Abstract
Social animals acquire information on predator identities through social learning, where individuals with no prior experience learn from experienced members of the group. However, a large amount of uncertainty is often associated with socially acquired information especially in cases of cross-species learning. Theory predicts that socially acquired information from heterospecifics should take more repetitions to develop in complex ecosystems where the number of participants is greater. Our work focuses on coral reef fish as their social and communal lifestyles, along with their complex life histories, make them an ideal model to test for socially acquired predator recognition. Specifically, we tested if Pomacentrus wardi were capable of transmitting the recognition of an unknown predator, Pseudochromis fuscus, to closely related Pomacentrus moluccensis and phylogenetically distant Apogon trimaculatus. Individuals of both species were able to learn the predator's identity from experienced P. wardi based on a single conditioning event. It is somewhat surprising how fast social learning occurred particularly for the distantly related cardinalfish. This study demonstrates the widespread nature of social learning as a method of predator recognition in biologically complex ecosystems, and highlights that the benefits of responding to uncertain information may override the costs associated with lost foraging opportunities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Whether choosing a mate, deciding where to breed, selecting a foraging area or knowing who to avoid, an individual’s decisions can disclose useful information to others. Therefore, under conditions where individuals can observe one another's actions, it is only logical that animals learn from one another. This interaction, where learning is influenced by the observation of, or interaction with another animal is referred to as social learning (Brown and Laland 2003; Crane and Ferrari 2013). Studies on social learning in animals date back to at least the nineteenth century (Romanes 1884), with a wide variety of animals (e.g. insects, birds, eutherian mammals, marsupials and fish) known to benefit from this type of information transmission (Brown and Laland 2001; Griffin 2004; Laland 2004; Manassa and McCormick 2012a).
Social learning allows animals to obtain knowledge on locally adaptive information without the risks associated with individual learning (Brown and Laland 2002). Exploring the environment individually is risky, due to the increased exposure to unknown predators and associated costs in both time and energy. Therefore, given the relatively low risks associated with social learning, it is likely that publically available information will spread rapidly within a population (Swaney et al. 2001). The ability to learn quickly may be the difference between life and death, especially for naïve individuals who are required to promptly learn new predators in order to avoid capture and almost certain death.
Predation pressure acts as a strong selective force shaping the behaviour, life history, morphology and distribution of prey animals (Wisenden and Harter 2001; Brown 2003). As such, the ability to assess local predation risk is crucial for survival. A variety of taxa have been shown to acquire information on predator identities through social learning (Griffin 2004; Ferrari and Chivers 2008), with the pattern of acquisition similar across taxa (Griffin 2004). In a typical scenario, an avoidance response is evoked in an individual following the simultaneous detection of a previously neutral stimulus with an alarm stimulus (e.g. alarm call, mobbing call, damage-released chemical cues) (Griffin 2004; Galef and Laland 2005). Following this, the individual who has learnt through association responds to the neutral stimulus in future encounters, thereby highlighting it as a threat to other individuals who learn by this experience.
Studies on social learning about risk have focused almost exclusively on the transmission of information between conspecifics (Ferrari and Chivers 2008); however, intraspecific social learning is only one way in which naïve individuals can learn the identities of novel predators. Animals may also learn unfamiliar predators by observing the behaviour of other species (interspecific learning) (Griffin 2004). While research has indicated that this type of learning is possible, such studies are rare and have all been conducted in rather ecologically simple ecosystems where the number of species that could act as tutors was relatively few (Vieth et al. 1980; Mathis et al. 1996; Ferrari and Chivers 2008). Ferrari and Chivers (2008) showed a cross-species response in tadpoles collected from a pond which contained only two species. Likewise, Mathis et al. (1996) showed cross-species responses between two freshwater prey fishes, but the lake contained only a handful of other prey fishes. Theory dictates that in biologically complex environments such as coral reefs, where biodiversity is at its highest, interspecific social learning may be commonplace but will take considerable time to develop. In the case of larval reef fish, for any given individual that recruits to the reef, there are dozens of potential heterospecifics that could provide learning opportunities. Therefore, with such an astonishing array of heterospecifics in the vicinity, which behaviours are relevant and which are not?
Coral reef fish are known to respond to the damage-released chemical cues of heterospecific individuals (Mitchell et al. 2012), with studies demonstrating learnt predator recognition following a single-conditioning event (Brown 2003; Ferrari et al. 2005; Holmes and McCormick 2010; McCormick and Manassa 2008). However, these direct learning methods come at a potential cost, as the prey must be in the vicinity of an actively foraging predator. Socially acquired information reduces this risk; however, a level of uncertainly surrounds the accuracy of the information. Differences in size, sex, body condition as well as parasite load and hunger levels could all influence differential perception of risk and create uncertainty (Milinski 1985; Mirza et al. 2001; Pollock et al. 2006). The sources of uncertainty are the same in both simple and complex ecosystems; however, the number of players is much larger. Consequently, the number of encounters required to acquire accurate information should be greater, especially for newly recruited coral reef fishes. As a significant difference in survival has been demonstrated between recruiting fish that forage at high or low levels, with the latter surviving better due to reduced exposure to predators (Lönnstedt et al. 2012), selection should cause newly settled reef fish to be sceptical of socially acquired information.
Our study explored social learning of predator recognition among three species of coral reef fishes: Pomacentrus wardi, Pomacentrus moluccensis (family: Pomacentridae) and Apogon trimaculatus (family: Apogonidae). Specifically, we investigated if individual P. wardi were capable of transmitting the recognition of a common predator odour to closely related P. moluccensis and phylogenetically distant A. trimaculatus. There should be a greater amount of uncertainty about the quality of socially acquired information in situations where learning occurs from more distantly related species; hence, our original goal was to test for an asymmetry in the amount of information required to establish predator recognition from closely and distantly related fishes. However, this proved unnecessary given the high efficiency of the one-time learning we observed.
Materials and methods
The experiment was conducted at Lizard Island Research Station (14°40′ S, 145°28′ E) on the Great Barrier Reef, Australia during November and December 2011. Newly settling damselfish; P. wardi and P. moluccensis as well as the cardinalfish A. trimaculatus were collected from light traps (see Meekan et al. 2001 for design) moored overnight near the reef crest during the summer larval recruitment pulse. Light trap caught individuals were maintained in 32 L aerated flow-through holding tanks (density: approx. 50–100 per 32 L) at ambient temperatures (26–29 °C), under a 12:12 light dark photoperiod. Fish were fed ad libitum twice a day with Artemia franciscana and Aquaculture Nutrition NRD 5/8 pellets (to ensure a stable hunger state); however, no feeding occurred once individuals were placed into the observation tanks.
The dottyback Pseudochromis fuscus, a common predator of newly settled fish (Feeney et al. 2012), were collected on scuba using hand nets and a clove oil/ethanol/seawater solution (as an anaesthetic). Individuals were maintained in separate compartments within 32 L aerated flow-through holding tanks (density: approx. 6–8 per 32 L). Individuals used to produce predator odours were fed twice daily with INVE Aquaculture Nutrition NRD G12 pellets (commercially manufactured diet); however, no feeding occurred 24 h prior to collection of predator odours.
Experimental protocol
To determine if social learning of a predator odour occurred between P. wardi and P. moluccensis and/or A. trimaculatus, a series of experiments using a three-stage process (refer to Manassa and McCormick 2012b for protocol) were conducted: (a) conditioning of a naïve tutor (P. wardi) to recognise the predator; (b) pairing of the tutor with an observer (P. moluccensis or A. trimaculatus); (c) testing for an anti-predator response in the observer (P. moluccensis or A. trimaculatus) (Fig. 1). Behavioural observations were conducted during stage ‘a’ to collect baseline data and stage ‘c’ to determine if social learning occurred. A series of controls were undertaken to ensure that P. moluccensis and A. trimaculatus did not demonstrate an innate response to the predator odour. If the observer displayed an anti-predator response in stage ‘c’ to the predator odour compared to the controls, it was seen as evidence that the fish had learnt that the predator odour represented a potential threat through social learning.
Stage ‘a’—conditioning of a naïve tutor (P. wardi) to recognise the predator
An individual P. wardi was acclimated to an observation tank for a period of 18 h. Prior to the initial observation period, the flow-through system was turned off, with 60 ml of tank water drawn up the stimulus injection tube and discarded to remove any stagnant water. A further 135 ml was collected and kept. Immediately prior to the initial observation period, 10 ml of live A. franciscana (∼2500 nauplii per tank) was injected into the tube, followed by 60 ml of previously collected tank water to flush the tube. The behaviour of the focal P. wardi was then recorded for 3 min. After initial observations, one of two treatments (a solution of 15 ml of the damage-released chemical cue (see below) and 60 ml of predator odour (P. fuscus) (see below) (CCPO) or a solution of 15 ml of the seawater and 60 ml of predator odour (P. fuscus) (SWPO)) was injected into the tank, along with a further 10 ml of live A. franciscana. Following this, 60 ml of previously collected tank water was injected to ensure all the cue was flushed through. This was followed by a final 3 min observation period, with 60 replicates undertaken for each treatment. Naïve individuals exposed to the CCPO treatment should have the opportunity to learn that P. fuscus is a predator, and hence become experienced tutors. Individuals exposed to the SWPO treatment should not have the opportunity to associate risk with the predator odour and hence act as non-experienced tutors.
Stage ‘b’—pairing for social learning opportunity
Immediately following the final observation period, the P. wardi individual from stage ‘a’ was dipped in clean seawater then transferred to another observation tank housing either a naïve P. moluccensis or A. trimaculatus (acclimated for 18 h). The two individuals were acclimated in the tank for 2 h before experiments commenced. After the acclimation period, the flow-through system was turned off, and 60 ml of tank water was drawn up the stimulus injection tube and discarded, with a further 60 ml collected and kept. A 60 ml aliquot of predator odour along with 10 ml of live A. franciscana was injected into the tank followed by 60 ml of previously collected tank water. Both P. moluccensis and A. trimaculatus have the opportunity to learn the identity of the predator odour based on the fright response of the P. wardi in the tank. P. wardi that are experienced tutors should have the ability to transmit the recognition to other species, but non-experienced tutors should not.
Stage ‘c’—testing for anti-predator response
Immediately following stage ‘b’, the naïve P. moluccensis or A. trimaculatus from that stage was rinsed in clean seawater then transferred to an empty observation tank and acclimated for 2 h before observations commenced. After the acclimation period, the flow-through system was turned off, and 60 ml of tank water was drawn up the stimulus injection tube and discarded, with a further 120 ml collected and kept. Immediately prior to the initial observation period, 10 ml of A. franciscana was injected into the tube followed by 60 ml of previously collected tank water, to flush the tube. The behaviour of the focal individual was then recorded for 3 min. After initial observations, one of two treatments (60 ml of predator odour (PO) or 60 ml of seawater (SW)) was injected into the tank, along with a further 10 ml of live A. franciscana. Following this, 60 ml of previously collected tank water was used to flush through the tube. This was followed by a final 3 min observation period, with 15 replicates undertaken for each species and treatment crossed with each treatment in stage ‘a’ (see Fig. 1). This resulted in a total of four observer testing stage combinations: predator odour stimulus following conditioning with an experienced P. wardi, predator odour stimulus following conditioning with a non-experienced P. wardi, seawater stimulus following conditioning with an experienced P. wardi and seawater stimulus following conditioning with a non-experienced P. wardi.
Observation tanks
Observation tanks (height 17 cm × length 27 cm, width 17 cm) were set-up with an air stone placed at the back corner of each tank and an additional piece of plastic tubing, for cue injection. A 2 cm deep substratum of sand and a shelter consisting of coral rubble (approx. 9.4×8.4 cm) was located at the opposite corner to the air stone, with each tank surrounded on three sides by black plastic to avoid test fish observing adjacent tanks. Individual P. wardi (mean SL ± SE; 15 ± 0.36 mm), P. moluccensis (mean SL ± SE; 15 ± 0.71 mm) and A. trimaculatus (mean SL ± SE; 14 ± 0.28 mm) were placed in the observation tanks 18 h prior to experimentation.
Stimulus preparation
Damage-released chemical cues were prepared (refer to Manassa and McCormick 2012a for protocol) with a total of 60 P. wardi (mean SL ± SE; 15 ± 0.36 mm) (one individual per 15 ml of seawater) sacrificed. Cue donors were euthanized by a quick blow to the head, with ten superficial (minor flesh damage) cuts made to the skin with a clean razor blade. Specimens were then rinsed in 15 ml of seawater, previously obtained from each test tank. Following this, the 15 ml of damage-released chemical cue was filtered prior to use, with the cues used no longer than 20 min after preparation.
Predator odours were collected in such a way that they were free of possible P. wardi damage-released chemical cues. This involved P. fuscus (up to 72.4 mm SL) being fed a diet of fish pellets (INVE Aquaculture Nutrition NRD G12) which are manufactured commercially and known to contain no trace elements of chemical cues. The flow-through aquaria system was turned off 2 h prior to experimentation to ensure the predator odours collected just prior to the experiment were concentrated within the holding tanks.
Quantification of behaviour
The behavioural responses of the tutors and observers were quantified by recording the frequency of two behaviours: the number of feeding strikes (measures foraging levels) and the number of line crosses (measures activity levels). These are well established experimental indicators of an anti-predator response in larval damselfishes (Holmes and McCormick 2010; Manassa and McCormick 2012b). The observation tanks were divided into four equal vertical areas and six equal horizontal areas (grid of 4.7×4.2 cm rectangles, drawn on waterproof paper at the back of the tank), with every line crossed by the fish recorded. The number of feeding strikes was recorded regardless of success (impossible to observe success given the size of the fish and the food source), with the controls in each experiment not expected to show any changes between initial and final observation periods for the variables measured.
Statistical analysis
The difference in the total counts of feeding strikes and line crosses between the initial and final observation periods were used for all analyses to control for individual differences. The behavioural responses of P. wardi to a solution of damage-released CCPO along with the responses of P. wardi, P. moluccensis and A. trimaculatus to SWPO were statistically tested using a one-factor MANOVA. Two one-factor MANOVAs were also used to examine the difference in total counts of feeding strikes and line crosses between the initial and final observation periods for each of the four observer testing stage combinations for each species (P. moluccensis and A. trimaculatus). To further explore the nature of the significant differences found by MANOVA, univariate ANOVAs were undertaken on both variables (feeding strikes and/or line crosses) followed by Tukey's HSD means comparison tests. Residual analysis found that the assumptions of normality and homogeneity of variance were satisfied.
Results
Controls
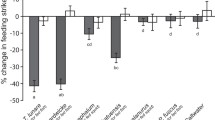
The behavioural response of P. wardi, P. moluccensis and A. trimaculatus to SWPO, along with the response of P. wardi to CCPO, was examined. During the trials (CCPO), P. wardi individuals made between 61 and 111 feeding strikes (mean ± SE; 94.3 ± 4.1) and between 38 and 89 line crosses (62.2 ± 4.5) during the initial observation period and between 25 and 80 feeding strikes (49.6 ± 3.9) and between 13 and 62 line crosses (38.3 ± 3.6) during the final observation period. There was a significant difference in the change in behaviour between treatments (MANOVA, Pillai's Trace = 0.817, F 6, 172 = 19.785, p < 0.001; Fig. 2), with a decrease in feeding strikes (F 3, 86 = 122.474, p < 0.001; Fig. 2) and line crosses (F 3, 86 = 16.439, p < 0.001; Fig. 2) when the damage-released chemical cue was injected compared to the controls.
Change in feeding strikes (foraging level) and line crosses (activity level) by Pomacentrus wardi, Pomacentrus moluccensis and Apogon trimaculatus between initial and final observation periods for fish exposed to a solution of seawater and predator odour (SWPO), along with the response of P. wardi to a solution of damage-released chemical cue and predator odour (CCPO), mean ± SE, n = 15 per treatment. Letters below the bars represent Tukey's HSD groupings of means
Stage ‘c’ response—P. moluccensis
The behavioural response of individual P. moluccensis to the four observer testing stage combinations was examined. There was a significant change in behaviour between the initial and final observation periods among the four cues (MANOVA, Pillai's Trace = 0.831, F 6, 112 = 13.241, p < 0.001; Fig. 3). This difference was caused by significant differences in both feeding strikes (F3, 56 = 47.994, p < 0.001; Fig. 3) and line crosses (F3, 56 = 12.407, p < 0.001; Fig. 3). Tukey's HSD means comparison tests highlighted a significant reduction in both feeding strikes and line crosses in response to the predator odour stimulus following conditioning with an experienced P. wardi, compared to the other three observer testing stage combinations. During this treatment stage, P. moluccensis individuals made between 24 and 101 feeding strikes (mean ± SE, 73.9 ± 4.9) and between 29 and 82 line crosses (58.2 ± 3.5) during the initial observation period and between 14 and 61 feeding strikes (29.9 ± 3.1) and between 20 and 69 line crosses (41.7 ± 3.8) during the final observation period.
Observer testing stage for anti-predator response: change in feeding strikes and line crosses by Pomacentrus moluccensis between initial and final observation periods for fish exposed to predator odour or seawater following conditioning with either a predator-experienced tutor (Pomacentrus wardi, exposed to a solution of damage-released chemical cue and predator odour (CCPO)) or a non-experienced tutor (P. wardi, exposed to a solution of seawater and predator odour (SWPO)). Data are means ± SE, n = 15 per treatment. Letters below the bars represent Tukey's HSD groupings of means
Stage ‘c’ response—A. trimaculatus
The behavioural response of individual A. trimaculatus to four observer testing stage combinations was examined. There was a significant change in behaviour between the initial and final observation periods among the four cues (MANOVA, Pillai's Trace = 0.851, F 6, 112 = 13.818, p < 0.001; Fig. 4). Significant differences in both feeding strikes (F3, 56 = 89.378, p < 0.001; Fig. 4) and line crosses (F3, 56 = 12.721, p < 0.001; Fig. 4) caused this difference. Tukey's HSD means comparison tests highlighted a significant reduction in both feeding strikes and line crosses in response to the predator odour stimulus following conditioning with an experienced P. wardi, compared to the other three observer testing stage combinations. During this treatment stage, A. trimaculatus individuals made between 40 and 76 feeding strikes (mean ± SE, 57.6 ± 2.8) and between 11 and 32 line crosses (21.1 ± 1.8) during the initial observation period and between 17 and 50 feeding strikes (32 ± 2.4) and between 7 and 21 line crosses (12.2 ± 1.1) during the final observation period.
Observer testing stage for anti-predator response: change in feeding strikes and line crosses by Apogon trimaculatus between initial and final observation periods for fish exposed to predator odour or seawater following conditioning with either a predator-experienced tutor (Pomacentrus wardi, exposed to a solution of damage-released chemical cue and predator odour (CCPO)) or a non-experienced tutor (P. wardi, exposed to a solution of seawater and predator odour (SWPO)). Data are means ± SE, n = 15 per treatment. Letters below the bars represent Tukey's HSD groupings of means
Discussion
This study highlights the use of social learning as a mechanism of acquiring information on predator identities among three species of coral reef fish. Specifically, we demonstrate that naïve P. wardi are capable of transmitting the recognition of a predator odour to another closely related damselfish P. moluccensis and a phylogenetically distant species A. trimaculatus through the process of social learning. While other studies have shown that social learning occurs between conspecifics (Ferrari et al. 2012; Manassa and McCormick 2012a, b), this is the first study to demonstrate the occurrence of interspecific social learning in species found naturally in highly diverse ecosystems. These results suggest that social learning may act as a useful mechanism for the spread of information between species, ultimately increasing the likelihood of survival (Manassa and McCormick 2012b).
Previously, studies have documented the occurrence of social learning in damselfish species (Ferrari et al. 2012; Manassa and McCormick 2012a,b), with this study extending our knowledge by demonstrating the use of this mechanism in cardinalfishes. As many species of coral reef fish live social lifestyles (Hoare and Krause 2003), it is expected that they could benefit greatly from this method of predator recognition, as it would allow for continuous updates and reinforcement of current predation events within the immediate area. Following a larval phase, coral reef fishes recruit to the reef in large numbers with many individuals settling onto the same habitat patches, resulting in both positive (e.g. schooling) and negative (e.g. competition) interactions (McCormick 2012). Selection of habitat patches is therefore crucial to survival, with those that settle into areas with high food availability, low occurrence of competition and minimal predators likely to have a considerable survival advantage (Feeney et al. 2012; McCormick 2012). During the first 48 h following settlement, predation by small piscivores (such as P. fuscus used in this study) is high, averaging ∼60 % (Almany and Webster 2006; Feeney et al. 2012). However, these fish settle with little knowledge of the identity of reef based predators (McCormick and Holmes 2006; Lönnstedt et al. 2012). Therefore, monitoring the behaviour of individuals that are similar in size, regardless of species, is likely to be beneficial especially within the first few hours following settlement when predator recognition is vital (Feeney et al. 2012; Lönnstedt et al. 2012). During these first few hours, it is likely that individuals are using information from all relevant sources in an attempt to survive. Fine-tuning of anti-predator behaviours is likely to follow with the assistance of direct learning methods. As such, a reliance on social learning in complex ecosystems with high species diversity, such as coral reefs, is likely immediately following settlement.
In complex ecosystems, there is greater uncertainty about the reliability of social information due to an increase in species diversity and, therefore, a greater number of heterospecifics to pay attention to. Thus, information will be variable in quality and relevance and may simply overwhelm an individual's ability to decipher the information in an ecologically relevant time frame. Given this, one may expect that individuals will need multiple learning opportunities to acquire information, particularly from distantly related heterospecifics. However, the results of this study demonstrate that both P. moluccensis and A. trimaculatus were able to learn the identity of the predator based on a single pairing with an experienced heterospecific. One may expect social transmission of predator avoidance amongst members of the same prey guild irrespective of phylogenetic relatedness; however, with the diversity of predators and constant ontogenetic shifts which occur on coral reef, this may not occur; therefore, additional research is required to further understand this topic. Further studies designed to manipulate the relative uncertainty of information, for example, by using individuals of different size, sex or body condition may also aid our understanding of social information use in complex ecosystems.
An anti-predator response to the alarm cues of other species is common throughout the animal kingdom with studies demonstrating an occurrence in birds, mammals, fishes, amphibians and insects (reviewed in Mitchell et al. 2012). However, the costs associated with this type of direct learning are significant, since individuals need to be in close proximity to a potential predator. As such, individuals may adopt a response that minimises risk by using information from all relevant sources. The use of interspecific social cues in predator recognition has been observed in studies conducted on birds (Vieth et al. 1980), larval amphibians (Ferrari and Chivers 2008) and Ostariophysan fishes (Krause 1993; Mathis et al. 1996). Since the number of potential information sources is greater in complex ecosystems, individuals who are able to detect and respond to social information provided by other ecologically similar species are likely to increase their chances of detecting actively foraging predators within their immediate vicinity. However, the mechanisms by which individuals select useful information may differ depending on the type of ecosystem. For example, on coral reefs, the intense predation pressures placed on newly settling reef fishes may drive a reliance on social information with the benefits outweighing the costs associated. This may contrast with simpler systems, such as freshwater lakes, where individuals may be more selective when choosing information sources because of the reduced number and type of predators present.
The ability to utilise social information gained from heterospecifics is likely to confer a significant survival advantage for coral reef fish, particularly during critical life history transitions (e.g. settlement) where predation pressure is spatially and temporally unreliable. Likewise, the capacity of individuals to socially learn after a single conditioning event has profound implications for predator–prey interactions. Along with highlighting the widespread nature of social learning as a method of predator recognition, this study documents the occurrence of interspecific learning in coral reef fishes, suggesting that the benefits of responding to uncertain information may override the costs associated with reduced foraging.
References
Almany GR, Webster MS (2006) The predation gauntlet: early post-settlement mortality in reef fishes. Coral Reefs 25:19–22
Brown C, Laland K (2001) Social learning and life skills training for hatchery reared fish. J Fish Biol 59:471–493
Brown C, Laland KN (2002) Social learning of a novel avoidance task in the guppy: conformity and social release. Anim Behav 64:41–47
Brown C, Laland KN (2003) Social learning in fishes: a review. Fish Fish 4(280):288
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4:227–234
Crane AL, Ferrari MCO (2013) Social learning of predation risk: a review and prospectus. In: Clark K (ed) Social learning theory: Phylogenetic considerations across animal, plant, and microbial taxa. Nova Science Publisher, New York
Feeney WE, Lönnstedt OM, Bosiger Y, Martin J, Jones GP, Rowe RJ, McCormick MI (2012) High rate of prey consumption in a small predatory fish on coral reefs. Coral Reefs 31:909–918
Ferrari MCO, Chivers DP (2008) Cultural learning of predator recognition in mixed-species assemblages of frogs: the effect of tutor-to-observer ratio. Anim Behav 75:1921–1925
Ferrari MCO, Manassa RP, Dixson DL, Munday PL, McCormick MI, Meekan MG, Sih A, Chivers DP (2012) Effects of ocean acidification on learning in coral reef fishes. PLoS One 7:1–10
Ferrari MCO, Trowell JJ, Brown GE, Chivers DP (2005) The role of learning in the development of threat-sensitive predator avoidance by fathead minnows. Anim Behav 70:777–784
Galef BG, Laland KN (2005) Social learning in animals: empirical studies and theoretical models. Bioscience 55:489–499
Griffin AS (2004) Social learning about predators: A review and prospectus. Learn Behav 32:131–140
Hoare DJ, Krause J (2003) Social organisation, shoal structure and information transfer. Fish Fish 4:269–279
Holmes TH, McCormick MI (2010) Smell, learn and live: the role of chemical alarm cues in predator learning during early life history in a marine fish. Behav Process 83:299–305
Krause J (1993) Transmission of fright reaction between different species of fish. Behaviour 127:37–48
Laland KN (2004) Social learning strategies. Learn Behav 32:4–14
Lönnstedt OM, McCormick MI, Meekan MG, Ferrari MCO, Chivers DP (2012) Learn and live: predator experience and feeding history determines prey behaviour and survival. Proc R Soc Lond B 279:2091–2098
Manassa RP, McCormick MI (2012a) Social learning and acquired recognition of a predator by a marine fish. Anim Cogn 15:559–565
Manassa RP, McCormick MI (2012b) Social learning improves survivorship at a life history transition. Oecologia. doi:10.1007/s00442-012-2458-x
Mathis A, Chivers DP, Smith RJF (1996) Cultural transmission of predator recognition in fishes: intraspecific and interspecific learning. Anim Behav 51:185–201
McCormick MI (2012) Lethal effects of habitat degradation on fishes through changing competitive advantage. Proc R Soc Lond B 279:3899–3904
McCormick MI, Holmes TH (2006) Prey experience of predation influences mortality rates at settlement in a coral reef fish, Pomacentrus amboinensis. J Fish Biol 68:969–974
McCormick MI, Manassa RP (2008) Predation risk assessment by olfactory and visual cues in a coral reef fish. Coral Reefs 27:105–113
Meekan MG, Wilson SG, Halford A, Retzel A (2001) A comparison of catches of fishes and invertebrates by two light trap designs, in tropical NW Australia. Mar Biol 139:373–381
Milinski M (1985) Risk of predation of parasitized sticklebacks (Gasterosteus aculatus L.) under competition for food. Behaviour 93:203–216
Mirza RS, Scott JJ, Chivers DP (2001) Differential responses of male and female red swordtails to chemical alarm cues. J Fish Biol 59:716–728
Mitchell MD, Cowman PF, McCormick MI (2012) Chemical alarm cues are conserved within the coral reef fish family Pomacentridae. PLoS One 7:1–7
Pollock MS, Pollock RJ, Chivers DP (2006) The effects of body size, body condition and reproductive state on the responses of fathead minnows to alarm cues. Can J Zool 84:1351–1357
Romanes GJ (1884) Mental evolution in animals. AMS Press, New York
Swaney W, Kendal J, Capon H, Brown C, Laland KN (2001) Familiarity facilitates social learning of foraging behaviour in the guppy. Anim Behav 62:591–598
Vieth W, Curio E, Ernst U (1980) The adaptive significance of avian mobbing. III. Cultural transmission of enemy recognition in blackbirds: cross-species tutoring and properties of learning. Anim Behav 28:1217–1229
Wisenden BD, Harter KR (2001) Motion, not shape, facilitates association of predation risk with novel objects by fathead minnows (Pimephales promelas). Ethology 107:357–364
Acknowledgments
We thank O. Lönnstedt, J. White and R. Brooker for assistance in the field and O. Lönnstedt and D. Dixson for providing useful comments on a draft of the manuscript. We also thank P. Manassa for his artistic contribution. This study was funded through the ARC Centre of Excellence for Coral Reef Studies.
Ethical standards
The experiment was performed in accordance with the National Health and Medical Research Council, Australian Code of Practise for the Care and Use of Animals for Scientific Purposes 7th Edition, 2004: (the Code) and in compliance with the Queensland Animal Care and Protection Act, 2001 (Act no. 64 of 2001) (the Act) and James Cook University guidelines under approval A1067.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Buston
Rights and permissions
About this article
Cite this article
Manassa, R.P., McCormick, M.I. & Chivers, D.P. Socially acquired predator recognition in complex ecosystems. Behav Ecol Sociobiol 67, 1033–1040 (2013). https://doi.org/10.1007/s00265-013-1528-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-013-1528-3