Abstract
Previous experiences can play a significant role in determining future behaviors. Winner and loser effects, where the outcome of previous aggressive encounters influences the behavioral approach to and outcomes of future conflicts, have been documented in many taxa and illustrate this phenomenon. These effects are prevalent in species that interact frequently because modulation of these potentially costly social interactions may influence fitness. Stalk-eyed flies of the dimorphic species Teleopsis dalmanni engage in frequent fights over food resources, as well as over access to harems of females, with larger males typically prevailing when size disparities exist. However, whether and how prior experience influences fighting decisions and outcomes remains unexplored. To test for winner and loser effects in stalk-eyed flies, sexually mature flies were paired in size-mismatched dyads to establish winning and losing experiences. After their first contest, the flies were paired with size-matched individuals and allowed to interact. We determined whether an initial winning or losing experience significantly altered the outcome probabilities in the second size-matched encounter. Initial winning experience did not significantly affect the second interaction, providing no evidence for a winner effect. However, initial losers were significantly more likely to lose a subsequent interaction which provides evidence for a loser effect in stalk-eyed flies. In addition, smaller males experienced an increased probability of losing their second interaction regardless of prior winning or losing experience. This effect was not seen in large males. Our data suggest that the loser effects we observed, which were more pronounced in small males, could result from the energetic costs of fighting that they were less able to absorb than large males.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aggression in animals is modulated by intrinsic and extrinsic factors (Dugatkin 1997). Intrinsic factors such as resource holding potential or the ability of an animal to win a fight may allow individuals to formulate an internal threshold in order to determine the intensity to which they will engage in physical contests (Dugatkin and Druen 2004). Extrinsic factors such as residency and prior experience may also play a role in determining initiation, escalation, and cessation of an encounter (Beaugrand et al. 1996; Grossman 1980; Small et al. 2009). In combination, the use of these factors may facilitate assessment of a rival’s resource holding potential relative to their own and, thus, help to mediate the use of aggression to gain access to limited resources or mates in the environment (Chen et al. 2002; Grossman 1980). Animals can learn to modify their behavior, including with regard to aggressive interactions, based on prior experience. Prior experience in aggressive interactions may allow individuals to gain information about potential opponents in the population by creating a baseline assessment of what to expect from other rivals in the area (Chen et al. 2002). Experience may also allow for better self-assessment and determination of one’s own strength relative to that of a rival’s. Furthermore, with prior experience, individuals can modify energy investments made to single encounters based on previous outcomes (Rutte et al. 2005; Whitehouse 1997).
Winner effects are defined as an increased probability of winning subsequent encounters after a winning experience, whereas loser effects are characterized as an increased probability of losing (or, equivalently, as a decreased probability of winning) subsequent encounters after a losing experience; both have been shown in a variety of taxa (Dugatkin 1997; Hsu et al. 2006; López and Martín 2001; Rutte et al. 2005; Whitehouse 1997). Winner and loser effects are thought to arise, in some cases, as a form of reinforcement learning which encourages increased or decreased aggression with other opponents (Hsu et al. 2006). Mechanistically, these effects may be mediated by physiological changes in neuromodulators and hormones (Oliveira et al. 2009), as well as by gains or losses of energy reserves (Hack 1997). Behavioral modulation based on previous experiences can shorten contests as winners display their abilities near the beginning of an interaction and losers retreat after a quick assessment shows the opponent to be stronger (Whitehouse 1997). Social cues expressed by opponents are also important in gaining information about the entire population (Frost et al. 2007). Winners who display winner effects in subsequent fights tend to be increasingly aggressive and initiate more fights (Hsu and Wolf 2001). This increased aggressive behavior can cause opponents to retreat (Rutte et al. 2005), but it may also force highly escalated interactions (Yurkovic et al. 2006).
Despite the prevalence of winner effects (Hsu and Wolf 1999), loser effects appear to be more important in determining contest outcomes across animal taxa because the consequences of a losing experience may be more influential on subsequent fights (Hsu et al. 2006; Rutte et al. 2005). For many taxa, the cost of fighting can manifest in the form of injuries which cause an opponent to retreat until healed (Chen et al. 2002). For other taxa that do not experience injuries, costs of fighting can stem from time lost foraging or mating, losing access to a resource, or energy costs associated with high-intensity fighting (Hsu and Wolf 2001; Wilkinson and Dodson 1997). Modulating behavior based on prior experience through either winner or loser effects can also save energy since encounters will be settled quickly with avoidance of escalation and injury (Hsu and Wolf 1999; Rutte et al. 2005). Saving time and energy may be especially important to smaller individuals, as energy reserves may have an inverse relationship with body size (Small et al. 2009). Individuals that have previously lost contests tend to initiate fewer contests than winners, participate in fewer interactions, and retreat when confronted with any aggressive display by an opponent (Hsu and Wolf 1999). Winner and loser effects are also time dependent, as large intervals of time between fights can decrease recognition, diminishing, or even negating the degree of winner and loser effects (Hsu et al. 2006). Consequently, an intermediate frequency and time between fights support the evolution of winner and loser effects (Rutishauser et al. 2004).
Teleopsis dalmanni is a sexually dimorphic species in the family Diopsidae, the stalk-eyed flies, which are characterized by eye stalks borne on cephalic appendages (Burkhardt and de la Motte 1983). These flies engage in aggressive interactions multiple times per day for access to food resources and, perhaps more importantly, for access to and dominance of local harems, ownership of which can change each night (De la Motte and Burkhardt 1983; Wilkinson and Dodson 1997). Stalk-eyed flies rely on eyestalk length as a reliable signal for mutual assessment (Burkhardt and de la Motte 1983; Egge et al. 2011; Panhuis and Wilkinson 1999; Small et al. 2009) with some degree of self-assessment likely (Brandt and Swallow 2009). Generally, large stalk-eyed male flies prevail in contests with smaller conspecifics; however, smaller males sometimes win interactions (Panhuis and Wilkinson 1999). A smaller male winning an interaction may be related to differences in the motivational states, which can change throughout an interaction or day depending on availability of resources of the individuals involved (Panhuis and Wilkinson 1999; Wilkinson and Dodson 1997). It is unknown whether or not stalk-eyed flies exhibit winner or loser effects, but they offer a suite of characteristics that make them ideal to test for effects of prior experience. Males engage in multiple interactions per day (Wilkinson 1993), with many of these interactions lasting for extended periods of time (Egge et al. 2011). Thus, the possibility exists that previous experience could alter motivational states and/or energy levels for subsequent interactions and manifest in the form or either winner or loser effects. Furthermore, tracking winner and loser effects may provide clues to the costs associated with fighting.
Methods
Subjects
T. dalmanni is a species of stalk-eyed fly native to the tropics of Asia (Burkhardt and de la Motte 1983; de la Motte and Burkhardt 1983). The current captive-bred populations of stalk-eyed flies were obtained from Gerald Wilkinson (University of Maryland—College Park). Flies in the laboratory were raised in plastic cages (40 × 20 × 22 cm) at 80% humidity and 23–25°C on a 12-h light/dark cycle (Wilkinson 1993). We allowed flies to feed and oviposit ad libitum on ~50 ml of pureed and autoclaved whole ears of corn provided in a plastic cup. Twice per week, these food cups were transferred into larger 500-ml containers lined with moist cotton and larvae were allowed to pupate. This rearing protocol allowed for larval densities and competition over food resources that produced sufficient body size variation in our captive population. Within 24 h of eclosion, the flies were placed in smaller (13.5 × 12 × 13 cm) clear cotton-lined plastic containers and reared individually, with each cage separated by an opaque barrier, and maintained on Ward’s Drosophila food with ad libitum access to water.
Contest protocol
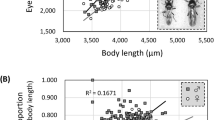
At 2 weeks of age, each fly was CO2 anesthetized and placed lying on its thoracic spines under a dissecting microscope at ×15–20 magnification and photographed using a digital camera. Using Scion Image (National Institutes of Health), we measured eye span and body length to the nearest 0.01 mm from digital images (following Ribak et al. 2009). Subjects were then reared in social isolation as described above and allowed to fully mature, which occurs by 25 days of age on average for male T. dalmanni (Baker et al. 2003). All testing occurred on flies that were between 25–35 days of age to control for potential age effects on contest outcome. Upon maturation, we conducted a two-stage experiment, with the first stage having size-mismatched opponents and the second stage having size-matched opponents based on eye span measurements (mean sizes, 7.76 mm; range 6.34–9.62 mm; Fig. 1). Because eye span and body length are highly correlated in stalk-eyed flies (e.g., Burkhardt and de la Motte 1983; Wilkinson 1993), eye span serves as an accurate measure of body size (see also Small et al. 2009). The first stage with size-mismatched flies was designed to provide reliable winning or losing experiences (Begin et al. 1996), and flies were paired with at least a 5% difference in sizes (average difference = 0.93 mm, range = 0.32–1.84 mm; Hsu et al. 2006). The size-matched flies in stage 2 varied 1% in size differences (average difference, 0.14 mm; range 0.005–0.20 mm; Beacham 2003). Two size-mismatched flies in stage 1 served to test for winner–loser effects in stage 2, whereas two additional naive flies were used as opponents during stage 2 size-matched interactions. Flies with smaller eye spans in each size-mismatched trial, are hereafter referred to as small flies (mean size, 7.64 mm; range, 6.34–8.65 mm) and those with larger eye spans in each size-mismatched trial are hereafter referred to as large flies (mean size, 8.48 mm; range: 6.88–9.62 mm). Each of the four flies used in the trials were painted a different color with an opaque paint pen on their thorax for identification.
Experimental design for testing winner and loser effects in stalk-eyed flies. Both stage 1 and stage 2 flies were subjected to 24 h of starvation. After 24 h, stage 1 flies (Fly 1 and 2), which differed by >5% body size, were presented with a food resource and allowed to interact for 10 min; interactions were video recorded for later analysis. Flies from stage 1 were then placed in new arenas and their corresponding size-matched flies were removed from their initial arenas and placed with their size-matched opponent, separated by a barrier (Fly 1 with Fly 3, Fly 2 with Fly 4). After a 1-h acclimation period, stage 2 flies were presented with a food resource and allowed to interact for 10 min; these interactions were also video recorded
In stage 1 of each trial, two size-mismatched flies (flies 1 and 2 in Fig. 1) were placed in an arena (11 × 6.5 × 5 cm) lined with moist filter paper and separated by a removable barrier. The arena consisted of three wooden walls painted white, a forward facing glass wall for increased lighting, and a removable glass top for introducing flies and through which video recording of interactions took place. The two flies that were size-matched to flies 1 and 2 (flies 3 and 4 in Fig. 1) were placed in separate arenas and treated in the same fashion for stage 2 of the trial. The four flies were then starved for 24 h and allowed to acclimate to the arena in the same climatic conditions in which they were raised. After 24 h, stage 1 flies were presented with a drop of corn medium (approximately 4 mm in diameter) in the center front of the arena using a sterile syringe needle and the partition was removed. The interaction was then digitally recorded for 10 min. Over the course of each 10-min trial, dyads interacted in multiple contests.
After 10 min, stage 1 flies were then paired with their size-matched rivals for stage 2 of the trials. Each fly was removed from their original arena and placed in a new arena (to avoid resident effects, Small et al. 2009) with an opaque barrier. Fly 1 was paired with fly 3 and fly 2 was paired with fly 4 for stage 2 of the trial (Fig. 1). The flies in stage 2 were allowed to acclimate to their new arenas for 1 h (Rutishauser et al. 2004; Whitehouse 1997). After 1 h, a similar drop of corn medium as in stage 1 was added to the arena and flies were allowed to interact for 10 min. The interactions were digitally recorded and multiple contests occurred between the dyads.
Each behavior was mutually exclusive and exhaustive, meaning only one behavior could occur and be recorded at any given time. Behaviors used were solely those which occurred during aggressive interactions as per Egge et al. (2011, as per Table 2 therein). The start of each aggressive contest was defined by either the parallel lining up of eye stalks or an individual’s approach of their opponent, and the end of each contest was determined when the flies were greater than one body length apart or not directly facing each other for three or more seconds. There were multiple contests per trial, as flies will approach an opponent and interact and subsequently move away several times within a 10-min time period of the trial.
Analysis
We scored all interactions using JWatcher, a free behavior analysis program (Blumstein et al. 2007). The behavior of each fly was scored independently of its opponent so that each video was scored twice, once for the winner and once for the loser. A fly was determined to be a loser if it turned away to retreat or if it quickly ran away from its paired conspecific more often than the rival fly over the course of the 10-min interaction (Hsu and Wolf 1999; Egge et al. 2011; Panhuis and Wilkinson 1999). Conversely, the fly that showed fewer retreat behaviors than its rival was scored as the winner. Draws were established when both flies had equal pursuit and retreat behaviors at the end of a contest and were included as a non-winning (i.e., losing) experience. Analyses are based on data from both individuals’ contest outcome in stage 1 and their subsequent outcome of stage 2. The proportion of subsequent interactions won or lost among prior winners and losers was analyzed with a sign test. A sign test was also used to compare the outcome of stage 2 based on size (small versus large), regardless of prior winning or losing experience. T tests were used to compare contest durations between stage 1 and stage 2 contest durations for both small and large flies.
Results
Forty stage 1 trials were conducted. Of those 40, 4 (10%) were draws, and some flies with initial experience were not tested again (N = 5) because flies in reserve died before they could be used in stage 2 trials. Thus, there were 36 size-mismatched interactions that yielded a clear winner and loser (Table 1). The smaller male lost 28 of 40 (70%) size-mismatched stage 1 trials (S = 12, p = 0.008; Table 2). To test for winner and loser effects, regardless of size, initial wins and losses (in stage 1) were compared to subsequent wins and losses (in stage 2) with a sign test. We had 34 (of 40) previous winner trials and 35 (of 40) previous loser trials in stage 2. Only 18 of the initial winners, approximately half, went on to win their size-matched stage 2 interactions (Table 3). However, initial losers were significantly less likely to win their second stage 2 interaction against a size-matched opponent (Table 3). Using a more conservative approach where draws were excluded, losers still lost significantly more of their second interactions (S = 9, p = 0.008), whereas winners did not win significantly more of their subsequent interactions (S = 16, p = 0.180).
When larger males from stage 1 were paired with a size-matched individual in stage 2, they won half of their interactions regardless of previous winning or losing experience (Table 2), meaning there was an equal chance that large experienced males could either win or lose during their second interaction (S = 17, p = 0.567). When the smaller males from stage 1 were paired with a size-matched rival, they only won a quarter of their interactions (Table 2) regardless of previous winning or losing experience, indicating that smaller experienced males were significantly less likely to win their second interaction (S = 10, p = 0.003).
Patterns of wins, losses, and draws for stages 1 and 2 were also summed for large and small flies (Table 1). For large flies, there was no significant relationship between the stage 1 experience and the stage 2 outcomes (S = 8, p = 0.270). For small males, those that initially lost their stage 1 interaction were also more likely to lose their stage 2 interaction (S = 6, p = 0.037). Small initial winners and those that had a draw had no significant association between stage 1 and stage 2 outcomes (S = 4, p = 0.377). Large stage 2 winners with previous experience fought an average of 0:58 in stage 1 fights and fought significantly longer in stage 2 fights (1:34; t 30 = 1.69, p = 0.050). Large stage 2 losers fought an average of 0:49 in stage 1 fights and 1:10 in stage 2 fights, which was not statistically different (t 22 = 1.37, p = 0.090). Large winners and losers did not fight for significantly different contest durations between stage 1 and stage 2 fights (t 26 = −0.80, p = 0.213, and p = 0.174, respectively). Small stage 2 winners fought an average of 1:10 in stage 1 fights and fought significantly shorter fights in stage 2 fights (0:47; t 18 = −1.86, p = 0.039). Small stage 2 losers fought an average of 0:54 in stage 1 fights and 1:13 in stage 2 fights, which was not a statistically significant difference (t 40 = 1.11, p = 0.136). Small stage 2 winners fought for longer contest durations in stage 1 than small stage 2 losers (t 29 = 1.97, p = 0.028). Small stage 2 winners and losers did not fight for significantly different contest durations in stage 2 (t 29 = −1.06, p = 0.148). When large stage 2 winners were compared with small stage 2 winners, large males fought significantly longer (1:34) in their stage 2 contests than small males did (0:47; t 24 = 1.74, p = 0.046).
Discussion
Stalk-eyed flies engage in many, potentially intense, aggressive contests throughout the day (Panhuis and Wilkinson 1999); consequently, incorporating previous experiences in the process of rival assessment and making fighting decisions may be useful for stalk-eyed flies, as in other systems (Hsu et al. 2006). Our data indicate that a previous winning experience does not influence the outcome of subsequent interactions but that a losing experience significantly reduces the likelihood of winning subsequent interactions. The loser effect was particularly pronounced for small males. In fact smaller experienced males also had a significantly decreased chance of winning subsequent interactions, regardless of prior winning or losing experience. For large males, experience did not influence the outcome of stage 2 contests, regardless of previous winning or losing experience, meaning that large flies could win, lose, or draw during their next interaction.
As has been previously reported for stalk-eyed flies (Burkhardt and de la Motte 1983; Panhuis and Wilkinson 1999; Small et al. 2009), small males significantly lost more interactions against large males in size-mismatched interactions. Such size effects are common in other taxa as well (Hack 1997) as the larger competitor in size-mismatched competitions most often either inflicts greater injuries or has energetic reserves to outlast their smaller opponent. Small males that lost their stage 2 interaction also had significantly shorter stage 1 fights than small males did that won their stage 2 interaction. As predicted by mutual assessment models of aggression (Egge et al. 2011; Enquist and Leimar 1983; Panhuis and Wilkinson 1999), these shorter first contests could indicate that smaller males quickly detected significant size differences with their rivals, and thus retreated after a short contest. Small stage 2 winners also fought for significantly longer durations in their stage 1 interactions than in stage 2. Similarly, large stage 2 winners also fought for longer durations in stage 1 than in stage 2. Thus, stage 2 winners could be using their previous experience to exploit their naive opponent. These results could also reflect a physiological or hormonal change due to losing, which was not explicitly tested here (Oliveira et al. 2009). When paired with size-matched individuals, small stage 2 winners also fought for shorter contests than large stage 2 winners. In this case, the smaller pair fought for significantly less time than the larger pair did, suggesting that larger males have ample energy resources left to fight a longer battle even after a primary interaction, while the small males do not have energy reserves to fight lengthy contests.
Fighting in stalk-eyed flies does not yield any detectable injuries, but energy reserves may be at a premium for small animals engaging in high-intensity encounters (Hsu and Wolf 2001). As our data indicate, stalk-eyed flies that have previously lost have a significantly lower probability of winning a subsequent interaction (Table 3). We propose two different potential mechanisms to explain loser effects in stalk-eyed flies. First, losing males could use their losing experience and make future decisions based on the previous outcome of their fight. Second, although detectable injuries may not occur during stalk-eyed fly interactions, energetic costs associated with aggressive interactions can quickly accrue impairing a fly’s ability to win contests, especially for small males. In either case, acting based on a previous losing experience could save energy losses associated with high-intensity interactions if the losing flies relinquish the resource more readily in exchange for less energetic loss. Energy costs, which are acquired with each fight, would add up and compound with each subsequent interaction throughout the day. In our experiment, we found that small males were more likely to lose a fight with a size-matched opponent if they had endured previous contest, regardless of the result (Table 2). A recent study indicated that pairs of small male stalk-eyed flies fight for a shorter duration than pairs of large males (Small et al. 2009). Together, these experiments suggest that smaller males have lower internal energy thresholds and will end fights more quickly, either due to quicker energy depletion or in order to save energy for other activities or future opponents. Smaller males may lack the energy reserves it takes to fight in multiple high-intensity encounters in a day, particularly if they expended more energy in an attempt to win against larger individuals. Flies coming off a recent encounter may choose to withdraw more quickly from a resource when paired against a similarly size-matched opponent, allowing them to save their energy reserves for their next opponent.
For larger males, previous experience did not influence the outcome of their second encounter. Even when their previous encounters were long or intense, larger males may still retain ample energy reserves to win subsequent encounters, even when paired against size-matched opponents. However, for those larger males that lost their second interaction, the decision to retreat may still have been influenced by the loss of energy reserves from their previous interactions. Large stage 2 winners fought significantly longer contests on average in stage 2 than in stage 1; these longer duration contests could likely be explained by a rival assessment hypothesis that includes increasing contest duration as size differences decrease (Enquist and Leimar 1983; Panhuis and Wilkinson 1999). However, for large males to win again after a first interaction, they must have the energetic capacity to perform for longer periods of time, as well as to match aggressive cues from a naive opponent. Naive animals are known to be highly aggressive, possibly because they do not have any idea of their opponent’s strength and may be more likely to initiate an intense interaction with an opponent (Whitehouse 1997); the experienced male, on the other hand, may observe cues of high-intensity behaviors and will choose to avoid a high-intensity interaction if their energy has been depleted or the assessed costs outweigh the benefit of the resource. Large males are not guaranteed a win at any stage of the contest, and losses in energy reserves can be the breaking point for contest resolution and a primary cost during aggressive interactions.
Stalk-eyed flies are aggressive and contests are often settled through high-intensity behaviors (Egge et al. 2011). While escalation to high-intensity behaviors are required to settle some contests, at times it may be more advantageous to avoid these energetically costly and potentially injurious behaviors altogether. One way to shorten contest duration and avoid escalation may be to rely on past encounters and employ winner and loser experiences to contest settlement. Our experiment clearly showed loser effects; losers had a significantly lower probability of winning subsequent interactions. On the other hand, we did not detect winner effects; winners did not have a significantly higher probability of winning subsequent interactions. Smaller males also had an increased probability of losing subsequent interactions, regardless of prior winning and losing experience, suggesting that the loser effects may be mediated through energetic constraints. Regardless of how they are mediated, previous experience effects we describe may be particularly important in stalk-eyed flies because they can engage in multiple contests throughout the day and the resultant energy-saving effect may be critical, especially for smaller males. The assessment of a current rival’s relative size and fighting abilities may be especially advantageous for larger stalk-eyed flies, whereas relying on internal energetic thresholds to determine contest duration may be more important for previous losers and smaller flies.
References
Baker RH, Denniff M, Futerman P, Fowler K, Pomiankowski A, Chapman T (2003) Accessory gland size influences time to sexual maturity and mating frequency in the stalk-eyed fly, Cyrtodiopsis dalmanni. Behav Ecol 14:607–611
Beacham JL (2003) Models of dominance hierarchy formation: effects of prior experience and intrinsic traits. Behaviour 140(10):1275–1303
Beaugrand JP, Payette D, Goulet C (1996) Conflict outcome in male green swordtail fish dyads (Xiphophorus helleri): interaction of body size, prior dominance/subordination experience, and prior residency. Behaviour 133:303–319
Begin J, Beaugrand JP, Zaya R (1996) Selecting dominants and subordinates at conflict outcome can confound the effects of prior dominance or subordination experience. Behav Process 36:219–226
Blumstein DT, Daniel JC, Evans CS (2007) JWatcher. http://www.jwatcher.ucla.edu/
Brandt Y, Swallow JG (2009) Do the elongated eye stalks of Diopsid flies facilitate rival assessment? Behav Ecol Sociobiol 63:1243–1246
Burkhardt D, de la Motte I (1983) How stalk-eyed flies eye stalk-eyed flies: observations and measurements of the eyes of Cyrtodiopsis whitei (Diopsidae, Diptera). J Comp Physiol 151:407–421
Chen S, Yeelin Lee A, Bowens NM, Huber R, Kravitz EA (2002) Fighting fruit flies: a model system for the study of aggression. PNAS 99:5664–5668
De la Motte I, Burkhardt D (1983) Portrait of an Asian stalk-eyed fly. Naturwiss 70:451–461
Dugatkin LA (1997) Winner and loser effects and the structure of dominance hierarchies. Behav Ecol 8(6):583–587
Dugatkin LA, Druen M (2004) The social implications of winner and loser effects. Proc Biol Sci 271(Suppl 6):S488–S489
Egge AR, Brandt Y, Swallow JG (2011) Sequential analysis of aggressive interactions in the stalk-eyed fly Teleopsis dalmanni. Behav Ecol Sociobiol 65(2):369. doi:10.1007/s00265-010-1054-5
Enquist M, Leimar O (1983) Evolution of fighting behaviour: decision rules and assessment of relative strength. J Theor Biol 102:387–410
Frost AJ, Winrow-Giffen A, Ashley PJ, Sneddon LU (2007) Plasticity in animal personality traits: does prior experience alter the degree of boldness? Proc R Soc B 274:333–339. doi:10.1098/rspb.2006.3751
Grossman GD (1980) Food, fights and burrows: the adaptive significance of intraspecific aggression in the bay goby (Pisces: Gobiidae). Oceologia 45:261–266
Hack MA (1997) The energetic costs of fighting in the house cricket, Acheta domesticus L. Behav Ecol 8(1):28–36
Hsu Y, Early RL, Wolf LL (2006) Modulating aggression through experience. In: Brown C, Laland K, Krause J (eds) Fish cognition and behaviour. Blackwell, Oxford, pp 96–118
Hsu Y, Wolf LL (2001) The winner and loser effect: what fighting behaviours are influenced? Anim Behav 61:777–786
Hsu Y, Wolf LL (1999) The winner and loser effect: integrating multiple experiences. Anim Behav 57:903–910
López P, Martín J (2001) Fighting rules and rival recognition reduce costs of aggression in male lizards, Podarcis hispanica. Behav Ecol Sociobiol 49:111–116
Oliveira RF, Silva A, Canario AVM (2009) Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc R Soc B 276:2249–2256
Panhuis T, Wilkinson GS (1999) Exaggerated male eye span influences contest outcome in stalk-eyed flies (Diopsidae). Behav Ecol Sociobiol 46:221–227
Ribak G, Egge AR, Swallow JG (2009) Saccadic head rotations during walking in the stalk-eyed fly (Cyrtodiopsis dalmanni). Proc R Soc B. doi:10.1098/rspb.2008.1721
Rutishauser RL, Basu AC, Cromarty SI, Kravitz EA (2004) Long-term consequences of agonistic interactions between socially naive juvenile American lobsters (Homarus americanus). Biol Bull 207:183–187
Rutte C, Taborsky M, Brinkhof MWG (2005) What sets the odds of winning and losing? Trends Ecol Evol 21:16–21
Small J, Cotton S, Fowler K, Pomiankowski A (2009) Male eyespan and resource ownership affect contest outcome in the stalk-eyed fly, Teleopsis dalmanni. Anim Behav 78:1213–1220
Whitehouse MEA (1997) Experience influences male-male contests in the spider Argyrodes antipodiana (Theridiidae: Araneae). Anim Behav 53:9113–9923
Wilkinson G (1993) Artificial sexual selection alters allometry in the stalk-eyed fly Cyrtodiopsis dalmanni (Diptera: Diopsidae). Genet Res Cam 62:213–222
Wilkinson G, Dodson G (1997) Function and evolution of antlers and eye stalks in flies. In: Choe C (ed) The evolution of mating systems in insects and arachnids. Cambridge University Press, Cambridge
Yurkovic A, Wang O, Basu AC, Kravitz EA (2006) Learning and memory associated with aggression in Drosophila melanogaster. Neuroscience 103(46):17519–17524
Acknowledgments
We thank Sarah Magdanz, Kassidy Boyd, and Ryan Moriarty for stalk-eyed fly care and maintenance. Thank you to Jerry Wilkinson for providing pupae for our own colonies of flies and Sol Redlin for construction of the arenas where the interactions took place. We acknowledge Jerry Husak and Cliff Summers for comments and critiques on the many drafts of the manuscript and Jake Kerby for his help with statistical analysis and comments. This work was funded by National Science Foundation Grant IOB0448060.
Ethical standards
The work in this study was carried out with the highest ethical standards according to the laws of the country in which the work was performed.
Conflicts of interest
The authors declare they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Siva-Jothy
Rights and permissions
About this article
Cite this article
Egge, A.R., Swallow, J.G. Previous experience matters in the stalk-eyed fly Teleopsis dalmanni . Behav Ecol Sociobiol 65, 1731–1737 (2011). https://doi.org/10.1007/s00265-011-1181-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-011-1181-7