Monitoring recovery status and age structure of cook inlet, Alaska belugas by skin color determination. University of Washington, Seattle, WA, USA, MS thesis
Lyamin O, Pryaslova J, Kosenko P, Siegel J (2007) Behavioral aspects of sleep in bottlenose dolphin mothers and their calves. Physiol Behav 92:725–733
MacNeilage PF, Rogers LJ, Vallortigara G (2009) Origins of the left and right brain. Sci Am 301:60–67
Mann J, Smuts BB (1999) Behavioral development of wild bottlenose dolphin newborns (Tursiops sp.). Behaviour 136:529–566
Manning JT, Chamberlain AT (1990) The left-side cradling preference in great apes. Anim Behav 39:1224–1227
Manning JT, Heaton R, Chamberlain AT (1994) Left-side cradling: similarities and differences between apes and humans. J Human Evol 26:77–83
Manning JT, Trivers RL, Thorhill R, Singh D, Denman J, Eklo MH, Anderton RH (1997) Ear asymmetry and left-side cradling. Evol Human Behav 18:327–340
Nagy M, Akos Z, Biro D, Vicsek T (2010) Hierarchical group dynamics in pigeon flocks. Nature 464:890–893
Negayama K, Kawai M, Yamamoto H, Tomiwa K, Sakakihara Y (2010) Behavioral development of infant holding and its laterality in relation to mothers’ handedness and child-care attitude. Infant Behav Dev 33:68–78
Nishida T (1993) Left nipple suckling preference in wild chimpanzees. Ethol Sociobiol 14:45–51
Ocklenburg S, Güntürkün O (2012) Hemispheric asymmetries: the comparative view. Front Psychol 3:5
Ocklenburg S, Ströckens F, Güntürkün O (2013) Lateralisation of conspecific vocalisation in non-human vertebrates. Laterality 18:1–31
Peirce JW, Leigh AE, Kendrick KM (2000) Configurational coding, familiarity and the right hemisphere advantage for face recognition in sheep. Neuropsychologia 38:475–483
Reissland N (2000) The cradling bias in relation to pitch of maternal child-directed language. Brit J Devl Psychol 18:179–186
Reissland N, Hopkins B, Helms P, Williams B (2009) Maternal stress and depression and the lateralisation of infant cradling. J Child Psychol Psychiatry 50:263–269
Robins A, Lippolis G, Bisazza A, Vallortigara G, Rogers LJ (1998) Lateralized agonistic responses and hindlimb use in toads. Anim Behav 56:875–881
Rosa Salva O, Regolin L, Mascalzoni E, Vallortigara G (2012) Cerebral and behavioural asymmetries in animal social recognition. Comp Cogn Behav Rev 7:110–138
Sakai M, Hishii T, Takeda S, Kohshima S (2006) Laterality of flipper rubbing behaviour in wild bottlenose dolphins (Tursiops aduncus): caused by asymmetry of eye use? Behav Brain Res 170:204–210
Saling MM, Cooke W (1984) Cradling and transport of infants by South African mothers: a cross-cultural study. Curr Anthropol 25:333–335
Saling M, Tyson G (1981) Lateral cradling preferences in nulliparous females. J Genet Psychol 139:309–310
Salk L (1960) The effects of the normal heartbeat sound on the behaviour of the new-born infant: implications for mental health. World Mental Health 12:168–175
Scola C, Vauclair J (2009) Infant holding side biases displayed by fathers in maternity hospitals. J Reprod Infant Psychol 28:3–10
Scola C, Vauclair J (2010) Is infant holding-side bias related to motor asymmetries in mother and child? Dev Psychobiol 52:475–486
Sieratzki JS, Woll B (1996) Why do mothers cradle babies on their left? Lancet 347:1746–1748
Siniscalchi M, Dimatteo S, Pepe AM, Sasso R, Quaranta A (2012) Visual lateralization in wild striped dolphins (Stenella coeruleoalba) in response to stimuli with different degrees of familiarity. PLoS One 7:e30001
Solovyev B, Glazov D, Chernook V, Nazarenko E, Chelintsev N, Rozhnov V (2012) Distribution and abundance of beluga whales (Delphinapterus leucas) in the White Sea and in the Southern part of the Barents Sea based on aerial counts in August 2011. In: Marine Mammals of the Holarctic. Conference papers, Suzdal, Russia, pp 264–269
Sovrano VA (2004) Visual lateralization in response to familiar and unfamiliar stimuli in fish. Behav Brain Res 152:385–391
Sovrano V, Andrew R (2006) Eye use during viewing a reflection: behavioral lateralization in zebrafish larvae. Behav Brain Res 167:226–231
Sovrano V, Rainoldi C, Bisazza VG (1999) Roots of brain specializations preferential left-eye use during mirror-image inspection in six species of teleost fish. Behav Brain Res 106:175–180
Sovrano VA, Bisazza A, Vallortigara G (2001) Lateralization of response to social stimuli in fishes: a comparison between different methods and species. Physiol Behav 74:237–244
Thieltges H, Lemasson A, Kuczaj S, Böye M, Blois-Heulin C (2011) Visual laterality in dolphins when looking at (un) familiar humans. Anim Cogn 14:303–308
Tomaszycki M, Cline C, Griffin B, Maestripieri D, Hopkins WD (1998) Maternal cradling and infant nipple preferences in rhesus monkeys (Macaca mulatta). Dev Psychobiol 32:305–312
Vallortigara G, Andrew RJ (1991) Lateralization of response by chicks to change in a model partner. Anim Behav 41:187–194
Vallortigara G, Rogers LJ (2005) Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28:575–589, discussion 589–633
Vallortigara G, Chiandetti C, Sovrano VA (2011) Brain asymmetry (animal). WIREs Cogn Sci 2:146–157
Vauclair J, Donnot J (2005) Infant holding biases and their relations to hemispheric specializations for perceiving facial emotions. Neuropsychologia 43:564–571
Ventolini N, Ferrero EA, Sponza S, Della Chiesa A, Zucca P, Vallortigara G (2005) Laterality in the wild: preferential hemifield use during predatory and sexual behaviour in the black-winged stilt. Anim Behav 69:1077–1084
Versace E, Morgante M, Pulina G, Vallortigara G (2007) Behavioural lateralization in sheep (Ovis aries). Behav Brain Res 184:72–80
Vervloed MPJ, Hendriks AW, van den Eijnde E (2011) The effects of mothers’ past infant-holding preferences on their adult children’s face processing lateralisation. Brain Cognition 75:248–254
von Fersen L, Schall U, Güntürkün O (2000) Visual lateralization of pattern discrimination in the bottlenose dolphin (Tursiops truncatus). Behav Brain Res 107:177–181
Williams TM, Friedl WA, Fong ML, Yamada RM, Sedivy P, Haun JE (1992) Travel at low energetic cost by swimming and wave-riding bottlenose dolphins. Nature 355:821–823
Yaman S, von Fersen L, Dehnhardt G, Güntürkün O (2003) Visual lateralization in the bottlenose dolphin (Tursiops truncatus): evidence for a population asymmetry? Behav Brain Res 142:109–114
Zhao D, Gao X, Li B, Watanabe K (2008) First wild evidence of neonate nipple preference and maternal cradling laterality in Old World monkeys: a preliminary study from Rhinopithecus roxellana. Behav Process 77:364–368
Zucca P, Sovrano VA (2008) Animal lateralization and social recognition: quails use their left visual hemifield when approaching a companion and their right visual hemifield when approaching a stranger. Cortex 44:13–20
Acknowledgments
We wish to thank the members of Marine Mammals Behaviour and Bioacoustics Laboratory at the P.P. Shirshov Institute of Oceanology, especially Vera Krasnova, Anton Chernetsky Vladimir Baranov, Alexander Bratanov, and Vsevolod Bel’kovich, as well as Bettina van Elk, and Alexandra Zheludkova for their organizational assistance and valuable help in the field. This work was supported by the Federal Grant-in-Aid Program "Human Capital for Science and Education in Innovative Russia" (Governmental Contract No. P2379). Aerial surveys were conducted as a part of scientific projects of Utrish Dolphinarium, Ltd, White whale Programme IPEE RAS (financing by Russian Geographical Society) and "Current status of the Sakhalin-Amur beluga aggregation (Okhotsk Sea, Russia): sustainability assessment" project (financing by Utrish Dolphinarium, Ltd, Ocean Park Corporation, Hong Kong; Georgia Aquarium Inc., USA; SeaWorld Parks & Entertainment, USA; Mystic Aquarium and Institute for Exploration, USA; Kamogawa Sea World, Japan). We thank Lisa Veikher for final English style check.
Ethical statement
This study does not include any study of human subjects or non-human primates, thus does not need any specific adherence to the Declaration of Helsinki or Weatherall report. As for the work with other subjects, this work, which only implies distant observations on animals, did not require any permission according to local rules and laws in Russia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. D. Twiss
Rights and permissions
About this article
Cite this article
Karenina, K., Giljov, A., Glazov, D. et al. Social laterality in wild beluga whale infants: comparisons between locations, escort conditions, and ages. Behav Ecol Sociobiol 67, 1195–1204 (2013). https://doi.org/10.1007/s00265-013-1545-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1545-2