Abstract
In some vertebrate species, parents create a large brood or litter then, in the event of unfavourable ecological conditions, apparently allow the number of offspring to be adaptively reduced through siblicide. But how is sibling aggression regulated so that deaths occur only in unfavourable conditions? One proposed mechanism is brood size-dependent aggression. Two experiments tested for this mechanism by reducing three-chick broods of blue-footed boobies either during or after the period of dominance hierarchy establishment. In neither experiment did aggression of the two eldest and highest ranking chicks decline after removal of the youngest broodmate, in comparison with controls. These results suggest that dominant booby chicks do not become less aggressive to each other after disappearance of their youngest broodmate and that this species does not show brood size dependent aggression. Elder blue-footed booby chicks increase their attacks on broodmates when they receive less food, and this mechanism may be sufficient to tailor brood size to food availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In numerous species of vertebrates, parents initially create and care for large broods or litters then prune them down to a more manageable size in the event of resources turning out to be inadequate to feed all members. This facultative brood reduction (Lack 1954) normally occurs through selective starvation of offspring resulting from begging competition and parental food allocations, but the process can also involve aggressive competition amongst broodmates or littermates and death through expulsion or enforced starvation (Mock and Parker 1997). Parents only rarely interfere obviously in broodmate aggression, so the question of its regulation arises. Assuming that facultative brood reduction is adaptive, how is the intensity of aggression regulated so that marginal offspring are eliminated when food supply is inadequate and allowed to live when it is adequate?
Two non-exclusive behavioural mechanisms have been proposed: aggression dependent on food amount and aggression dependent on brood size. The food amount hypothesis holds that a chick’s tendency to attack its broodmates increases with food deprivation. It has been supported by studies of several avian species in which aggression of dominant broodmates increased when their food ingestion was experimentally restricted (review in Drummond 2001a) and by comparative observations of spotted hyaena Crocuta crocuta litters suggesting that siblicidal aggression is greater when food availability is poorer (Smale et al. 1995; Golla et al 1999; review in Drummond 2006).
The brood size hypothesis holds that chicks respond to the number of accompanying broodmates per se: Aggressiveness increases with brood size and declines when the number of broodmates falls. It is supported by two experiments in which cattle egret chicks (Bubulcus ibis) reduced their aggression after a chick was removed from a brood of three (Mock and Lamey 1991). However, this study is inconclusive because in one experiment, aggression declined in control broods similar to the way it declined in reduced broods, and in the other experiment, aggression may have declined in response to increased food ingestion rather than disappearance of the broodmate (Drummond 2001b). In several avian species, aggression appears to be more intense in large broods than in small broods (Mock et al. 1987), but this could be because per capita ingestion is lower in larger broods (reviews in Lessells 1993; Ploger 1997). In the absence of further experiments, we do not know whether any parentally fed vertebrate infants increase their aggressiveness when their littermates or broodmates are more numerous.
Personal food ingestion and brood size are distinct classes of stimuli to which a nestling could respond by modifying its aggressiveness. Either or both mechanisms could exist (see likely scenarios in “Discussion”), and facultatively siblicidal species whose aggression is food dependent could show brood size dependent aggression as an alternative or backup mechanism (Mock and Lamey 1991). Importantly, when brood size changes naturally or experimentally, the food ingestion of individual brood members is likely to change in consequence, complicating the interpretation of which mechanism accounts for any observed change in aggressiveness.
We made two tests of the brood size hypothesis by analysing the effects of artificial reduction of three-chick broods of the blue-footed booby (Sula nebouxii), a tropical marine pelecaniform that produces broods of one–three chicks. Broods of three chicks show staggered hatching at roughly 4-day intervals, linear dominance hierarchies generally following age hierarchies (Valderrábano-Ibarra et al. 2007), and facultative brood reduction involving greater mortality of second- and, especially, third-hatched chicks (B-chicks and C-chicks). The two highest ranking broodmates are the ones responsible for nearly all aggression in a three-chick booby brood. Experiments on this booby have shown that aggressiveness to broodmates varies with hatch interval (=age/size difference; Osorno and Drummond 1995), previous agonistic experience (winning versus losing against broodmates; Drummond and Osorno 1992) and food ingestion, with pecking rates increasing severalfold when ingestion of parentally supplied food was thwarted (Drummond and García Chavelas 1989). We therefore asked whether blue-footed boobies also adjust their aggression to the number of broodmates present in the nest. Specifically, does aggression between the two highest ranking chicks diminish after the lowest ranking chick disappears? The first experiment used chicks of an age when dominance relationships are being established through intense agonism; the second experiment used chicks of an age when the brood dominance hierarchy is already established and aggression has receded.
Both experiments controlled for possible effects of differential food ingestion in reduced and non-reduced broods. In the first experiment, we compared aggression in three-chick broods temporarily reduced by removal of the C-chick with aggression in three-chick broods that remained intact. To prevent food ingestion becoming a confound in the event of per capita ingestion increasing after experimental brood reduction, we inhibited experimental and control chicks from ingesting parentally provided food for the duration of the 24-h treatments. The second experiment was similar except that we monitored aggression of the two highest ranking chicks during 5 days after removal of the C-chick and allowed normal parental feeding in all broods, experimental and control. The longer trial allowed scope for expression of a delayed response to change in number of broodmates, and normal parental feeding potentially allowed us to reach broader conclusions. In experiment 2, we planned to control for differential ingestion/growth by including weight gain in our analyses. In the event, this was unnecessary because experimental and control chicks showed similar weight gain.
Materials and methods
Study species
In March 2006, we studied the blue-footed boobies on Isla Isabel, off the Pacific coast of Mexico (21°52′ N, 105°54′ W). Eggs are laid on the ground in bare scrapes and both parents incubate the clutch then feed chicks by mouth to mouth regurgitation three–five times per day during a period of 3–4 months (Drummond et al. 1986). Broods of three are always a minority and in many years are scarce, but in 2006 (a non-El Niño year with high, but not exceptional, breeding success; Drummond unpublished data), they were common. Facultative brood reduction in three-chick broods of the study colony is documented by the mortality observed over a period of 23 years: 217 three-chick broods fledged 75% of A-chicks, 69% of B-chicks and 31% of C-chicks, and in 20% of broods, all three broodmates fledged (unpublished data). Deaths of C-chicks occurred throughout the 70-day nestling period, but most occurred at age 1–15 days.
Dominance relationships in three-chick broods are established over the first 4 weeks of life and usually involve the elder chick in each of the three dyads eliciting submissive postures from the younger one by pecking, biting and threatening (Valderrabano-Ibarra et al. 2007). C-chicks seldom peck broodmates at any age because they receive frequent beatings and acquire a submissive personality. In a minority of A–B dyads in broods of two or three chicks, the younger chick establishes dominance over the elder or inverts dominance at some stage before fledging by increasing its attacking over a period of several days (Drummond et al. 1991). Under experimental food deprivation, rates of aggression by dominant chicks in young broods of two chicks increase by several hundred percent (Drummond and García Chavelas 1989).
Experimental samples
During routine population monitoring, all nests in our study area were checked every 3 days from shortly after the start of hatching in the colony, and all chicks were given an individual banding identity. Ages of chicks were either known from hatching between nest checks or, if chicks were present on first inspection of the nest, estimated from growth curves. We assembled samples for both experiments by detecting pairs of three-chick broods of roughly the same age, assigning one brood randomly to experimental treatment (removal of C-chick) and the other to control treatment (non-removal of C-chick), then running the two trials on the same dates.
As revealed by baseline observations on day 1 (see below), all C-chicks were subordinate to both broodmates, but in eight of 42 broods age ranks and dominance ranks did not coincide because the B-chick dominated the A-chick (attacked it more frequently than vice versa). Our analyses categorise chicks according to dominance rank rather than age rank, and we use “dominants” and “subordinates” to refer to the ranks of the two eldest chicks in each brood in relation to each other.
General protocol
At 0650 hours on day 1, the two eldest chicks were paintmarked on head and rump for individual identification using randomly assigned colors (blue, yellow and red) and promptly returned to their nests. Experimental brood reductions involved removing C-chicks from experimental broods during 1 day (experiment 1) or 5 days (experiment 2) after baseline observations on day 1. After weighing of chicks at roughly 1810 hours on day 1 (see below), C-chicks of experimental broods were moved to foster nests and C-chicks of control broods were replaced in their natal nests. On every observation day of each experiment, including day 1, all broods were observed in their natal nests during 6 h, from 0700 to 1000 hours and 1500 to 1800 hours (the hours of greatest nestling activity). An observer sat quietly about 6 m away from one or two focal broods, recording the absolute frequencies of aggressions (pecks+bites) between the two eldest chicks, using the behavioural criteria in Drummond et al. (2003b) and noting which chick attacked. Immediately after observations on day 1 and the final day of each experiment, chicks were weighed, permitting us to calculate changes in mass of the two eldest chicks over the period of experimental or control treatment. Change in mass was assumed to be an index of food ingestion during the treatment period
Experimental C-chicks were inserted into distant foster nests at roughly 1815 hours on day 1 and re-inserted into their home nests at roughly1815 hours on the last day of each experiment. After both insertions, we observed them for 30 min to ensure that they were accepted by the family, and during the period of fostering, we monitored them regularly. Problems arose when three C-chicks that were re-inserted into their natal nests showed insufficient attachment to their natal territory (after 5 days absence) and attempted to wander away. These were monitored closely and returned to the natal territory every time they departed from it until, after 3 days, attachment was re-established.
Experiment 1
Thirteen pairs of three-chick broods were observed on two consecutive days (days 1 and 2), over a block of eight calendar days for the 13 pairs. A-chicks ages were 16.9 ± 1.00 days [X ± standard error (se)] in experimental broods and 16.8 ± 1.16 days in control broods. To limit ingestion, micro-pore adhesive tape was fastened around the necks of A-chicks and B-chicks in experimental and control broods at 1800 hours on day 1 and removed at 1800 hours on day 2.
Tapes did not constrict chicks’ necks but prevented them expanding to pass food. Previous experiments have shown that taping necks in this way prevents most ingestion whilst doing no harm to chicks’ necks (Drummond and García Chavelas 1989). After trying unsuccessfully to swallow food transferred into their mouths by parents, taped chicks shake the food out of their mouths and continue begging, and the food is eventually scavenged by gulls, whiptail lizards or ants. Booby chicks recover well from food deprivation. Young blue-footed booby chicks are fed only three–five times per 12-h day and chicks appear to recover completely from a few days of food deprivation (Drummond and García Chavelas 1989). Furthermore, although younger/subordinate chicks consume substantially less food than their broodmates during the first few weeks of life and grow slowly (Guerra and Drummond 1995), they reach a similar size and mass by fledging (Drummond et al. 1991) and even appear to outperform their broodmates during the first 10 years after fledging (Drummond et al. 2003a). When chicks are artificially deprived of food and dominant broodmates become more aggressive, parents seem a little agitated and increase the frequency of feeding attempts (Drummond and García Chavelas 1989), but in the present experiment, these effects occurred in both experimental and control treatments.
Experiment 2
Eight pairs of three-chick broods were both observed on days 1, 2, 5 and 6 of a 6-day period, over a block of eight calendar days for the eight pairs. No tapes were applied. On day 1, A-chicks ages were 31.7 ± 0.88 days (X ± se) in experimental broods and 30.6 ± 0.26 days in control broods.
Statistical analysis
Using analysis of covariance (ANCOVA), we compared the frequencies of aggressive behaviours of experimental dominants versus control dominants on the days when experimental and control treatments were applied; aggression of each chick on day 1 (baseline) was included as a covariate. A second ANCOVA compared the summed aggression of the two eldest experimental chicks (dominants+subordinates) versus the two eldest control chicks (dominants+subordinates); aggression of each pair of eldest chicks on day 1 was the covariate. These two comparisons were chosen because they are the comparisons most likely to detect a difference, since dominants are responsible for most attacking within A–B dyads (80–94% of attacks at ages 15–30 days; Valderrabano-Ibarra et al. 2007), and because increased attacking by a subordinate dyad member provokes increased attacking by the dominant dyad member (Drummond and Osorno 1992). In experiment 2, days (2, 5 and 6) were treated as a repeated measures factor.
When aggression frequencies were not normally distributed (experiment 1 only), they were log transformed. Weights of experimental and control chicks were compared using t tests, or with the Mann–Whitney U test when variances were unequal. When mean behavioural scores were not significantly different, we report 95% confidence intervals (CI; Colegrave and Ruxton 2003), except in experiment 2, where the repeated measures design does not allow this.
Results
Experiment 1
Weight loss over the 24-h period when experimental and control treatments were applied did not differ between experimental and control dominants (4.4 ± 2.79% versus 6.4 ± 1.23%, respectively, U = 69.50, n 1 = 13, n 2 = 13, p = 0.44, Mann–Whitney U test) or between experimental and control subordinates (6.04 ± 1.22% versus 4.50 ± 1.82%, respectively, U = 72, n 1 = 13, n 2 = 13, p = 0.52). Hence, ingestion was successfully constrained by tapes and probably similar in experimentals and controls.
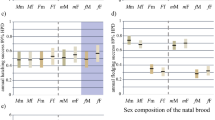
Frequencies of aggression on day 2 did not differ between experimental and control dominants (F 1,23 = 0.561, p = 0.46, CI −0.077 to +0.165; Fig. 1) or between experimental and control pairs of eldest chicks (57.38 ± 19.88 versus 72.46 ± 32.40 aggressions, respectively; F 1,23 = 0.270; p = 0.61, CI −0.086 to +0.144), when aggression on day 1 was taken into account by inclusion as a covariate (Fig. 1). Thus, the evident increase in aggression from day 1 to 2 in our samples was similar in experimental and control chicks, whether we considered only the most dominant chick (Fig. 1) or the two eldest chicks in each brood. Furthermore, we can have some confidence in this negative result since the confidence intervals of the transformed data (shown above) are narrow in relation to the transformed mean scores of experimentals and controls, respectively (dominants—1.39, 1.48; two eldest chicks—1.47, 1.57; see Colegrave and Ruxton 2003).
Experiment 2
Weight increase over the 5-day period when treatments were applied was similar in experimental versus control dominants (21.9 ± 3.08% versus 20.7 ± 2.78%, respectively; t = 0.186, df = 13, p = 0.85) and in experimental versus control subordinates (21.4 ± 3.15 versus 16.7 ± 2.33%, t = −1.217, df = 14, p = 0.24). Hence, removal of the C-chick apparently did not result in the two eldest broodmates receiving more food or growing faster, presumably because parents delivered less food to reduced broods, as generally occurs in other avian species (Ploger 1997).
Frequencies of aggression across days 2, 5 and 6 did not differ between experimental and control dominants (Table 1; Fig. 2) or between experimental and control pairs of eldest chicks (15.9 ± 9.68 versus 9.8 ± 2.68 aggressions, respectively, across days 2, 5 and 6; Table 1), when aggression on day 1 was taken into account. Although aggression of dominant chicks in both treatments seemed to decline a little over the 6 days of the experiment, the apparent effect was similar in experimentals and controls and there was no treatments x days interaction (Table 1; Fig. 2). A similar pattern was evident in pairs of eldest chicks, for which the treatments x days interaction was also non-significant (Table 1). Control dominants showed high variance in aggression scores on days 1 and 2, due to a temporary struggle for dominance between the two eldest chicks of a single brood.
Discussion
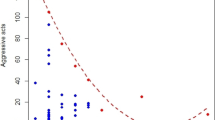
We tested for response to artificial reduction of three-chick broods in different circumstances. The two eldest (and highest ranking) chicks were either at the age when they show the intense aggression characteristic of dominance establishment (experiment 1) or at the age when dominance is already established and low intensity agonism maintains the status quo (experiment 2), as confirmed by lower aggression scores in experiment 2 (cf. Figs. 1 and 2). Senior chicks were either fed normally (experiment 2) or experienced sufficient artificial food deprivation to elicit an apparent increase in aggression (experiment 1; Fig. 1). In none of these circumstances did the data indicate that artificial brood reduction elicited a decrease in aggression by eldest chicks, and nor did a supportive trend appear when behavioural monitoring was extended from 1 to 5 days. We conclude that the two eldest chicks in blue-footed booby broods of three reduce their aggression little or not at all in response to disappearance of C-chicks. Extrapolating to natural unreduced broods, we further suggest that the aggression of A- and B-chicks may not differ between broods of two versus three chicks in response to brood size.
The experiment was carried out in a year when three-chick broods suffered relatively little mortality and experienced an apparently high rate of dominance inversion. Over 23 seasons 80% of three-chick broods in the study colony were reduced (lost 1–3 chicks), whereas in the year of our study, only 21% were reduced and 100%, 93% and 79% of A-, B- and C-chicks, respectively, survived until fledging (age 70 days). And in the year of our study, eight of 42 three-chick broods showed dominance inversion between the two eldest chicks, compared to none of the 18 three-chick broods observed by Valderrabano-Ibarra et al. (2007; p = 0.09, Fisher’s test) in a year when 96% of three-chick broods were reduced (unpublished data). Strictly, our finding that the two eldest blue-footed booby chicks do not reduce their aggression after loss of the C-chick applies to benign ecological circumstances in which mortality of eldest chicks is rare and a substantial minority of broods undergo dominance inversion. However, it may hold for other ecological circumstances; in a small sample of three-chick brown pelican (Pelecanus occidentalis) broods observed under conditions of severe food shortage in which most chicks died, Ploger (1997) similarly found that the two eldest chicks did not reduce their aggression after experimental removal of C-chicks. Nonetheless, we cannot rule out the possibility that brood size affects aggressiveness of blue-footed booby chicks only during food shortage, functioning in that context to accelerate starvation-induced siblicide in three-chick broods.
In functional terms, limiting siblicide to appropriate ecological circumstances seems likely to be better achieved if aggressiveness is cued by supply side variables such as ingestion, hunger or growth than if it responds to demand side variables such as brood size (Mock and Lamey 1991), and the aggressiveness of blue-footed booby chicks is indeed cued by the amount of food recently ingested (Drummond and García Chavelas 1989). However, Mock and Lamey (1991) suggested, reasonably, that in food-sensitive species, brood size dependence could still be valuable as an alternative or backup system for regulating aggression and siblicide. Our results suggest that this is not the case in the blue-footed booby, although our data do not allow us to conclude that the (extremely rare) presence of a fourth chick would not elicit more attacking.
In no vertebrate species has it been conclusively shown that dependent offspring adjust their aggression to the number of sibling competitors, but only three tests have been made (Mock and Lamey 1991; Ploger 1997 and the present study). Where might we most profitably seek brood-size dependent aggression? Species with unpredictable food availability is one suggestion: Egrets and herons appear not to adjust their aggression to the amount of food ingested (Mock et al. 1987, but see Drummond 2001a) and may not do so because recent ingestion by nestlings could be a poor predictor of future parental provision in ardeids (Mock et al. 1987). Another plausible context is where the number of nestmates can exceed the maximum that parents could possibly raise to viable independence, for example, after eggdumping by conspecific or allospecific parasites. To the extent that extra offspring are not needed to replace possible victims of developmental failure or predation during parental care (cf., the insurance and progeny quality hypotheses; Forbes and Mock 1994), automatic elimination of such super-numerary nestmates is likely to be adaptive. A third context is where the number of offspring can be so low (but still more than one!) that no parents in any circumstances could fail to raise them all to viable independence, although higher numbers can exceed parental feeding capacity. That is, when the brood is so small that there is no prospect of feeding competition, presumably sibling aggression can be safely turned off or reduced to levels needed for other functions (such as assuring dominance over siblings after independence; Drummond 2006). Such small broods might result from early losses of eggs or chicks through inviability, predation or deficient parental care. Finally, as suggested by Mock and Lamey (1987), any species for which it is known that broodmate aggression does not vary with food ingestion (if there are such) is a likely candidate. Further tests of the brood size hypothesis should probably be made in these four contexts.
References
Colegrave N, Ruxton GD (2003) Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav Ecol 14:446–450
Drummond H (2001a) A revaluation of the role of food in broodmate aggression. Anim Behav 61:517–526
Drummond H (2001b) The control and function of agonism in avian broodmates. In: Slater PJB, Rosenblatt JS, Snowdon CT, Roper TJ (eds) Advances in the study of behavior. Academic, USA, pp 261–301
Drummond H (2006) Dominance in vertebrate broods and litters. Q Rev Biol 81:3–32
Drummond H, García Chavelas C (1989) Food shortage influences sibling aggression in the blue-footed booby. Anim Behav 37:806–819
Drummond H, Osorno JL (1992) Training siblings to be submissive losers: dominance between booby nestlings. Anim Behav 44:881–893
Drummond H, Gonzalez E, Osorno JL (1986) Parent-offspring cooperation in the blue-footed booby (Sula nebouxii). Behav Ecol Sociobiol 19:365–372
Drummond H, Osorno JL, García C, Torres R, Merchant H (1991) Sexual dimorphism and sibling competition: implications for avian sex ratios. Am Nat 138:623–641
Drummond H, Torres R, Krishnan VV (2003a) Buffered development: resilience after aggressive subordination in infancy. Am Nat 161:794–807
Drummond H, Rodríguez C, Vallarino A, Valderrábano C, Rogel G, Tobón E (2003b) Desperado siblings: uncontrollably aggressive junior chicks. Behav Ecol Sociobiol 53:287–296
Forbes LS, Mock DW (1994) Proximate and ultimate determinants of avian brood reduction. In: Parmigiani S, Vom Saal FS (eds) Infanticide and parental care. Harwood Chur, Switzerland, pp 237–256
Golla W, Hofer H, East ML (1999) Within-litter aggression in spotted hyaenas: effect of maternal nursing, sex and age. Anim Behav 58:715–726
Guerra M del C, Drummond H (1995) Reversed sexual size dimorphism and parental care: minimal division of labour in the blue-footed booby. Behaviour 132:479–496
Lack D (1954) The natural regulation of animal numbers. Clarendon, Oxford
Lessells CM (1993) The cost of reproduction: do experimental manipulations measure the edge of the options set? Etologia 3:95–111
Mock DW, Lamey TC (1991) The role of brood size in regulating egret sibling aggression. Am Nat 138:1015–1026
Mock DW, Parker GA (1997) The evolution of sibling rivalry. Oxford University Press, Oxford
Mock DW, Lamey TC, Williams CF, Ploger BJ (1987) Proximate and ultimate roles of food amount in regulating egret sibling aggression. Ecology 68:1760–1772
Osorno JL, Drummond H (1995) The function of hatching asynchrony in the blue-footed booby. Behav Ecol Sociobiol 37:265–273
Ploger BJ (1997) Does brood reduction provide nestling survivors with a food bonus? Anim Behav 54:1063–1076
Smale L, Holekamp KE, Weldele M, Frank LG, Glickman SE (1995) Competition and cooperation between litter-mates in the spotted hyaena, Crocuta crocuta. Anim Behav 50:671–682
Valderrábano-Ibarra C, Brumón I, Drummond H (2007) Development of a linear dominance hierarchy in nestling birds. Anim Behav 74:1705–1714
Acknowledgements
Fieldwork was financed by a grant from CONACYT (47599Q) and logistical support was provided by the Mexican Navy, the staff of Isla Isabel National Park and the generous fishermen of San Blas and Boca de Camichín. We are grateful to Cristina Carmona, Natalia Lifshitz, Oscar Sanchez, Jaime Villareal and Myrna Hernandez for valuable and dedicated help with field observations and to Jaime Zaldivar, Alejandro Gonzalez, Regina Macedo and Fabrice Dentressangle for helpful comments on the manuscript. Permission for fieldwork was granted by SEMARNAT (permit 0357/06) and experiments complied with relevant Mexican legislation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Leonard
Rights and permissions
About this article
Cite this article
Drummond, H., Rodríguez, C. No reduction in aggression after loss of a broodmate: a test of the brood size hypothesis. Behav Ecol Sociobiol 63, 321–327 (2009). https://doi.org/10.1007/s00265-008-0664-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-008-0664-7