Abstract
Birdsong differs from other sexual traits in that the acquisition process involves learning. Especially in close-ended learning species like the Bengalese finch, conditions experienced during the critical song-learning period can have a profound influence on song quality. Therefore, to understand song evolution from a life-history perspective, we investigated early ontogenetic effects on song quality. In particular, we focused on maternal effects and sibling competition. In asynchronously hatching bird species, the age hierarchy among nestlings affects physical development due to competition for food; mothers may influence this competition by adjusting their investment in each egg according to its sequence in the laying order. To independently assess these effects, chicks of the Bengalese finch were cross-fostered so that the age hierarchies formed in fostered broods were independent of the laying order. Our results indicate that song quality partially reflects early ontogenetic conditions, whereas song duration and note-type repertoire were independent of either laying order or age hierarchy. The syntactical complexity of note order declined over the laying sequence. This finding suggests that the song learning ability is influenced by within-clutch variation in maternal investment toward eggs. Considering that song syntactical complexity is subject to female preference in the Bengalese finch, it is likely that maternal resource allocation strategies play a role in song evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Birdsong is a sexually selected trait that consists of multiple factors. Female songbirds show a preference for various song features, such as performance and elaboration, which have driven the evolution of songs (Catchpole and Slater 2008). Indicator mechanisms of sexual selection suggest that exaggerated sexual traits are preferred by females; such traits indicate male quality by signaling that the male can pay the underlying cost of expressing the trait (Andersson 1994; Andersson and Simmons 2006). In a similar fashion, it is likely that both song performance and elaboration incur costs and thus reflect male quality (reviewed by Gil and Gahr 2002).
Oscine juveniles learn their songs based on model songs of adult males that are heard during a critical period, and the acquired acoustical song features change little after song crystallization, especially in close-ended learners (Marler 1990). Thus, it is possible that a male sings attractive, complex songs simply because he was exposed to good song models in early life. However, most recent literature does not regard song elaboration as completely cost free; it is highly likely that neural and endocrinological controls of song learning impose costs on the acquisition of song complexity (Gil and Gahr 2002; Podos et al. 2004); in this regard, the developmental stress hypothesis predicts that early growth conditions during the song-learning phase affect neural development and therefore song elaboration (Nowicki et al. 1998; Nowicki and Searcy 2004; Nowicki and Searcy 2005). Many studies have shown that the growth of the song control nuclei is obstructed by early developmental stresses such as food restriction and stress hormone administration (Nowicki et al. 2002; Buchanan et al. 2004; Spencer et al. 2005; MacDonald et al. 2006; but see also Gil et al. 2006). Although direct evidence showing the effect of early developmental stress on song elaboration is rather scarce (but see Spencer et al. 2003), Zann and Cash (2007) reported that early dietary restrictions can have detrimental effects on the song complexity, calculated as a principal component of six song variables in the zebra finch, Taeniopygia guttata. Moreover, Soma et al. (2006a) found that the intensity of sibling competition resulting from variable brood composition affects song quality in the Bengalese finch, Lonchura striata var. domestica, probably because sibling competition is tied to chick stress and nutrition levels. Specifically, male birds that were reared in large broods had small adult body size and sang songs with short bout durations, while males from large and male-biased broods sang less complex songs regardless of body size in comparison with the birds from small or female-biased broods (Soma et al. 2006a). These results indicate the possibility that neural development associated with song learning is partially independent of physical development.
Another parameter that might influence the development of song and its neural mechanisms is that of asynchronous hatching. In many avian groups, including songbirds, asynchronous hatching results from the onset of incubation before clutch completion, creating an age and size hierarchy within a brood; this hierarchy may cause higher stress levels (Masello and Quillfeldt 2004) and reduced growth or survival (reviewed by Krebs 1999) of nestlings that hatch later in the hierarchy, due in part to disadvantages in the competition for food. Therefore, in a wide range of asynchronously hatching bird species, mothers skew their investment in eggs by investing more (or less in some cases) in eggs that are laid later than in those that are laid earlier (Schwabl 1993; Schwabl et al. 1997; Gil et al. 1999; Reed and Vleck 2001; Pilz et al. 2003; Soma et al. 2007). Numerous studies have identified relationships between laying sequence and maternal investment in both egg size (e.g., Rutkowska and Cichoń 2002; Magrath et al. 2003) and yolk androgens (e.g., Schwabl 1993; Groothuis and Schwabl 2002). In particular, within-clutch variation in yolk androgen hormones (e.g., testosterone) has been well investigated and can have a profound influence on phenotypic differences in early life such as growth, competitive ability, and begging behavior (Groothuis et al. 2005).
In general, the early development of asynchronously hatching songbirds can depend considerably on laying and hatching order because of biases in both maternal investment and sibling competition. However, these two factors can have opposite effects on development when maternal investment favors later-hatched chicks to compensate for the disadvantages in sibling competition. Soma et al. (2007) conducted a cross-fostering experiment to independently assess the confounding influences of maternal effect (i.e., egg order) and sibling competition (i.e., age hierarchy in the foster brood) on physical development. Specifically, birds from eggs that were laid later in the laying order could grow larger when reared in older positions in the age hierarchy in fostered broods. Surprisingly, however, when reared in younger positions in the age hierarchy in fostered broods, birds from eggs that were produced earlier in the laying order grew larger than birds from later eggs. This phenomenon could not be explained by within-clutch variation in egg size (i.e., earlier eggs being smaller in size). Presumably, the phenomenon that individuals from earlier laying order eggs had heavier adult body mass under intense sibling competition (younger position in the age hierarchy) is attributable to the qualitative differences among eggs such as androgens that are assumed to enhance the competitive ability of chicks (Schwabl 1996; Lipar and Ketterson 2000; Eising et al. 2001; Rutkowska et al. 2007). In the zebra finch, which is closely related to the Bengalese finch (Zeng et al. 2007), yolk testosterone levels in male eggs decline with laying order (Gilbert et al. 2005), whereas egg mass increases with laying order, at least in a non-wild population (Rutkowska and Cichoń 2002; but also see Zann and Runciman 2003). In contrast, long-term maternal effects from yolk androgens have recently gained attention because sex steroids are strongly involved in the expression of sexual traits (Owens and Short 1995; Kimball and Ligon 1999) and in sexual differentiation of the brain and behaviors (Clark and Galef 1995; Schlinger 1998; also see Godsave et al. 2002). Several studies have experimentally demonstrated the link between maternally derived androgens and morphological secondary sexual traits (Strasser and Schwabl 2004; Eising et al. 2006; Rubolini et al. 2006), whereas behavioral traits have been less well studied, except for an inter-specific comparison suggesting the involvement of yolk testosterone in song evolution (Garamszegi et al. 2007).
We investigated the effects of early ontogenetic conditions on both song output (duration) and complexity (note-type repertoire and syntax of note order). Because we expected maternal effects in relation to laying sequence, in addition to the age hierarchy among brood mates (Soma et al. 2007), nestlings were preliminarily checked for egg order and cross-fostered to experimentally controlled broods. To simulate a natural social environment in a simple experimental setting and to avoid the ceiling effect of a single repertoire from the foster father’s song, an unrelated adult male (subtutor) was introduced into the breeding cage around the time of fledging. These breeding situations allowed us to independently assess the potential influences of maternal effects (i.e., egg order) and rearing conditions (i.e., age hierarchy in the foster brood) on song traits.
Materials and methods
Breeding procedures
Bengalese finches maintained in our laboratory (Brain Science Institute, RIKEN) were used as parents. These birds did not include siblings nor parent–offspring. They were paired in individual breeding cages (45 × 45 × 45 cm) equipped with one nest box. Each cage was isolated visually, but not audibly. Throughout the study, the birds were maintained in a controlled environment suitable for breeding (temperature 24 ± 3°C, humidity 30–50%, 14 L:10D photoperiod) and provided with finch seed mixture, foxtail millet coated with egg yolk, water, shell grit, and green vegetables ad libitum. Each nest was checked every morning (i.e., 10:00–11:00 a.m.), and newly laid eggs were marked with nontoxic waterproof colored pens. Newly hatched chicks were marked in the same way and sexed molecularly using DNA extracted from toe clippings (for details see Soma et al. 2007). In most cases, only one chick hatched in a single day in a nest, but in rare cases when two or more chicks hatched on the same day, hatchlings could be assigned to eggs based on the dampness of their nestling down.
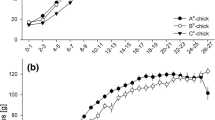
In total, 64 nestlings (32 males and 32 females) from 16 broods of 11 pairs were cross-fostered to create 16 broods. Because two male chicks died around the fledging period, a total of 30 males were analyzed. The cross-fostering was designed to create broods with two males and two females. The pairs of nestlings of the same sex were selected randomly to form a size and age hierarchy in each brood to simulate the situation of sibling competition caused by hatching asynchrony (Fig. 1) After cross-fostering, each brood included an older male (mass [mean ± SD] = 4.8 ± 1.5 g, median age = 9 days), an older female (mass = 5.1 ± 1.4 g, median age = 9 days), a younger male (mass = 2.8 ± 0.9, median age = 6), and a younger female (mass = 3.3 ± 1.6, median age = 5), but this size hierarchy among nestlings gradually faded around fledgling period (see also Soma et al. 2007 for the details of physical development). The age hierarchy formed in fostered broods was independent of egg order to distinguish the effect of egg order and sibling competition caused by age hierarchy (Spearman’s rank correlation between egg order and age hierarchy: r s = 0.292, n = 32, P = 0.12). Nine adult pairs served as foster parents, and seven of them reared two broods at 3-month intervals. An additional nine adult males were used as subtutors and were introduced into the breeding cages around the time of fledging (median age = 27 days) of the subject chicks. Cross-fostered subjects were kept in the breeding cages with foster parents and subtutors until maturity when songs were recorded.
Song measurements
Bengalese finch males have a repertoire of one song, which is only used in courtship displays and not for between-male competition (Okanoya 2004b). Individual songs consist of discrete syllables called notes and can be characterized by the type and order of notes included in a song (Honda and Okanoya 1999; Okanoya 2004a; Soma et al. 2006b). Experimental studies have demonstrated that Bengalese finch females prefer syntactically complex songs (Okanoya 2004b).
When the subject males were fully mature (130–140 days of age), their songs were recorded. Each bird was placed individually in a soundproof room, and its vocal output was recorded using a directional microphone (Sony, ECM-MS975) and a digital audio recorder (Marantz, PMD390) with a sampling rate of 44.1 kHz and 16-bit resolution. At least ten bouts of undirected songs were obtained for each individual. In total, 344 song bouts for 30 subjects were analyzed using the sound analysis software Raven 1.2 (Charif et al. 2004). Based on the computed sonograms, song bout duration and note-type repertoire were calculated for each song bout and averaged for each individual. To estimate the syntactical complexity of note orders, we also calculated the entropy of a first-order Markov model. This model was originally developed in information theory, but is gaining popularity in linguistic analysis and studies of songs sung by Bengalese finch (Nakamura and Okanoya 2004; Soma et al. 2006b) and other songbirds (American redstart, Setophaga ruticilla: Lemon et al. 1993; European starling, Sturnus vulgaris: Gentner and Hulse 2000). The entropy for a song that contains k types of notes is
where P(A i ∩A j ) is the probability that note A i to A j transition is observed among transitions, and P(A i |A i ∩A j ) is the probability that note A i is followed by note A j in transitions that are proceeded by A i . The distinct feature of the entropy model is that it considers transition probabilities. The index will be larger if the note-to-note transition is more versatile (i.e., complex).
The experimental procedures and housing conditions were approved by the Institute’s Animal Experiments Committee. All of the birds were cared for and treated humanely in accordance with the Institutional Guidelines for Experiments using animals (Brain Science Institute, RIKEN).
Statistical analyses
We used mixed effect models to investigate the effect of early ontogenetic conditions on individual differences in three song variables (i.e., average song duration, average note-type repertoire, and song complexity measured as entropy) while considering the non-independence of the data because of the shared rearing conditions. Specifically, we analyzed each song variable using a linear mixed-effect (LME) model in which the identity of the foster brood was treated as a random effect, and the two variables associated with early ontogenetic conditions, i.e., egg order and age hierarchy (older or younger) in the foster brood, and their interaction were treated as fixed effects. To assess the statistical significance of the fixed effects in each model, we first built maximum models containing all of the fixed effects and then used a backward stepwise procedure to sequentially remove nonsignificant terms. The statistical significance of a term was assessed by the change in deviance (which approximates a chi-square distribution) associated with dropping that term from the model. All statistics were performed using R 2.6.1 (R Development Core Team 2007).
Results
The cross-fostering manipulation revealed a partial impact of early ontogenetic conditions on song elaboration by adult birds. Song duration and note-type repertoire were independent of the foster brood identity (p > 0.999) and were not affected by egg order or age hierarchy in the foster brood (p > 0.4, Table 1). However, early ontogenetic conditions had an influence on the syntactical complexity of note order measured as entropy. Although age hierarchy and the interaction of age hierarchy × egg order did not have statistically significant effects on song complexity (LME: age hierarchy: χ 2 = 0.073, p = 0.787; age hierarchy × egg order: χ 2 = 0.814, p = 0.367; Table 1), egg-order influenced the syntactical complexity of songs (LME: χ 2 = 5.941, p = 0.015; Table 1). Birds from eggs that were laid earlier in the egg order acquired more complex songs than did birds from eggs that were laid later, regardless of their position in the age hierarchy of the foster brood (Figs. 2 and 3). In addition, the random effect of brood identity was almost significant, suggesting that song complexity tended to be similar among brood mates (LME: χ 2 = 2.956, p = 0.086; Table 1).
Song examples from a pair of unrelated brood mates that learned songs from the same tutors. The egg order of bird B22 is second and that of bird S4 is fourth. a, b Sonograms of the birds’ songs; the note-type classification is indicated by lowercase letters. c, d Transition diagrams showing the patterns of note-to-note transition for the two songs. The song syntactical complexity was higher for bird B22 (H = 1.243) than for bird S4 (H = 0.924)
Discussion
Song quality partially reflected early ontogenetic conditions in the close-ended learner, the Bengalese finch. A performance-related trait (song duration) was independent of either egg order or age hierarchy in the foster brood, whereas an elaboration-related trait (i.e., syntactical complexity of note order, but not note-type repertoire) was influenced by egg order and also tended to be similar among unrelated brood mates (Table 1). Overall, these results imply that the conditions experienced in earlier periods of ontogeny can have critical effects on the signal development of individual males because their song’s syntactical complexity changes little after sexual maturity (Brenowitz and Beecher 2005) and plays a primary role in mate choice in this species (Okanoya 2004a). Playbacks of experimentally controlled song stimuli have shown that song complexity elicits more reproductive behavior in females (Okanoya 2004b) and that more females choose complex songs in choice tests (Morisaka et al. 2008). Hence, these female preferences for song complexity should provide a trigger for song evolution.
Effect of sibling competition on songs
Indicator mechanisms of sexual selection predict that high-quality signals that attract females should be costly to produce (Andersson 1994; Andersson and Simmons 2006). With regard to song complexity, the developmental stress hypothesis posits that stresses experienced during nestling and fledgling periods can adversely affect brain development and song learning ability (Nowicki et al. 2002; Nowicki and Searcy 2004; Nowicki and Searcy 2005). Although the idea has been tested and supported by experimental manipulations of nutritional conditions and stress hormone levels (Buchanan et al. 2004; Spencer et al. 2004; Zann and Cash 2007), the underlying stress-related ecological factors have received less attention. However, our examinations of the role of sibling competition as such an ecological factor that may affect song learning have produced contrasting results. We previously found that song duration was not the only factor that was affected by poor physical development caused by larger brood size; song syntactical complexity was also impaired in large and male-biased broods (Soma et al. 2006a). However, when we controlled brood size and composition in the present study, we found no significant effect of sibling competition (resulting from age hierarchy) on any song traits. Combining these results, we conclude that age disparities between brood mates do not have as critical an effect on song development as does brood size.
Maternal effect on songs
Our most notable finding is the potent maternal effect on song complexity. Specifically, syntactical complexity, measured as entropy, declined over the laying sequence, but was not affected by the brood’s age hierarchy (Table 1, Fig. 2). Although several previous studies focused on the phenomenon of sibling competition among asynchronously hatched brood mates and its effect on physical growth (reviewed by Krebs 1999), the laying order was not assessed independently because it is naturally confounded with the age hierarchy unless the brood composition is controlled experimentally. However, the amounts of resources such as androgens, carotenoids, vitamins, and immune factors that a mother deposits into her eggs varies with the laying sequence in a wide range of avian species (e.g., Schwabl et al. 1997; Reed and Vleck 2001; Rutkowska and Cichoń 2002; Saino et al. 2002) and such skewed investment within a clutch affects the fitness of the hatched chicks (reviewed by Gil 2003; Groothuis et al. 2005). Testosterone in particular has adaptive effects in some species, but causes harm in others (Groothuis et al. 2005). In zebra finch, which is related to the Bengalese finch, testosterone levels decrease with laying order (Gil et al. 1999; Gilbert et al. 2005), and experimentally elevated yolk testosterone in males contributes to survival in early developmental stages (von Engelhardt et al. 2006). Unfortunately, maternal effects on sexual traits have yet to be investigated in estrildid finches. However, a quantitative genetics study of cross-fostered zebra finch found that female choosiness during mate selection declines in relation to laying sequence (Forstmeier et al. 2004). The authors suggested that this phenomenon resulted from variation in egg content derived from the laying order, but they did not investigate song components. Therefore, ours is the first study to suggest a link between within-clutch variation in maternal effects and song traits. Future studies should examine whether maternal investment, especially of testosterone, is responsible for offspring phenotypic divergence in sexual behavior in adult altricial birds.
The Bengalese finch is a domesticated strain of the white-rumped munia (Lonchura striata) that resulted from selective breeding for plumage color, but without artificial selection for song. The Bengalese finch sings syntactically complicated songs compared to those of the ancestral strain (Honda and Okanoya 1999; Okanoya 2004a). This greater song complexity probably resulted from a shift in song learning ability. Although Bengalese finch females prefer complex songs, such a change in song complexity may not have directly resulted from female choice because aviculturists would have limited free breeding opportunities in the process of domestication. Maternal effect is one candidate factor that could mediate such intersexual selection (Qvarnström and Price 2001). Our current findings illuminate within-clutch variation, but among-clutch variation in maternal effects is also worth considering. In particular, when females mate with males that have attractive songs and the song features are inherited by the sons through vocal learning, a greater investment in reproduction is expected through the production of more offspring (possibly more sons) or heavier, higher-quality eggs. For example, canary (Serinus canaria) females that are exposed to playbacks of more attractive songs lay larger eggs (Leitner et al. 2006) or produce eggs with greater amounts of testosterone (Gil et al. 2004). Similarly, such indirect parental effects may be involved in the evolution of song traits and the song-learning capacity of the Bengalese finch.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson M, Simmons LW (2006) Sexual selection and mate choice. Trends Ecol Evol 21:296–302
Brenowitz EA, Beecher MD (2005) Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci 28:127–132
Buchanan KL, Leitner S, Spencer KA, Goldsmith AR, Catchpole CK (2004) Developmental stress selectively affects the song control nucleus HVC in the zebra finch. Proc R Soc B 271:2381–2386
Catchpole CK, Slater PJB (2008) Bird song: biological themes and variations, 2nd edn. Cambridge University Press, Cambridge
Charif RA, Clark CW, Firstrup KM (2004) Raven 1.2 user’s manual. Cornell Laboratory of Ornithology, New York
Clark MM, Galef BG (1995) Prenatal influences on reproductive life-history strategies. Trends Ecol Evol 10:151–153
Eising CM, Eikenaar C, Schwabl H, Groothuis TG (2001) Maternal androgens in black-headed gull (Larus ridibundus) eggs: consequences for chick development. Proc R Soc B 268:839–846
Eising CM, Müller W, Groothuis TG (2006) Avian mothers create different phenotypes by hormone deposition in their eggs. Biol Lett 2:20–22
Forstmeier W, Coltman DW, Birkhead TR (2004) Maternal effects influence the sexual behavior of sons and daughters in the zebra finch. Evolution 58:2574–2583
Garamszegi LZ, Biard C, Eens M, Møller AP, Saino N (2007) Interspecific variation in egg testosterone levels: implications for the evolution of bird song. J Evol Biol 20:950–964
Gentner TQ, Hulse SH (2000) Female European starling preference and choice for variation in conspecific male song. Anim Behav 59:443–458
Gil D (2003) Golden eggs: maternal manipulation of offspring phenotype by egg androgen in birds. Ardeola 50:281–294
Gil D, Gahr M (2002) The honesty of bird song: multiple constraints for multiple traits. Trends Ecol Evol 17:133–141
Gil D, Graves J, Hazon N, Wells A (1999) Male attractiveness and differential testosterone investment in zebra finch eggs. Science 286:126–128
Gil D, Leboucher G, Lacroix A, Cue R, Kreutzer M (2004) Female canaries produce eggs with greater amounts of testosterone when exposed to preferred male song. Horm Behav 45:64–70
Gil D, Naguib M, Riebel K, Rutstein A, Gahr M (2006) Early condition, song learning, and the volume of song brain nuclei in the zebra finch (Taeniopygia guttata). J Neurobiol 66:1602–1612
Gilbert L, Rutstein AN, Hazon N, Graves JA (2005) Sex-biased investment in yolk androgens depends on female quality and laying order in zebra finches (Taeniopygia guttata). Naturwissenschaften 92:178–181
Godsave SF, Lohmann R, Vloet RP, Gahr M (2002) Androgen receptors in the embryonic zebra finch hindbrain suggest a function for maternal androgens in perihatching survival. J Comp Neurol 453:57–70
Groothuis TG, Schwabl H (2002) Determinants of within- and among-clutch variation in levels of maternal hormones in Black-Headed Gull eggs. Funct Ecol 16:281–289
Groothuis TG, Müller W, von Engelhardt N, Carere C, Eising C (2005) Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Behav Rev 29:329–52
Honda E, Okanoya K (1999) Acoustical and syntactical comparisons between songs of the white-backed munia (Lonchura striata) and its domesticated strain, the Bengalese finch (Lonchura striata var. domestica). Zool Sci 16:319–326
Kimball RT, Ligon JD (1999) Evolution of avian plumage dichromatism from a proximate perspective. Am Nat 154:182–193
Krebs EA (1999) Last but not least: nestling growth and survival in asynchronously hatching crimson rosellas. J Anim Ecol 68:266–281
Leitner S, Marshall RC, Leisler B, Catchpole CK (2006) Male song quality, egg size and offspring sex in captive canaries (Serinus canaria). Ethology 112:554–563
Lemon RE, Dobson CW, Clifton PG (1993) Songs of American Redstarts Setophaga ruticilla: sequencing rules and their relationships to repertoire size. Ethology 93:198–210
Lipar JL, Ketterson ED (2000) Maternally derived yolk testosterone enhances the development of the hatching muscle in the red-winged blackbird Agelaius phoeniceus. Proc R Soc B 267:2005–2010
MacDonald IF, Kempster B, Zanette L, MacDougall-Shackleton SA (2006) Early nutritional stress impairs development of a song-control brain region in both male and female juvenile song sparrows (Melospiza melodia) at the onset of song learning. Proc R Soc B 273:2559–2564
Magrath MJL, Brouwer L, Komdeur J (2003) Egg size and laying order in relation to offspring sex in the extreme sexually size dimorphic brown songlark, Cinclorhamphus cruralis. Behav Ecol Sociobiol 54:240–248
Marler P (1990) Song learning: the interface between behaviour and neuroethology. Phil Trans R Soc Lond B 329:109–114
Masello JF, Quillfeldt P (2004) Are haematological parameters related to body condition, ornamentation and breeding success in wild burrowing parrots Cyanoliseus patagonus ? J Avian Biol 35:445–454
Morisaka T, Katahira K, Okaoya K (2008) Variability in preference for conspecific songs with syntactical complexity in female Bengalese Finches: towards an understanding of song evolution. Ornithol Sci 7:75–84
Nakamura KZ, Okanoya K (2004) Neural correlates of song complexity in Bengalese finch high vocal center. Neuroreport 15:1359–1363
Nowicki S, Searcy WA (2004) Song function and the evolution of female preferences—why birds sing, why brains matter. Ann NY Acad Sci 1016:704–723
Nowicki S, Searcy WA (2005) Song and mate choice in birds: how the development of behavior helps us understand function. Auk 122:1–14
Nowicki S, Peters S, Podos J (1998) Song learning, early nutrition and sexual selection in songbirds. Am Zool 38:179–190
Nowicki S, Searcy WA, Peters S (2002) Brain development, song learning and mate choice in birds: a review and experimental test of the “nutritional stress hypothesis". J Comp Physiol A 188:1003–1014
Okanoya K (2004a) Song syntax in Bengalese finches: proximate and ultimate analyses. Adv Stud Behav 34:297–346
Okanoya K (2004b) The Bengalese finch—a window on the behavioral neurobiology of birdsong syntax. Ann NY Acad Sci 1016:724–735
Owens IPF, Short RV (1995) Hormonal basis of sexual dimorphism in birds: implications for new theories of sexual selection. Trends Ecol Evol 10:44–47
Pilz KM, Smith HG, Sandell MI, Schwabl H (2003) Interfemale variation in egg yolk androgen allocation in the European starling: do high-quality females invest more? Anim Behav 65:841–850
Podos J, Huber SK, Taft B (2004) Bird song: the interface of evolution and mechanism. Annu Rev Ecol Evol Syst 35:55–87
Qvarnström A, Price TD (2001) Maternal effects, paternal effects and sexual selection. Trends Ecol Evol 16:95–100
R Development Core Team (2007) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Reed WL, Vleck CM (2001) Functional significance of variation in egg-yolk androgens in the American coot. Oecologia 128:164–171
Rubolini D, Romano M, Martinelli R, Leoni B, Saino N (2006) Effects of prenatal yolk androgens on armaments and ornaments of the ring-necked pheasant. Behav Ecol Sociobiol 59:549–560
Rutkowska J, Cichoń M (2002) Maternal investment during egg laying and offspring sex: an experimental study of zebra finches. Anim Behav 64:817–822
Rutkowska J, Wilk T, Cichoń M (2007) Androgen-dependent maternal effects on offspring fitness in zebra finches. Behav Ecol Sociobiol 61:1211–1217
Saino N, Bertacche V, Ferrari RP, Martinelli R, Møller AP, Stradi R (2002) Carotenoid concentration in barn swallow eggs is influenced by laying order, maternal infection and paternal ornamentation. Proc R Soc B 269:1729–1733
Schlinger BA (1998) Sexual differentiation of avian brain and behavior: current views on gonadal hormone-dependent and independent mechanisms. Annu Rev Physiol 60:407–429
Schwabl H (1993) Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA 90:11446–11450
Schwabl H (1996) Maternal testosterone in the avian egg enhances postnatal growth. Comp Biochem Physiol A 114:271–276
Schwabl H, Mock DW, Gieg JA (1997) A hormonal mechanism for parental favouritism. Nature 386:231
Soma M, Takahasi M, Ikebuchi M, Yamada H, Suzuki M, Hasegawa T, Okanoya K (2006a) Early rearing conditions affect the development of body size and song in Bengalese finches. Ethology 112:1017–1078
Soma M, Takahasi M, Hasegwa T, Okanoya K (2006b) Trade-offs and correlations among multiple song features in the Bengalese Finch. Ornithol Sci 5:77–84
Soma M, Saito DS, Hasegawa T, Okanoya K (2007) Sex-specific maternal effect on egg mass, laying order, and sibling competition in the Bengalese finch (Lonchura striata var. domestica). Behav Ecol Sociobiol 61:1695–1705
Spencer KA, Buchanan KL, Goldsmith AR, Catchpole CK (2003) Song as an honest signal of developmental stress in the zebra finch (Taeniopygia guttata). Horm Behav 44:132–139
Spencer KA, Buchanan KL, Goldsmith AR, Catchpole CK (2004) Developmental stress, social rank and song complexity in the European starling (Stumus vulgaris). Proc R Soc B 271:S121–S123
Spencer KA, Buchanan KL, Leitner S, Goldsmith AR, Catchpole CK (2005) Parasites affect song complexity and neural development in a songbird. Proc R Soc B 272:2037–2043
Strasser R, Schwabl H (2004) Yolk testosterone organizes behavior and male plumage coloration in house sparrows (Passer domesticus). Behav Ecol Sociobiol 56:491–497
von Engelhardt N, Carere C, Dijkstra C, Groothuis TG (2006) Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc R Soc B 273:65–70
Zann R, Cash E (2007) Developmental stress impairs song complexity but not learning accuracy in non-domesticated zebra finches (Taeniopygia guttata). Behav Ecol Sociobiol 62:391–400
Zann R, Runciman D (2003) Primary sex ratios in zebra finches: no evidence for adaptive manipulation in wild and semi-domesticated populations. Behav Ecol Sociobiol 54:294–302
Zeng SJ, Szekely T, Zhang XW, Lu K, Liu L, Zuo MX (2007) Comparative analyses of song complexity and song-control nuclei in fourteen oscine species. Zool Sci 24:1–9
Acknowledgements
We thank the members of the Biolinguistics team for help in maintaining the birds, and two anonymous reviewers for the comment of the manuscript. This study was supported financially by JSPS Research Fellowships for Young Scientists (DC2-17-10949 and PD-19-7732).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Podos
Rights and permissions
About this article
Cite this article
Soma, M., Hiraiwa-Hasegawa, M. & Okanoya, K. Early ontogenetic effects on song quality in the Bengalese finch (Lonchura striata var. domestica): laying order, sibling competition, and song syntax. Behav Ecol Sociobiol 63, 363–370 (2009). https://doi.org/10.1007/s00265-008-0670-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0670-9