Abstract
When animals forage in groups, they can search for food themselves (producer tactic), or they can search for opportunities to exploit the food discoveries of others (scrounger tactic). Both theoretical and empirical work have shown that group-level use of these alternative tactics is influenced by environmental conditions including group size and food distribution, and individual tactic use can be influenced by several measures of individual state, including body condition. Because body condition has been shown to be heritable for various species, social foraging tactics may also be heritable. We looked for evidence of heritability in social foraging tactic use in the zebra finch (Taeniopygia guttata) by testing whether: (1) natural variation in body condition correlates with tactic use, (2) there are family-related differences in body condition, and (3) there are family-related differences in observed tactic use. Tactic use in the zebra finch was significantly related to body condition; individuals with lower body condition scores had a significantly higher use of the scrounger tactic as predicted from variance-sensitive producer–scrounger models. Body-condition scores differed significantly between families, suggesting that this aspect of individual state may have a heritable component. Finally, we recorded significant family-related differences in the use of producer and scrounger alternatives. These results are consistent with heritability in observed tactic use resulting from an inheritance of individual state, in this case body condition, which itself influences tactic use. Understanding how and why individuals differ in their use of alternative tactics is fundamental as it may provide important insights into inter-individual variation in fitness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social foraging is one of the best-studied examples where individuals use alternative tactics (Giraldeau and Caraco 2000). When animals forage in groups, individuals can search for food themselves (producer tactic) or they can search for other individuals that have located food in order to exploit their discovery (scrounger tactic) (Giraldeau and Caraco 2000). Both tactics are maintained in groups via frequency dependence because each tactic does better relative to the other when it is rare (theoretical: Vickery et al. 1991; Giraldeau and Caraco 2000; empirical: Mottley and Giraldeau 2000). Consequently, at some intermediate frequency, the payoff to both tactics is equal. This point is known as the stable equilibrium frequency (SEF) (Mottley and Giraldeau 2000).
The SEF of tactic use within groups is influenced by several conditions. Increasing group size and/or increasing food patch size favour a higher use of the scrounger tactic (theoretical: Vickery et al. 1991; empirical: Coolen 2002; Coolen and Giraldeau 2003). Several empirical studies have noted that when the SEF changes, individuals retain consistent differences in their use of producer–scrounger alternatives (Koops and Giraldeau 1996; Beauchamp 2001; Morand-Ferron et al. 2007). Individuals with a relatively low use of scrounger in condition A continue to have a low use of scrounger relative to their group mates in condition B and vice versa. This suggests that tactic use may also depend on an individual's phenotype. In fact, several individual state variables have been identified that influence individual tactic use, including levels of energy reserves (Lendvai et al. 2004; Wu and Giraldeau 2004; Lendvai et al. 2006), basal metabolic rate (Mathot et al. 2009), vulnerability to predation (Barta et al. 2004; Mathot and Giraldeau 2008), dominance (Liker and Barta 2002; Lendvai et al. 2006), and foraging efficiency (Beauchamp 2006). Given that some of the measures of individual state known to influence tactic use may also be heritable (dominance status: Boag and Alway 1981; body condition: Phillips and Furness 1998; Gosler and Harper 2000; Merilä et al. 2001; Blanckenhorn and Hosken 2003; basal metabolic rate: Rønning et al. 2007), producer–scrounger foraging provides a tractable system for investigating potential for heritability of condition-dependent tactic use (Hazel et al. 1990; Gross and Repka 1998a, b).
Here, we test whether natural variation in body condition, as indicated from the residuals of a regression of body mass against tarsus length (Pärt 1990), is correlated with tactic use decisions in the zebra finch, Taeniopygia guttata. Dynamic variance-sensitive foraging theory predicts that lower levels of energy reserves early in the day should favour a higher use of the scrounger tactic (Barta and Giraldeau 2000). Consistent with this prediction, previous studies have shown that individuals increase their investment in the scrounger tactic following an experimental reduction in energy reserves (Lendvai et al. 2004, 2006). However, it is unclear whether natural variation in body condition is sufficient to generate individual differences in tactic use. We also test whether there are family-related differences in body condition because body condition has been reported to be heritable in other systems (Phillips and Furness 1998; Gosler and Harper 2000; Merilä et al. 2001; Blanckenhorn and Hosken 2003). If producer–scrounger tactic use decisions are influenced by body condition, and body condition is heritable, then observed tactic use may also show heritable variation (Hazel et al. 1990; Gross and Repka 1998a, b). Although we predict family-related differences in tactic use based on earlier studies that have shown heritable variation in some of the measures of condition (i.e. body condition) known to influence producer–scrounger tactic use, heritable variation in tactic use could also arise independent of any condition dependence.
To date, empirical demonstrations of inheritance of tactic use under a conditional strategy are scarce (but see Garant et al. 2003; Thériault et al. 2007), and further investigations into how and why individuals differ in their use of alternative tactics is warranted. If tactic use decisions are influenced by individual state variables which are inherited, it may provide important insights into inter-individual variation in fitness.
Methods
Study subjects and aviaries
Breeding was carried out between January and June 2008 using outbred domesticated zebra finches obtained from a commercial supplier. Adult zebra finches were paired randomly, and each pair was housed in an individual cage (57 × 29 × 42 cm) to allow unambiguous parentage assignment. Each cage contained two perches, one reed nest and nesting materials. Birds were maintained on a 12:12 h light:dark cyle (lights on from 600 to 1800 hours) at 22°C to 24°C. During breeding and outside experimental periods, birds had ad libitum access to water, vitamin-supplemented commercial millet seed mixture, cuttlefish bone, and crushed oyster shells. Additionally, birds were provided with fresh fruits and vegetables three times per week and a protein supplement once per week.
Chicks were removed from their natal cage at independence (\( {\hbox{mean}}\pm {\hbox{SE}} = {42}.{3}\pm 0.{7}\;{\hbox{day}} \) after hatching) and housed in single sex groups with non-family members for a period of 1.5 to 5 months prior to experiments. Thus, the environment experienced by the young after independence was shared among all individuals and was not confounded with family id. Birds were not used in experiments until they had completed development (Zann 1996) and ranged in age from 87–229 days old at the onset of experiments.
Full siblings were not always produced from the same clutch, and therefore ‘age’ and ‘family id’ were not confounded in this study. Individuals from the same family differed in age by 55 ± 21 days at the time of testing (range 14 to 128 days). We formed five mixed-sex flocks of five birds each; the sex ratio in each flock was 2:3 male:female. Each flock was comprised of non-siblings, and six families were replicated between two and five times (by individuals from that family being present in different flocks). Three individuals were used which had no other full sibling in any other flock, and these individuals were excluded from analyses. Within each group, individuals were provided with a unique leg flag color.
Experimental procedure
During experiments, flocks were placed in indoor aviaries (1.5 × 3.8 × 2.3 m high). Immediately before introducing flocks into the aviaries, we measured tarsus length and body mass of each individual to obtain an index of body condition (see below). We used a single measure of body mass in order to minimise handling of the birds during the experiments. Although zebra finches undergo marked diurnal variation in mass (Dall and Witter 1998), data from 34 zebra finches collected in an earlier experiment indicate that despite a significant effect of time of day on body mass (linear mixed effects (LME), F 1,33 = 30.32, p < 0.001), individuals show consistent differences in body mass (log-likelihood ratio test of LME model with and without ‘individual’ as a random effect, p < 0.0001, KJM unpublished data). Thus, given that tarsus length is fixed, between-individual differences in body-condition scores would be consistent over time. All body-mass values used to calculate body-condition scores in this study were taken between 1300 and 1400 hours in order to control for diurnal variation in body mass.
Each aviary contained two large perches and a foraging grid. The foraging grid consisted of two plywood boards, positioned side by side, so that their combined dimensions were 2.2 × 1.1 m, in which a total of 200 wells 1.5 cm in diameter, 0.8 cm deep and spaced at 10 cm intervals were drilled. Foraging grids were placed on tables approximately 90 cm above the aviary floor, which allowed a seated observer to videotape the birds through a one-way mirror using a digital video camera mounted on a tripod. The foraging grid was covered by a sheet of black opaque plastic at all times except during the foraging trials. The perches were placed at the far end of the aviary away from the grids preventing perching birds from seeing directly into the wells.
Birds were given 2 days to become familiar with the aviaries. Food was removed at 1800 hours on the evening of the second day and each evening thereafter (evenings 2 through 9). Trials commenced at 800 hours the following mornings (days 3 through 10). Thus, the birds were deprived of food during the 12 h dark phase, plus an additional 2 h after lights on, durations that were necessary given that they store seeds in their extensible crops for overnight use. Because foraging trials were carried out early in the day, leaving foragers with much time to meet their energy requirements following the trials, we predicted that lower body-conditions scores would be associated with a higher use of the scrounger tactic.
Foraging trials were conducted for 8 days (days 3 through 10), five times per day at 1 h intervals. Before each foraging trial, ten millet seeds were placed in each of 20 randomly selected wells. Trials typically lasted circa 5 min, after which time, all the patches on the foraging grid had been exploited and the birds returned to the perches. Birds were given ad libitum access to food following the final foraging trial each day (from 1240 to 1800 hours).
Each foraging trial was videotaped as the observer called out the location of individuals into the audio channel of the camera to facilitate the identification of the individuals during the playbacks from which data were recorded.
Video analysis
Each video file of foraging trials was assigned a coded name in order to allow a single observer (KJM) to score the videos while remaining blind to the family identity and body-condition score of the individual being observed. Videos were scored in random order using Noldus Observer 5.0 Video Pro. We scored the finding and joining events of each flock member up until the tenth patch discovery of the flock. This procedure was adopted to standardise the effect of patch depletion between trials, by controlling for variation in the time required to locate patches either between flocks or across trials. A finding event was defined as an event where the focal individual was the first to encounter and feed at a patch and can be seen as the outcome of the producer tactic. A joining event was defined as an event where the focal individual moved towards a patch with at least one other bird already there and can be seen as the outcome of the scrounger tactic. Because there were few finding and joining events for a given individual per trial (finding events, range 0 to 7 per individual per trial; joining events, range 0 to 10 per individual per trial), we summed the total number of finding and joining events in a given day to calculate the daily proportion of patches scrounged for each individual (N joining / (N finding + N joining)).
Statistical analyses
All statistical analyses were carried out using R v.2.8.0 (R Development Core Team 2007). We tested whether individual differences in the proportion of patches scrounged related to body condition. Body condition was estimated as the residual from a linear regression of body mass on tarsus length, and each unit of body condition corresponds to a 1-g deviation from the allometrically expected mass (Pärt 1990). Although female zebra finches are structurally larger than males (Zann 1996), there were no sex-related differences in body-condition scores (ANOVA, F 1,20 = 1.03, p = 0.32).
We constructed a LME model with the proportion of patches scrounged as the dependent variable, following arcsine square root transformation to normalise the data (Zar 1999). ‘Body condition’ was included as a fixed effect. Flock, id nested within flock, and day nested within id nested within flock (∼1|flock/individual/day) were included as random effects to account for the non-independence of repeated measures on the same individuals and of individuals tested in the same flock (Pinheiro and Bates 2000). We also tested whether there were significant family-related differences in body condition using an ANOVA with ‘family’ as a fixed effect (‘aov’ function).
Finally, we tested whether family identity was a significant predictor of the use of the scrounger tactic, as indicated by the proportion of patches joined using LME models (‘lme’ function from the ‘nlme’ library, Pinheiro et al. 2008). The proportion of patches joined was used as the dependent variable in the analyses, following an arcsine square root transformation to normalise the data. ‘Family’ was included as a fixed effect. Flock, id nested within flock, and day nested within id nested within flock (∼1|flock/individual/day) were included as random effects.
All tests were carried out using data from 22 individuals over 8 observation days to exclude the 3 individuals that had no full siblings present in other flocks.
Results
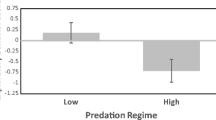
We recorded body-condition indices ranging from −3.7 to 4, and body condition was significantly related to the proportion of patches scrounged (LME, F 1,16 = 5.25, p = 0.035; Fig. 1). Individuals with high body-condition indices had a lower proportion of joining than individuals with low body-condition indices. Body-condition indices differed significantly between families (ANOVA, F 1,5 = 7.51, p < 0.001; Fig. 2). We also recorded significant family-related differences in tactic use (LME, F 1,5 = 3.53, p = 0.034; Fig. 3).
The proportion of patches joined (an index of investment in the scrounger tactic) as a function of ‘body condition’. Values presented are means ± 1 SE calculated over 8 days. Note that while the model calculations were performed on arcsine square root transformed proportions, untransformed proportions are illustrated in the figure. Individuals from the same families are indicated by the same letter
Family-related differences in body condition. Body condition was estimated as the deviation from a linear regression of body mass (g) on tarsus length (mm). Thus, each unit represents 1 g deviation from the allometrically expected mass. Families were ordered on the x-axis from lowest to highest mean body condition, and family names (A through F) are the same as in Fig. 1. The number of individuals per family is indicated in parentheses above the x-axis
The proportion of patches joined (an index of investment in the scrounger tactic) as a function of ‘family’. Note that while the model calculations were performed on arcsine square root transformed proportions, untransformed proportions are illustrated in the figure. Family names (A through F) are the same as in Fig. 1
Discussion
We found that natural variation in body condition was related to differences in the use of producer–scrounger alternatives. Individuals with higher body-condition scores scrounged less than individuals with low body-condition scores, similar to findings from earlier studies on house sparrows (Passer domesticus) which experimentally manipulated body condition (Lendvai et al. 2004, 2006). We also found significant family-related differences in body condition, suggesting that this measure of individual state could be heritable in the zebra finch, as has been reported in other species (Phillips and Furness 1998; Gosler and Harper 2000; Merilä et al. 2001; Blanckenhorn and Hosken 2003). Finally, we recorded family-related differences in tactic use. Thus, taken together, these results are consistent with heritability in observed tactic use resulting from an inheritance of individual state, in this case body condition, which itself influences tactic use.
We used the residuals from a regression of body mass against tarsus length as an index of body condition in this study. There are at least two potential criticisms of the body-condition index used in this study. First, tarsus length alone may be insufficient to account for among-individual variation in structural body size (Gosler and Harper 2000; Green 2001), which would result in inflated family-related differences in residual mass. However, no further inter-individual variance in residual mass could be accounted by wing length (r 2 = 0.005, t 1,23 = 165.81, p = 0.73), which suggests that tarsus length was sufficient to account for among-individual variation in structural body size in our sample of 25 birds (Gosler and Harper 2000). A second criticism of this body-condition index is that residual body-mass values do not identify the specific body components (i.e. fat versus muscle) contributing to the residual variation (Gosler and Harper 2000; Green 2001). However, studies have shown that residuals of body mass against body size reflect differences in the amounts of both muscle and fat (Schulte-Hostedde et al. 2005 and references therein). Given that both muscle and fat can serve as endogenous energy sources for birds (Swain 1992), positive residuals will generally indicate better body condition than negative residuals.
Consistent with our interpretation that the body-condition index used here reflects variation in energy reserves, we observed that individuals with lower body-condition scores scrounged more than individuals with higher body-condition scores. This result can be understood in the context of variance-sensitive foraging behaviour, with lower energy reserves prompting individuals to adopt the tactic that yields less variable rewards, scrounger (Barta and Giraldeau 2000). Although the observed relationship between energy reserves and tactic use in this study is correlative, it is qualitatively similar to results obtained in studies which did experimentally manipulate the level of energy reserves (Lendvai et al. 2004, 2006). To our knowledge, this is the first study to show that natural (i.e. unmanipulated) variation in body condition is associated with differences in social foraging tactic use.
Body condition differed significantly between families, suggesting the possibility that such differences are heritable. Heritability of body condition has been reported elsewhere (Gosler and Harper 2000 and references therein); however, these studies focused on animals under natural conditions. As such, heritable variation in body condition has been interpreted as potentially reflecting heritable variation in the ability to compete for resources (Gosler and Harper 2000). In this study, the differences in body condition reported were measured following an extended period of ad libitum feeding (2 to 5 months post-fledging), and therefore do not reflect differences in access to food. Given that body-condition indices differed significantly between families, this suggests the intriguing possibility that there is heritable variation in the level of energy reserves that individuals maintain. Maintaining energy reserves can be seen as a form of insurance to buffer against uncertainty in future feeding opportunities (Dall and Johnstone 2002). Thus, our finding of family-related differences in the propensity to maintain energy reserves may reflect heritable differences in how animals manage uncertainty.
We recorded significant family-related differences in a trait (body condition) that influences tactic use (producing vs. scrounging) as well as family-related differences in tactic use. It should be noted that the presence of family-related differences in tactic use does not imply that tactic use is fixed, but that a proportion of the variance in tactic use among individuals can be explained by their shared family effect. The study design employed here, comparisons between full siblings, does not allow us to disentangle the cause of family-related differences in body condition and tactic use. Although our results are consistent with heritable variation in tactic use, full siblings in this study shared common parental, environmental and genetic effects, each of which may have contributed to similarities in body condition, and hence tactic use, between siblings. Further studies will be needed to separate these effects.
Although we provide evidence that body condition is related to social foraging tactic use, we recognise that individual tactic-use decisions are influenced by a suite of factors, including factors which are known to show heritable variation (basal metabolic rate: Rønning et al. 2007; Mathot et al. 2009). Nonetheless, several important inferences can be drawn from our findings. Although individuals are able to adjust their investment in producer–scrounger alternatives according to the prevailing conditions (Mottley and Giraldeau 2000; Coolen 2002; Morand-Ferron et al. 2007), individual differences in tactic use resulting from a conditional strategy indicate that individual flexibility in tactic use may be limited. Consequently, the frequency of alternative tactics in a group may vary depending on the specific individuals making up the group (Repka and Gross 1995). Since the payoffs to producer and scrounger alternatives are frequency-dependent (Giraldeau and Caraco 2000; Mottley and Giraldeau 2000), differences in the frequency of tactics between groups would result in differences in intake rates between groups. Although the experiment presented here does not address the process of group formation, our results suggest that individuals would benefit from gauging the condition or tactic use of others in order to join groups of individuals whose use of producer and scrounger tactics best complement their own behavioural profile. More explicit consideration of the process of group formation may provide new insights into producer–scrounger foraging dynamics.
The potential for heritability in observed tactic use under a conditional strategy has been recognised for some time (Hazel et al. 1990; Gross and Repka 1998a, b), but there have been few demonstrations to date. Studies which have found evidence for heritability in tactic use involved tactics which, once adopted by an individual, become fixed (Garant et al. 2003; Thériault et al. 2007). Our results differ in this sense, as they show that genetic and/or shared parental and environmental effects early in life can generate consistent individual differences in a flexible behavioural trait.
References
Barta Z, Giraldeau L-A (2000) Daily patterns of optimal producer and scrounger use under predation hazard: a state-dependent dynamic game analysis. Am Nat 155:570–582
Barta Z, Liker A, Mónus F (2004) The effects of predation risk on the use of social foraging tactics. Anim Behav 67:301–308
Beauchamp G (2001) Consistency and flexibility in the scrounging behaviour of zebra finches. Can J Zool 79:540–544
Beauchamp G (2006) Phenotypic correlates of scrounging behavior in zebra finches: role of foraging efficiency and dominance. Ethology 112:873–878
Blanckenhorn WU, Hosken DJ (2003) Heritability of three condition surrogates in the yellow dung fly. Behav Ecol 14:612–618
Boag DA, Alway JH (1981) Heritability of dominance status among Japanese quail: a preliminary report. Can J Zool 59:441–444
Coolen I (2002) Increasing foraging group size increases scrounger use and reduces searching efficiency in nutmeg mannikins (Lonchura punctulata). Behav Ecol Sociobiol 52:232–238
Coolen I, Giraldeau L-A (2003) Incompatibility between antipredatory vigilance and scrounger tactic in nutmeg mannikins, Lonchura punctulata. Anim Behav 66:657–664
Dall SRX, Witter MS (1998) Feeding interruptions, diurnal mass changes and daily routings of behaviour in the zebra finch. Anim Behav 55:715–725
Dall SRX, Johnstone RA (2002) Managing uncertainty: information and insurance under the risk of starvation. Philos Trans R Soc Lond B Biol Sci 357:1519–1526
Garant D, Dodson JJ, Bernatchez L (2003) Differential reproductive success and heritability of alternative reproductive tactics in wild Atlantic salmon (Salmo salar L.). Evolution 57:1133–1141
Giraldeau L-A, Caraco T (2000) Social foraging theory. Princeton University Press, Princeton, New Jersey
Gosler AG, Harper DGC (2000) Assessing the heritability of body condition in birds: a challenge exemplified by the great tit Parus major L. (Aves). Biol J Linn Soc 71:103–117
Green AJ (2001) Mass/length residuals: measures of body condition or generators of spurious results? Ecology 82:1473–1483
Gross MR, Repka J (1998a) Game theory and inheritance in the conditional strategy. In: Dugatkin LA, Reeve HK (eds) Game theory and animal behavior. Oxford University Press, New York, pp 168–187
Gross MR, Repka J (1998b) Stability with inheritance in the conditional strategy. J Theor Biol 192:445–453
Hazel WN, Smock R, Johnson MD (1990) A polygenic model for the evolution and maintenance of conditional strategies. Proc R Soc Biol Sci Ser B 242:181–187
Koops MA, Giraldeau L-A (1996) Producer–scrounger foraging games in starlings: a test of rate-maximizing and risk-sensitive models. Anim Behav 51:773–783
Lendvai ÁZ, Barta Z, Liker A, Bókony V (2004) The effect of energy reserves on social foraging: hungry sparrows scrounge more. Proc R Soc Biol Sci Ser B 271:2467–2472
Lendvai ÁZ, Liker A, Barta Z (2006) The effects of energy reserves and dominance on the use of social-foraging strategies in the house sparrow. Anim Behav 72:747–752
Liker A, Barta Z (2002) The effects of dominance on social foraging tactic use in house sparrows. Behaviour 139:1061–1076
Mathot KJ, Giraldeau L-A (2008) Increasing vulnerability to predation increases preference for the scrounger foraging tactic. Behav Ecol 19:131–138
Mathot KJ, Godde S, Careau V, Thomas DW, Giraldeau L-A (2009) Testing dynamic variance-sensitive foraging using individual differences in basal metabolic rates of zebra finches. Oikos 118:545–552
Merilä J, Kruuk LEB, Sheldon BC (2001) Natural selection on the genetic component of variance in body condition in a wild bird population. J Evol Biol 14:918–929
Morand-Ferron J, Giraldeau L-A, Lefebvre L (2007) Wild Carib grackles play a producer–scrounger game. Behav Ecol 18:916–921
Mottley K, Giraldeau L-A (2000) Experimental evidence that group foragers can converge on predicted producer–scrounger equilibria. Anim Behav 60:341–350
Pärt T (1990) Natal dispersal in the collared flycatcher: possible causes and reproductive consequences. Ornis Scand 21:83–88
Phillips RA, Furness RW (1998) Measurement of heritability of hatching date and chick condition in parasitic jaegers. Can J Zool 76:2290–2294
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer Verlag, New York
Pinheiro JB, Bates D, DebRoy S, Sarkar D, R Core Team (2008) nlme: Linear and nonlinear mixed effects models. In: R package version 3.1–89
R Development Core Team (2007) R: A language and environment for statistical computing. R Foundation for Statistical Computing. In. See http://www.R-project.org, Vienna, Austria
Repka J, Gross MR (1995) The evolutionarily stable strategy under individual condition and tactic frequency. J Theor Biol 176:27–31
Rønning B, Jensen H, Moe B, Bech C (2007) Basal metabolic rate: heritability and genetic correlations with morphological traits in the zebra finch. J Evol Biol 20:1815–1822
Schulte-Hostedde AI, Zinner B, Millar JS, Hickling GJ (2005) Restitution of mass-size residuals: validating body condition indices. Ecology 86:155–163
Swain SD (1992) Flight muscle catabolism during overnight fasting in a passerine bird, Eremophila alpestris. J Comp Physiol B 162:383–392
Thériault V, Garant D, Bernatchez L, Dodson JJ (2007) Heritability of life-history tactics and genetic correlations with body size in a natural population of brook charr (Salvelinus fontinalis). J Evol Biol 20:2266–2277
Vickery WL, Giraldeau L-A, Templeton JJ, Kramer DL, Chapman CA (1991) Producers, scroungers, and group foraging. Am Nat 137:847–863
Wu G-M, Giraldeau L-A (2004) Risky decisions: a test of risk sensitivity in socially foraging flocks of Lonchura punctulata. Behav Ecol 16:8–14
Zann RA (1996) The zebra finch: a synthesis of field and laboratory studies. Oxford University Press, London
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Upper Saddle River
Acknowledgments
We are grateful to Neeltje Boogert for help with the breeding of the zebra finches, Jan Wijmenga for constructing the foraging grids and for comments on an earlier version of the MS, and Denis Réale and members of the Giraldeau lab for helpful discussions. KJM was supported by an Natural Sciences and Engineering Research Council of Canada (NSERC) graduate scholarship, an NSERC Discovery grant to L.-A.G, and a Bourse d'Excellence de l'UQAM.
Statement of integrity of research and reporting
These experiments conform to guidelines of the Canadian Council for Animal Care and were approved by the UQAM University Animal Care Committee (0108-601-0109). The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Gibson.
Rights and permissions
About this article
Cite this article
Mathot, K.J., Giraldeau, LA. Family-related differences in social foraging tactic use in the zebra finch (Taeniopygia guttata). Behav Ecol Sociobiol 64, 1805–1811 (2010). https://doi.org/10.1007/s00265-010-0992-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-010-0992-2