Abstract
Flight initiation distance, the predator–prey distance when escape begins, is predicted by escape theory to decrease if fleeing entails loss of benefits. Shortening of flight initiation distance during social interactions is known only in males and only in a few species. In a previous study, male, but not female, Sceloporus virgatus lizards had shorter flight initiation distance when interacting with tethered conspecifics. Females in that study were not gravid or close to ovulating. I predicted that flight initiation distance would be shorter in gravid females that perform sidle-hopping displays to reject courtship than in lone females. I tested this prediction and examined effects of social interactions by males with free-ranging conspecifics to ensure that previous findings were not artifacts of tethering and experimental introduction of conspecifics. Flight initiation distance was shorter in females when interacting with males than when alone; it was also shorter in males interacting with either sex. Thus, when beneficial for reproductive reasons, social interaction affects flight initiation distance in females, but at other times, it does not. Lesser shortening of flight initiation distance in females than males may be a consequence of greater social benefit to males and protection of reproductive investment by females.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escape from an approaching predator must be given a high priority, but predation risk is only one of many factors that affect fitness. When prey is engaged in activities that enhance fitness when confronted by predators, its behavior should reflect compromises between escape and the beneficial activity. Optimal escape theory predicts that a prey’s decision regarding how close a predator is allowed to approach before it begins to flee, the flight initiation distance, is based on a tradeoff between predation risk and benefits of any activity that might enhance fitness if the prey does not flee (Cooper and Frederick 2007). The optimal flight initiation distance, which maximizes the prey’s expected fitness when the encounter ends, is based primarily on the prey’s initial fitness, opportunity costs (benefits lost by fleeing), and predation risk, but also on other costs of fleeing, such as energy expenditure and risk of injury (Cooper and Frederick 2007). Loss of opportunity to forage due to escape has the predicted effect of shortening flight initiation distance (Cooper 2000; Cooper and Pérez-Mellado 2004), and the degree of shortening increases with the magnitude of the food reward that must be abandoned (Cooper et al. 2006).
Loss of benefits from social behavior also can be a major opportunity cost of fleeing. Both optimal escape theory and an earlier cost-benefit model (Ydenberg and Dill 1986) predict that flight initiation distance is shorter when prey must abandon social opportunities than when these opportunities are not present. These predictions of tradeoffs of social behaviors with escape behavior have been supported by limited empirical studies.
Social behaviors strongly affect flight initiation distance, but few studies have documented this effect for aggressive, sexual, and parental behaviors. In the lizards Plestiodon (Eumeces) laticeps, Tropidurus hispidus, and Sceloporus virgatus, males have shorter flight initiation distance when interacting aggressively with other males than when alone (Díaz-Uriarte 1999; Cooper 1999; Cooper and Wilson 2007a). In the lizards P. laticeps, Psammodromus algirus, and S. virgatus, males have shorter flight initiation distance when guarding mates or interacting with tethered females than when alone (Cooper 1997a, 1999; Martín and López 1999; Cooper and Wilson 2007a). Effects of parental behavior on escape have been established for some birds and mammals. Among birds, flight initiation distance (flush distance in the bird literature) by incubating females decreased as number of days incubating increased in several species of waterfowl (Forbes et al. 1994; Osiejuk and Kuczyński 2007). In Zenaida doves, parents with chicks had shorter flight initiation distance than incubating parents (Burger et al. 1989). All of these findings are consistent with the hypothesis that risk-taking increases when the cost of losing offspring increases as eggs become more likely to survive to hatch and become chicks. In some species, but not others, flight initiation distance decreased as clutch size increased (Forbes et al. 1994; Osiejuk and Kuczyński 2007), which is also consistent with greater shortening as cost of losing the clutch increased. Among mammals, female African ungulates had longer flight initiation distances when accompanied by neonates that cannot flee quickly than at other times (Caro 2005), as did female groups of female sheep (Ciuti et al. 2008). Although few in number, the cited findings suggest a taxonomically widespread influence of social costs on escape decisions.
Only one study has examined possible effects of lost social opportunities on escape by females of any animal species. Although male striped plateau lizards (S. virgatus) had shorter flight initiation distance when in the presence of either introduced males or females, females had similar flight initiation distance when alone and in the presence of introduced conspecific males or females (Cooper and Wilson 2007a). However, females in that study did not have the orange throat coloration that develops around the time of ovulation and is retained throughout the breeding season (Vinegar 1972; Weiss 2006). Neither were they observed to mate or perform sidle-hops, which are courtship rejection displays used by many female phrynosomatid lizards to indicate their readiness to aggressively deter courtship by males, and which occur in S. virgatus only in females having orange throat coloration (Carpenter and Ferguson 1977; personal observations). Thus, social interaction may have failed to affect flight initiation distance because females were not sexually receptive and were not likely to be harassed by courting males during the study. Therefore, interacting with males was unlikely to have been beneficial. An alternate possibility is that females, unlike males, did not assess the experimentally introduced lizards as appropriate objects of social behavior.
I studied effects of interaction with conspecifics on flight initiation distance in S. virgatus, focusing on naturally occurring interactions and supplementing them by tethered introductions of males to unrestrained females. Based on the greater cost of fleeing while engaging in social behavior, optimal escape theory predicts that flight initiation distance would be (1) shorter for males in the presence of conspecific males or females and (2) shorter for females when performing sidle-hopping rejection displays to males than when not engaging in social behavior.
Materials and methods
Animals and study site
S. virgatus is a small (maximum snout–vent length 71 mm) ambush-foraging phrynosomatid lizard that occupies ground and rocks and climbs trees, rock faces, and steep slopes (Cooper et al. 1994, 2001; Stebbins 2003). When on the ground or on rocks, it escapes by running, stopping on the surface, or entering refuges, which are usually crevices under rocks or in grass clumps at ground level, or trees where it hides on the far side of trunks (Cooper and Wilson 2007a, b; Cooper 2009).
Data were collected during the second half of May 2008 during the breeding season in the western division of the Coronado National Forest in the Chiricahua Mountains of southeastern Arizona (31°52′N, 109°14′W). The study site is at an elevation of approximately 1,800 m and included rocky creek beds and surrounding open woods. All lizards in the study were adults initially sighted on the ground or on low rocks not suitable for use as refuges due to lack of crevices. Observations were made on warm (28.0–32.0°C), sunny days when lizards were fully active (after completion of morning basking) in morning and early afternoon (0900–1400 Mountain Standard Time).
Simulation of predatory attacks and data collection
I simulated an approaching predator to induce escape by the lizards. This method is effective and has been used extensively in studies of S. virgatus, other lizards, and prey from diverse vertebrate and invertebrate taxa (Cooper 2003a, 2008 (review); for lizards including S. virgatus: Cooper and Wilson 2007a, b; Cooper et al. 2009). To begin a trial, I located a lizard by searching visually while walking slowly along transects through the study site. Upon sighting a lizard engaged in social behavior, I moved very slowly to a position where the lizard had a clear view of me and approached at a practiced speed of 75.9 ± 0.7 m/min (n = 10). Data here and throughout are expressed as mean ± 1.0 SE. I continued to approach until the lizard began to flee and then stopped immediately. I then measured flight initiation distance to the nearest 0.1 m.
Several factors that might affect flight initiation distance were controlled. Starting distance affects flight initiation distance in S. virgatus, but only slightly and only at a faster approach speed than used in this study. Starting distances were 5–8 m. Distance between a lizard and the nearest refuge can affect flight initiation distance (Cooper 1997b), but all S. virgatus in this study were very close to refuges, within ca. 1 m. Furthermore, distance to refuge did not affect flight initiation distance in a previous study of this species (Cooper and Wilson 2007b). Because some lizards in campgrounds have uncharacteristically short flight initiation distances due to habituation to human presence (Cooper 2009), data were collected outside campgrounds in areas where lizards are much less frequently exposed to human beings.
Experimental design and analysis
Two experiments were conducted to determine the effects of engaging in social behavior on flight initiation distance, both using independent groups’ designs. Because individuals were observed alone more frequently than while performing sexual or aggressive behaviors, the sequence of trials was not counterbalanced among groups, and more data were collected for lizards not performing social behaviors. To ensure that environmental factors such as time of day and temperature did not bias the findings, all data were collected on days and at times when social behavior occurs. Each lizards was tested only once, and pseudoreplication was avoided by walking along a transect once and then moving to another area. After collecting data for one individual, I observed where it fled and then moved a minimum of 15 m along the transect from the previous trial or 15 m past the post-trial position of the nearer of the two lizards involved in the trial to the transect. None of the tested lizards interacted with the next lizard tested.
In the experiment on effects of male social behavior on flight initiations distance, three groups were tested. These were males interacting with other males by fighting or performing pushup displays (male group, n = 7), males interacting with females by courting or attempting copulation (female group, n = 8), and males not in the presence of a conspecific (nonsocial group, n = 15). Males in the nonsocial group were more than 8 m from the nearest detectable lizard and not interacting if another was visible. In all trials, the focal lizard and any other lizard present were free rather than tethered.
In the experiment on effects of female social behavior on flight initiation distance, only two groups were studied because no females were observed to interact aggressively with other females. The two groups were females that were performing sidle-hopping displays to males, in two cases while males were performing courtship displays (male group) and females not interacting socially (nonsocial group, n = 15). Because only three females were observed responding to free-ranging males, data were collected for an additional seven females responding to males introduced on tethers. These males were captured by noosing. They were tethered by a 0.5-m string attached to the trunk of the body by tape on one end and to the end of a 1.5-m rod on the other. To introduce a tethered male, I approached very slowly and lowered the male to the substrate within 0.3 m of the female to be tested. I then slowly backed away 5–6 m from the focal female and stood immobile for 15 s before approaching.
Analysis of variance (ANOVA) for a single-factor experiment having an independent groups design was used to analyze data in the experiments for each focal sex. Following detection of significant main effects, differences between pairs of groups were tested for significance using Newman–Keuls tests. Because the sample size of females interacting with free-ranging males was small, I performed one ANOVA using only free-ranging males, another ANOVA to examine possible differences in responses by females interacting with free-ranging versus tethered males, and a final ANOVA in which data for trials with free-ranging and tethered males were pooled. In addition, I conducted a 2 × 2 factorial ANOVA comparing the responses of the two focal sexes to males and when alone. The assumptions of heterogeneity of variance and normality of the distribution of flight initiation distance were examined using Levene’s tests and Kolmogorov–Smirnov tests, respectively. For each ANOVA performed, the variances were homogeneous, and distributions did not depart significantly from normality. All of the above calculations were performed using Statistica. Effect sizes were reported as η 2, which ranges from 0.0–1.0, 1.0 being the maximum possible effect size in which all variance is attributable to the difference between groups (Cohen 1992). Tests of significance were two-tailed, with α = 0.05.
Results
Flight initiation distance by males differed significantly among conditions (F 2,27 = 44.15, P < 0.001, Fig. 1). The effect size was large, η 2 = 0.77. Newman–Keuls tests showed that flight initiation distance was significantly shorter when males were engaged in social activities with either other males or females (P < 0.001 each) than when alone. Flight initiation distances did not differ significantly when males were interacting with other males or females (P = 0.82).
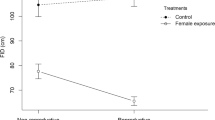
Flight initiation distance was significantly shorter (F 1,16 = 8.24, P = 0.011) for sidle-hopping females in response to free males than for females that were alone. The effect size was intermediate (η 2 = 0.34). Flight initiation distance did not differ significantly between females sidle-hopping in response to free males and tethered males (F 1,8 = 0.12, P = 0.74; η 2 = 0.01). Flight initiation distance was significantly shorter for females sidle-hopping in the presence of males (pooled) than for females that were alone (F 1,23 = 24.57, P = 0.001; Fig. 2). The effect size was η 2 = 0.52 (moderate-large) and corresponded to a flight initiation distance by sidle-hopping females that was only 0.47 than for females that were alone.
The interaction between focal sex and group (male versus alone) was significant (F 1,43 = 6.39, P = 0.015). The main effect of group (F 1,43 = 74.45, P < 0.001) was larger than that of focal sex (F 1,43 = 2.89, P = 0.096). Newman–Keuls tests showed that flight initiation distance did not differ between sexes when no other lizards were nearby (P = 0.56). In both sexes, flight initiation distance was significantly shorter in the presence of other males (males, P = 0.001; females, P < 0.001). When with other males, focal females had significantly longer flight initiation distance than focal males (P = 0.0047). In addition, mean flight initiation distances were significantly longer for males alone than for females with males (P = 0.00016) and for females alone than for males with males (P < 0.001). The clear source of interaction was that flight initiation distances were similar in the sexes when alone, but was longer for focal females than focal males in the presence of males (Figs. 1 and 2).
Discussion
Large effects of social behavior on flight initiation distance were observed in both sexes. Shorter flight initiation distance than when alone was associated in males with close proximity to a conspecific male or female and in females to close proximity to males. These results are consistent with the predictions of optimal escape theory (Cooper and Frederick 2007) under the assumption that social interactions may enhance fitness.
For a male interacting with another male, it may be important to establish or maintain dominance, exclude an intruder or rival from his territory, or to establish a territory, thus gaining advantage in access to females that may lead to higher reproductive success. For a male interacting with a female, obvious advantages that may accrue are mating immediately, inducing newly arrived females to stay in the territory, and maintaining or establishing pair bonds. These advantages are very important in S. virgatus because most hatchlings are sired by the territorial male having closest proximity to a female (Abell 1997). Male mating success in S. virgatus increases with the degree of home range overlap with females (Abell 1999). These factors may contribute to reduction of flight initiation distance by males during the breeding season and during the period prior to seasonal mating when male social behavior may affect later mating opportunities. The importance of such activity in the weeks before mating is indicated by the reduced flight initiation distance in male S. virgatus interacting with conspecifics of either sex in a previous study conducted shortly before breeding began (Cooper and Wilson 2007a).
The shorter flight initiation distance of male S. virgatus in social situations is consistent with all previous work in this and other species for interactions with both males (Díaz-Uriarte 1999; Cooper 1999; Cooper and Wilson 2007a) and females (Cooper 1997b, 1999, 2003b; Martín and López 1999; Cooper and Wilson 2007a). Previous studies had been conducted in experimental enclosures (Díaz-Uriarte 1999) or by introducing tethered individuals to focal lizards (Cooper and Wilson 2007a). The present findings show that the reduction of flight initiation distance in S. virgatus is not an artifact of using tethered stimulus lizards and are consistent with the shorter flight initiation distance by mate-guarding than solitary males in P. laticeps (Cooper 1997b, 1999) and P. algirus (Martín and López 1999).
An intriguing indication that male lizards take social opportunity costs of fleeing into account in making escape decisions is that flight initiation distance differs between color morphs of male Urosaurus ornatus that use different reproductive tactics (Thaker et al. 2009). Males of a morph having a blue throat patch on an orange background are territorial and have shorter flight initiation distance than males that lack the blue patch and are nonterritorial (Thaker et al. 2009). Territorial males defend their territories to restrict access by other males to resident females, which are assets that nonterritorial males lack. Therefore, the cost of fleeing is greater for territorial males that may temporarily lose the ability to defend resident females against mating attempts from other males. Clark’s (1994) asset protection principle and optimal escape theory (Cooper and Frederick 2007) predict that flight initiation distance is shorter in territorial males with blue throat patches than in nonterritorial males lacking blue throat coloration.
Female S. virgatus exhibited consistent reduction of flight initiation distance when interacting socially with free-ranging and tethered males. These findings are consistent with the prediction from optimal escape theory, suggesting that loss of the opportunity to reject male advances by sidle-hopping results in shorter flight initiation distance than in females not interacting with males. Alternative hypotheses are that females permitted closer approach due to dilution of risk by the male and that females are unable to respond optimally to the predator while rejecting males, possibly due to constraints on detection or attention. Risk dilution can be excluded because flight initiation distance by females did not differ between trials with tethered conspecifics stimulus lizards conducted before the breeding season (Cooper and Wilson 2007a). The effect of social contact on flight initiation distance appears to be limited to times when loss of social contact by fleeing is costly, supporting the predictions of escape theory (Ydenberg and Dill 1986; Cooper and Frederick 2007).
In the lizards Holbrookia propinqua and Crotaphytus collaris, females develop bright coloration slightly before being fertilized and begin to sidle-hop and aggressively reject courtship shortly thereafter (Yedlin and Ferguson 1973; Cooper 1992, 1988; Cooper and Crews 1987, 1988). Sidle-hopping may reduce harassment by multiple males that have home ranges overlapping with those of females. If so, females that fail to deter males may be subjected to additional courtship later, making escape costly. I did not test escape responses by females to other females in the present study. Because females rarely direct agonistic displays to other females or interact with them aggressively even in the breeding season, it may be predicted that introduction of tethered females does not affect the flight initiation distance of breeding females.
Breeding, courtship rejection, and orange throat coloration are associated. In 2008, the breeding season came slightly earlier than in previous years, beginning in the second half of May when data were collected: females mated, sidle-hopped, and had orange throat patches. In the first half of May 2006, no females were observed to mate or sidle-hop, and orange throat coloration was absent (Cooper and Wilson 2007a). The lack of relationship between flight initiation distance and presence of males at that time was presumably a consequence of females not being in a reproductive state in which sidle-hopping occurs; courtship rejection then is unnecessary (reviewed by Cooper 1992). Restriction of the effect of male presence on flight initiation distance to times when females sidle-hop supports the contention that female permit closer approach while sidle-hopping because failure to reject males is costly.
Even if failure to reject courtship is costly, females might be less likely to detect a predator while sidle-hopping. However, lizards had undoubtedly detected my approach before sidle-hopping began and presumably continued to monitor my presence. In unrecorded observations in which I moved a short distance away after a trial and then introduced a male again, females repeatedly exhibited short flight initiation distances when interacting with males despite my obvious movements seconds earlier. Ability to respond simultaneously to a male and a predator may be limited, but lizards are able to simultaneously evaluate multiple risks and cost factors (Cooper 2009), including approaches by multiple predators from differing angles (Cooper et al. 2007). Attention deficits during sidle-hopping cannot be excluded, but their effects presumably would be factored into decisions to sidle-hop in close proximity to a predator. Attention deficit has been excluded as an explanation for shortened flight initiation distance by male P. laticeps during social encounters (Cooper 1999).
Male presence leads to a somewhat greater reduction in flight initiation distance in males than females. This suggests that males have more to gain from defeating rivals than females have to gain by rejecting courtship. For females that are fertilizable, rejection might be a means of enforcing mate choice; for gravid females, rejection to reduce harassment in one encounter might have less influence on reproductive success. Males may benefit by interacting with other males to maintain or establish social dominance that may lead to greater reproductive success. Females of many species have less to gain than males by interacting with a male, because mating opportunities are abundant, and males provide no parental care. Because females that sidle-hop have large follicles or have already been fertilized, they have a large asset to protect (Clark 1994). In optimal escape theory, flight initiation distance increases as initial fitness increases (Cooper and Frederick 2007), and assets contribute to initial fitness. Another possibility is that differences in responses by stimulus males to focal lizards affected flight initiation distance.
Some lizards and mammals delay fleeing, when doing so, requires prey to forgo social interactions. In some birds and mammals, flight initiation distance by parents is reduced when their offspring are exposed to predation, potentially reducing parental reproductive success. However, few species belonging to limited taxonomic groups and few factors affecting parental investment have been investigated. Casual observations suggest that similar effects may occur in parental crocodilians, and they may be anticipated in other prey that exhibit parental care. Degree of kinship with offspring might affect escape decisions of helpers and parents. Quantitative studies are needed to assess the effects of breeding condition, dominance status, parental status, and kinship, and other factors that affect opportunity costs on escape decisions.
References
Abell AJ (1997) Estimating paternity with spatial behaviour and DNA fingerprinting in the striped plateau lizard, Sceloporus virgatus. Behav Ecol Sociobiol 41:217–226
Abell AJ (1999) Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphib-Reptil 20:185–194
Burger J, Go8chfeld M, Saliva JE, Gochfeld D, Gochfeld D, Morales H (1989) Antipredator behaviour in nesting Zenaida doves (Zenaida aurita): parental investment or offspring vulnerability. Behaviour 111:129–143
Caro TM (2005) Antipredator defenses in birds and mammals. University of Chicago Press, Chicago
Carpenter GC, Ferguson GW (1977) Variation and evolution of stereotyped behavior in reptiles. In: Tinkle DW, Gans C (eds) Biology of the Reptilia, Vol. 7. Physiology and behaviour A, Vol. 7. Academic, London, pp 335–554
Ciuti S, Pipia A, Ghiandai F, Grignolio S, Apollonio M (2008) The key role of lamb presence in affecting flight response in Sardinian mouflon (Ovis orientalis musimon). Behav Processes 77:408–412
Clark CW (1994) Antipredator behavior and the asset-protection principle. Behav Ecol 5:159–170
Cohen J (1992) A power primer. Psychol Bull 112:155–159
Cooper WE Jr (1988) Aggressive behavior and courtship rejection in brightly and plainly colored female keeled earless lizards (Holbrookia propinqua). Ethology 77:265–278
Cooper WE Jr (1992) Reptilian coloration and behavior. In: Gans C, Crews D (eds) Biology of the Reptilia, Vol. 18. Physiology E, hormones, brain, and behavior. University of Chicago Press, Chicago, pp 298–422
Cooper WE Jr (1997a) Factors affecting risk and cost of escape by the broad-headed skink (Eumeces laticeps): predator speed, directness of approach, and female presence. Herpetologica 53:464–474
Cooper WE Jr (1997b) Escape by a refuging prey, the broad-headed skink (Eumeces laticeps). Can J Zool 75:943–947
Cooper WE Jr (1999) Tradeoffs between courtship, fighting, and antipredatory behavior by a lizard, Eumeces laticeps. Behav Ecol Sociobiol 47:54–59
Cooper WE Jr (2000) Tradeoffs between predation risk and feeding in a lizard, the broad-headed skink (Eumeces laticeps). Behaviour 137:1175–1189
Cooper WE Jr (2003a) Shifted balance of risk and cost after autotomy affects use of cover, escape, activity, and foraging in the keeled earless lizard (Holbrookia propinqua). Behav Ecol Sociobiol 54:179–187
Cooper WE Jr (2003b) Social behavior and antipredatory defense in lizards. In: Fox SF, McCoy JK, Baird TA (eds) Lizard social behavior. Johns Hopkins University Press, Baltimore, pp 107–141
Cooper WE Jr (2008) Visual monitoring of predators: occurrence, cost and benefit for escape. Anim Behav 76:1365–1372
Cooper WE Jr (2009a) Optimal escape theory predicts escape behaviors beyond flight initiation distance: risk assessment and escape by striped plateau lizards (Sceloporus virgatus). Curr Zool 55(2):123–131
Cooper WE Jr (2009b) Fleeing and hiding under simultaneous risks and costs. Behav Ecol 20(3):665–671
Cooper WE Jr, Crews D (1987) Hormonal induction of secondary sexual coloration and rejection behaviour in female earless lizards, Holbrookia propinqua. Anim Behav 35:1177–1187
Cooper WE Jr, Crews D (1988) Sexual coloration, plasma concentration of sex steroid hormones, and responses to courtship in the female keeled earless lizard (Holbrookia propinqua). Horm Behav 22:12–25
Cooper WE Jr, Frederick WG (2007) Optimal flight initiation distance. J Theor Biol 244:59–67
Cooper WE Jr, Pérez-Mellado (2004) Tradeoffs between escape behavior and foraging by the Balearic lizard (Podarcis lilfordi). Herpetologica 60:321–324
Cooper WE Jr, Wilson DS (2007a) Sex and social costs of escaping in the striped plateau lizard Sceloporus virgatus. Behav Ecol 18:764–768
Cooper WE Jr, Wilson DS (2007b) Beyond optimal escape theory: microhabitats as well as predation risk affect escape and refuge use by the phrynosomatid lizard Sceloporus virgatus. Behaviour 144:1235–1254
Cooper WE Jr, Vitt LJ, Caldwell JP (1994) Movement and substrate tongue flicks in phrynosomatid lizards. Copeia 1994:234–237
Cooper WE Jr, Vitt LJ, Caldwell JP, Fox SF (2001) Foraging modes of some American lizards: relationships among measurement variables and discreteness of modes. Herpetologica 57:65–76
Cooper WE Jr, Pérez-Mellado V, Vitt LJ (2004) Ease and effectiveness of costly autotomy vary with predation intensity among lizard populations. J Zool 262:243–255
Cooper WE Jr, Pérez-Mellado V, Hawlena D (2006) Magnitude of food reward affects escape behavior and acceptable risk in Balearic lizards, Podarcis lilfordi. Behav Ecol 17:554–559
Cooper WE Jr, Perez-Mellado V, Hawlena D (2007) Number, speeds, and approach paths of predators affect escape behavior by the Balearic lizard, Podarcis lilfordi. J Herpetol 41:197–204
Cooper WE Jr, Hawlena D, Pérez-Mellado V (2009) Interactive effect of starting distance and approach speed on escape behavior challenges theory. Behav Ecol 20(3):542–546
Díaz-Uriarte R (1999) Anti-predator behaviour changes following an aggressive encounter in the lizard Tropidurus hispidus. Proc R Soc Lond B Biol Sci 266:2457–2464
Forbes MRL, Clark RG, Weatherhead PJ, Armstrong T (1994) Risk-taking by female ducks: intra- and interspecific tests of nest defense theory. Behav Ecol Sociobiol 34:79–85
Martín J, López P (1999) When to come out from a refuge: risk-sensitive and state-dependent decisions in an alpine lizard. Behav Ecol 10:487–492
Osiejuk TS, Kuczyński L (2007) Factors affecting flushing distance in incubating female greylag geese Anser anser. Wildlife Biol 13:11–17
Stebbins RC (2003) A field guide to western reptiles and amphibians, 3rd edn. Houghton Mifflin, Boston
Thaker M, Lima SL, Hews DK (2009) Alternative antipredatory tactics in tree lizard morphs: hormonal and behavioural responses to a predator encounter. Anim Behav 77:395–401
Vinegar MB (1972) The function of breeding coloration in the lizard Sceloporus virgatus. Copeia 1972:660–664
Weiss S (2006) Female-specific color is a signal of quality in the striped plateau lizard (Sceloporus virgatus). Behav Ecol 17:726–732
Ydenberg RC, Dill LM (1986) The economics of fleeing from predators. Adv Study Behav 16:229–249
Yedlin IN, Ferguson GW (1973) Variations in aggressiveness of free-living male and female collared lizards, Crotaphytus collaris. Herpetologica 29:268–275
Acknowledgments
I thank D. S. Wilson and the staff of the American Museum of Natural History’s Southwestern Research Station for hospitality and assistance during the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Downes
Rights and permissions
About this article
Cite this article
Cooper, W.E. Flight initiation distance decreases during social activity in lizards (Sceloporus virgatus). Behav Ecol Sociobiol 63, 1765–1771 (2009). https://doi.org/10.1007/s00265-009-0799-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-009-0799-1