Abstract
Male–male competition has historically been considered the major force driving sexual selection. However, female choice and inter-sexual conflict are increasingly recognized as important influences affecting differential mating and reproductive success. Many females exhibit preferences for particular males; however, male strategies may conflict with females’ ability to obtain their mate preferences. To influence paternity, females must affect both (1) whether or not sexual interactions occur, particularly during the periovulatory period (POP) and (2) the outcome of sexual interactions. This study focuses on the effectiveness of female choice in wild chimpanzees (Pan troglodytes verus). Over 2,600 h of data were collected on two habituated chimpanzee communities in the Taï National Park, Côte d’Ivoire. Female mate preferences were measured by quantifying proceptive and resistance behavior toward males in both the periovulatory period and non-POP phases of estrus. The efficacy of female preference was measured both (1) by measuring success rates of female proceptivity and resistance behaviors and (2) by determining how well measures of female mate preference (proceptivity and resistance rates) predict male mating success. Though male chimpanzees are clearly dominant to females, the results indicate that females could effectively resist male solicitations and, in most cases, unwanted copulations were averted. Both female proceptivity and resistance rates correlate (positively and inversely, respectively) with male mating success in POP. Outside POP, female proceptivity rates corresponded with male mating success, but resistance rates did not. Males (irrespective of rank) that were preferred by females obtained higher mating success compared to other males during the POP, suggesting that females were effective in their mate choice and that, despite clear male dominance, female choice influences paternity in wild chimpanzees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sexual selection was historically thought to be driven predominantly by male–male competition (reviewed by Andersson 1994). However, female choice and male coercion are also considered influential mechanisms for sexual selection (e.g., Trivers 1972; Parker 1979; Brown 1997; Partridge and Hurst 1998), and these factors are increasingly recognized as causes of differential mating and reproductive success among individuals in a wide variety of taxa (Cunningham 1986; Smuts and Smuts 1993; Clutton-Brock and Parker 1995; Wiley and Poston 1996). There is good reason to presume effective female choice is adaptive because, when female mate choice is successful, offspring reap reproductive, developmental, and fitness benefits. Laboratory experiments with mice and fish have demonstrated that females mating with preferred compared to non-preferred partners have higher reproductive success (Drickamer et al. 2000, 2003; Reynolds and Gross 1992). Many other studies demonstrate the enhancement of fitness through female choice (Partridge 1980; Norris 1993; Petrie 1994, Eberhard 1996; Promislow et al. 1998; Cunningham and Russell 2000; Kolm 2001; Gowaty et al. 2002; Hine et al. 2002). In addition, experimentally eliminating female counter-strategies in sexual conflict can be detrimental to females (Rice 1996).
Conflict arises when female and male strategies do not coincide. Male sexual coercion is one possible outcome. Male sexual coercion is the use or threat of force in the form of forced copulation, harassment, restricting female access to other males, interference, and intimidation to increase the probability that a female will mate at some cost to herself (Smuts and Smuts 1993; Clutton-Brock and Parker 1995). Because male dominance is prevalent in many mammalian social systems and males are often substantially larger than females, males have the potential to force females to mate against their will. The ability of females to influence paternity depends on their ability to attain their mate preferences despite of the possibility of male coercion.
Females of many species have well-developed behavioral and physiological sexual strategies to counter the sexual strategies of males and facilitate mate choice (Hrdy 1979; Janson 1984; Manson 1992; van Schaik et al. 2000, 2004; Stumpf and Boesch 2005; Setchell and Kappeleler 2003, for review). Female proceptivity and/or other proxies for female choice such as responsibility for proximity and resistance rates were associated with mating and reproductive success in a variety of male-dominant primates (Dunbar 1984; Wallen and Winston 1984; Keddy 1986; Boinski 1987; Bercovitch 1991; Manson 1992; Perloe 1992; Soltis et al. 1997; Buck 1998; but see Soltis et al. 2001; Birky 2002; see Paul 2002, for review), and similar results are found in other taxa (e.g. Burley et al. 1996). Consequently, although female mate choice may often be compromised by male mating strategies (Smuts and Smuts 1993), female counter-strategies may be effective, allowing females to influence paternity and constituting an important selective force (Tutin 1979; Cords et al. 1986; Huffman 1987; Small 1989, 1990; Manson 1992; Strier 1997).
The relative importance of female choice and male coercion varies across species, and in many taxa both components can affect the selection of sex-specific characteristics (e.g., Howard et al. 1997). To understand how these components of sexual selection influence a species’ evolution, it is important both to identify male and female sexual strategies and to determine their effectiveness in influencing non-random mating. While much is known about male mating strategies (see Cowlishaw and Dunbar 1991, for a review), female strategies, particularly their effectiveness, have received far less attention (Paul 2002).
Although female chimpanzees mate promiscuously (Goodall 1986; Boesch and Boesch-Achermann 2000), females do exhibit preferences for certain males and clear dislike of others (Tutin 1979; Pusey 1980; Nishida et al. 1985, 1990; de Waal 1982; Goodall 1986; Takahata et al. 1996; Stumpf and Boesch 2005). This study focuses on the efficacy of female choice in wild chimpanzees (Pan troglodytes verus). Studies of the effectiveness of female choice are necessary in order to determine the extent to which females influence paternity and have an evolutionary effect on male genetic contribution to future generations.
Individual females may vary in their ability to influence mating outcomes. Chimpanzee males are clearly dominant to females and may hinder females from achieving their reproductive goals. Males may affect female options by aggressively herding them, harassing them, or directing their movements (Goodall 1986). Specifically more aggressive, high-ranking, and persistent males may be able to manipulate, coerce, and intimidate females into mating with them against their will (Tutin 1979; Goodall 1986). High-ranking or older females may be less susceptible to coercion by males and may be more capable of rejecting male solicitations (Goodall 1986; Harcourt 1989; Pusey et al. 1997). Similarly peri-ovulatory (POP) females may be more able to achieve their mating preferences than non-POP females because females may risk higher costs of choice in this phase as the benefits are higher. Alternatively POP females may be less successful because males may benefit more by prevailing in inter-sexual conflict during this phase.
Hypotheses and predictions
In a previous study, we determined individual female mate preferences by identifying individual males toward whom females acted more proceptively and from whom females resisted fewer mating solicitations, compared to their average response to all males (Stumpf and Boesch 2005). We found that, although females mate promiscuously, they do prefer particular males (see also Tutin 1979). However, to successfully exert their mating preferences and potentially affect paternity, females must be able to influence whether or not sexual interactions occur when they are most likely to conceiveFootnote 1. In this paper, we extend our previous study by examining (1) whether females are effective at implementing their mate preferences. If female mating strategies are effective, we expect preferred males to obtain high POP mating success relative to other males to increase their likelihood of paternity. We wish to understand (a) if female mating preferences are effective, (b) how their preferences are achieved, and (c) whether the efficacy of female choice differs between POP and non-POP. Females may attempt to influence paternity through copulation durations if, as expected, there is minimum intromission duration needed for ejaculation to occur. Female ability to implement mate preference should vary with increasing likelihood of conception. Females are expected to be choosier and males more competitive during the fertile periovulatory period than in the non-fertile period, when other strategies such as paternity confusion are expected (e.g. Nunn 1999; Stumpf 2004; Stumpf and Boesch 2005). By comparing sexual interactions between the initial maximum swelling phase (non-POP) and near ovulation (POP), we can investigate if mating patterns reflect differences in the efficacy of female preference across estrus.
We also examine (2) whether there is individual variation in the extent to which females can implement their mate preferences and (3) to what extent male strategies are effective in diminishing the influence of female choice. A female’s success in influencing the outcome of sexual interactions should be positively correlated with female rank and age, negatively correlated with male rank and age, and negatively correlated with measures of male aggression. In contrast, the efficacy of female choice may be greater with males who show more affiliative behaviors. In addition, females may have more or, alternatively, less influence when other females are cycling, as male mate choice may also affect the ability of a female to obtain their preferred reproductive outcome. When more than one female is in estrus, males may prefer one female over another and the non-preferred female may have more difficulty in soliciting a mate. A single estrous female (or preferred female) may be alternatively subject to more intense male competition, limiting her ability to exert choice of mate. Finally, the presence of a higher-ranking male may also positively or negatively affect a female’s ability to influence the outcome of a sexual interaction. Female proceptivity may be less successful because lower-ranked males may be intimidated by higher-ranked males and thus ignore female solicitations (e.g. Matsumoto-Oda 1999). The presence of a higher-ranked male may alternatively increase a female’s ability to resist another male’s solicitations, as a higher-ranked male may suppress coercion attempts by a resisted male.
In summary, a female’s ability to influence the outcome of a sexual interaction should (1) be positively correlated with female factors, such as female rank, age, and ovulatory state; (2) be negatively correlated with male factors, such as male rank and age; and (3) depend in less predictable ways upon other factors, such as the simultaneous cycling of other estrous females and the presence of higher-ranking males.
Materials and methods
Wild chimpanzees live in stable multi-male, multi-female communities. Males are dominant to females and philopatric, while females generally emigrate during adolescence and remain within their new community throughout their lifetime (Goodall 1986). Chimpanzees have a fission–fusion social system in which the community splits into smaller parties and rejoins throughout the course of the day.
More than 2,600 h of data were collected on two habituated chimpanzee (P. t. verus) communities between September 1998 and December 2000 in the Taï National Park in Côte d’Ivoire (see Boesch and Boesch-Achermann 2000, for a detailed description of the study site). At the beginning of the study, the South and North communities included 62 and 32 individuals, respectively, with 25 and 11 adult females and 4 and 3 adult males, respectively. All-day focal follows (Altmann 1974) were conducted on 14 parous cycling females of different ages and ranks (Table 1). The aim was to sample each female during early, middle, and late stages of her maximal swelling, which lasts an average of 10–12 days (Tutin and McGinnis 1981). Data from multiple estrous periods were collected for ten females. During continuous focal animal sampling, RMS recorded all behaviors preceding, during, and after a sexual interaction between the target and an adult male. Data recorded included: (1) the sequence of behaviors, (2) the sexual initiator (male or female), (3) detailed response (resist or cooperate), (4) the outcome (copulation or not), (5) post-mating interactions between the pair, (6) other individuals present, and (7) the behavior of other individuals toward the pair. The end of a sexual interaction sequence was marked by a completed copulation, initiation of a different activity (such as feeding), or one of the two subjects leaving the party. Within the same dyad, successive solicitations were counted independently only if they were separated by more than 10 min from the last solicitation.
The ranks of males and females were determined by the unidirectionality of pant–grunts, which are submissive vocalizations (Bygott 1979; Goodall 1986). Ranks in males were linear. Throughout the text, the name of the male is followed by his rank (in parenthesis). Females were grouped into five rank categories, from high to low, based on all group females. When relative rank between two females could not be determined, they were regarded as occupying the same rank (n=8 dyads). Males and females were grouped into four age categories based on long-term data records (Table 1). Observations were recorded using a Psion Workabout handheld computer (Psion LTC, London, England), with Observer 3.0 software (Noldus, Wageningen, The Netherlands).
Chimpanzee estrus or sexually active phase can be divided into a longer non-periovulatory phase (referred to here as non-POP), during which conception is unlikely, and a 3- to 4-day periovulatory period (POP) during which conception most likely occurs (Elder and Yerkes 1936). Urine was collected daily for hormonal analyses and ovulation was detected using Ovuquick test kits (Quidel, San Diego, CA,USA), which reliably indicate reproductive status and timing of ovulation in apes (Knott 1997; Czekala et al. 1987). Ovulation was presumed to occur on the day the luteinizing hormone surge was detected. These tests were supplemented by laboratory hormonal analyses of urine samples for ovulation based on a sustained PdG rise (see Deschner et al. 2003). Ovulation was detected hormonally in 70% of cycles. Comparisons of presumed ovulatory days based on the use of both measures (N=12) were accurate to ±1 day in 11 of 12 cycles (91.7%) and to within 2 days in 100% of cycles. The limited time period for a copulation to lead to conception is dependent upon sperm and extra-follicular egg viability (Gomendio and Roldan 1993; France 1981; Wilcox et al. 1995), and studies of fertility patterns in apes and humans suggest that most pregnancies result from copulations 1 to 3 days before and including ovulation (Elder and Yerkes 1936; France et al. 1992; Wilcox et al. 1995). Based on this, the periovulatory period was defined in this study as 3 days before and including ovulation. When hormonal testing was not available (nine cycles), POP was defined as 5 days before the first day of detumescence, as hormonal testing of our samples and concurrent detumescence was compatible with this.
The measures used in this study are listed in Table 2. Information for how preference measures were determined is explained in a previous paper (Stumpf and Boesch 2005). Individual males are defined as preferred, eschewed, or neutral by a female (Table 2). For each female, these categories could apply to more than one male (i.e., females may prefer more than one male).
In a sexual interaction initiated by females, males can either resist a female or copulate. In a sexual interaction initiated by males, females can either respond cooperatively (rapidly approaching the soliciting male and presenting for copulation) or resist a male (ignore the solicitation, avoid the male, scream, or leave) (Tutin 1979; for a detailed description of chimpanzee courtship and copulatory behavior, see Tutin 1980). Female resistance does not imply that copulation did not occur. It only indicates female response to male solicitation (Fig. 1). Direct measures of preference, such as variation in female proceptivity and resistance success to male solicitations, are used when estimating the extent to which females are able to exert their preferences. In Fig. 1, sequences 8 and 11 indicate female proceptivity was effective, while sequences 1, 3, and 5 indicate effective resistance. Copulations resulting from sequences 2, 4, 6, 9, and 10 show lack of ability on the part of the female to influence the outcome of a sexual interaction. For each female, all sexual interaction sequences were summarized and placed in a two-by-two table (Table 3) that depicts whether or not the female was able to influence the outcome of a sexual interaction. The efficacy of female preference was measured by both determining how well measures of female mate preference (proceptivity and resistance rates) reflect male mating success and by quantifying the ratio of proceptivity success to proceptivity attempts and the ratio of resistance success to resistance attempts. Copulation duration (in seconds) was measured from time of intromission to disengagement.
All summarized sexual interaction sequences (initiator, response, and result) initiated by either males (left) or females (right). Numbers represent types of sexual interaction sequences (see text). For example, within one sexual interaction, sequence 4 indicates that the male solicited, the female was uncooperative, the male aggressed the female, and the female acquiesced and copulated. Circles indicate that the sequence ended in copulation. Hexagons indicate sequence that the did not end in copulation. Cooperative, individual B responds by presenting or commencing to mount directly after a sexual solicitation from individual A; uncooperative, individual B responds by ignoring, avoiding, or aggressing individual A directly after a sexual solicitation from individual A; persistent, after an uncooperative response from individual B, individual A continues to solicit individual B; forced, male manhandles female and obliges her to copulate; compliant, individual B eventually cooperates after persistent solicitation; noncompliant, individual B continues to avoid copulation solicitation
Because of the small sample sizes, non-parametric tests were applied (SPSS version 13.0, SPSS, Chicago). The only exceptions were in linear correlation analyses where bootstrap techniques were applied to obtain robust test statistics (Rabe-Hasketh and Everitt 2004; Manly 1997; see below). Because some males never associated with nor solicited some estrous females, female resistance behavior could not be expressed for some dyads. We are aware that matrix tests are a more appropriate method for statistical analysis of multiple dyadic interactions; however, such analyses require more columns (Hemelrijk et al. 1999) (i.e., males) than were present in either of the Taï chimpanzee groups, so matrix tests could not be performed on our data. These data were analyzed in two ways. First, to analyze the relationship between two variables such as female proceptivity or resistance and male mating success, we obtained linear correlations between the two variables. To correct for the within subject (female) correlation due to repeated measurements, we clustered the observations within each female (Rabe-Hasketh and Everitt 2004). Because the theoretical distribution of the test statistics is unknown (due to the small sample sizes), we used bootstrap standard error estimates (with 1,000 iterations) to obtain robust test statistics (Rabe-Hasketh and Everitt 2004; Manley 1997). Because the data were negatively skewed, we transformed the data by natural logarithm (ln (x+1)) to obtain normal distributed data with equal variances. The reported results include both N=number of clusters/females and n=number of dyads. These analyses were conducted in STATA 9.1 (StataCorp, College Station, TX, USA).
Second, Wilcoxon signed-ranks tests (Siegel and Castellan 1988) were used to determine whether preferred males obtained more copulations than eschewed males (exact probabilities are reported). One male (MA) died while two females (MY and PE) were still cycling. Thus, for these two females, comparisons of copulation counts among males included only those preceding MA’s demise.
Spearman rank correlations (r s) and Wilcoxon signed-ranks tests were used to test for relationships between female efficacy rate and factors such as female rank, age, stage in estrus, and male rank, age, aggressiveness, and affiliativeness. To examine the influence of male aggressive or affiliative behavior on the efficacy of female choice, all bouts of male aggression (or affiliation) aimed at the target female within 5 min before or during a sexual interaction sequence were compared to sequences in which no male aggression (or affiliation) occurred. Male aggressive behavior was defined as hitting, biting, slapping, threatening, or displaying at a female. Male affiliation was defined as grooming, food sharing, or support in interactions with conspecifics. The efficacy of female choice in interactions with more aggressive (or affiliative) males (as measured by aggression or affiliation/dyadic association time) was also examined. Non-parametric (Loess) regression lines are given to indicate trends on graphs.
To test whether the presence of a male more dominant than the paired male (or the existence of a simultaneously cycling female) influences the efficacy of female choice in a sexual interaction sequence, all sequences occurring in the presence of a more dominant male (or another estrous female) were compared to all sequences in the absence of a more dominant male (or other estrous females). When non-POP and POP results were similar, both test results were reported but only POP results were presented graphically.
Binomial tests were used to determine if the observed frequencies of behaviors such as proceptivity success differed from expected frequencies under chance conditions. To study the influence of two or more variables (such as phase, female and male rank and age, male aggression, male grooming, and dyadic association time) on a continuous dependent variable such as resistance or proceptivity success, mixed-model multiple regressions were performed (Tabachnik and Fidell 2001). The assumptions of multiple regression were met through analyses of residual scatterplots for normality, homoscedasticity, and linearity between the residuals (female proceptivity and resistance rates in each phase) and predicted dependent variables, such as rank and age (Tabachnik and Fidell 2001). To control for multiple testing of the same individuals, the variable “individual” (female and male) was used to reduce dependency. Model selection was based on an adjusted r 2 value, the amount of which denoted the percentage of variance accounted for by the independent variables, while accounting for sample size and the number of variables. For all tests, the α-level of significance was 0.05 (two-tailed).
Results
Data for this study included a total of 1,449 sexual interactions. In 206 cases, the complete sequence of events in a sexual interaction (initiator, response, and outcome) was unknown. Of the remainder, 13 additional sexual interactions were excluded due to interruptions by a third adult before the respondent’s reaction. Thus, the results from this study are based on a total of 1,230 sexual interactions. From these, on average (summary over all 14 individuals), females initiated 23.7% of sexual interactions. Most female solicitations were accepted by males (78.7%), thus leading to copulation, while 21.3% were rejected (sign test, P<0.05).
Males initiated the majority of sexual interactions (76.3%, N=938). Females responded cooperatively to 71.7% of male solicitations and attempted to resist 28.3%. When females resisted male solicitations, they were successful in avoiding copulation in the majority of cases (69.2%) (sign test, P<0.05).
Are females successful in implementing their mate preferences?
Female proceptivity rates correlated significantly with male mating success in POP (POP, r=0.521, z=4.84, N=12, n=38, P=0.001; (Fig. 2a). Female resistance rates were significantly correlated with copulations in POP (r=−0.381, z=−3.00, N=13, n=31, P=0.003). Excluding dyads in which the male rarely solicited (less than three solicitations), resulted in an even stronger correlation (r=−0.470, z=−3.50, N=13, n=25, P=0.001) (Fig. 2b).
During non-POP, female proceptivity rates correlated significantly with male mating success (r=0.554, z=7.00, N=14, n=47, P=0.0001; Fig. 3a), but resistance rates did not (r=−0.130, z=−0.61, N=14, n=40, P=0.541; Fig. 3b).
Are all individual females able to implement mate choice?
To test if all individual Taï chimpanzee females are able to influence copulation counts, we controlled for individual effects in both POP and non-POP. In POP, pairwise comparisons of eschewed and preferred males for each female suggest that preferred males (based on resistance rates) obtained more copulations than eschewed males (T=1, N=9, P=0.023; Fig. 4a). Based on proceptivity rates, the majority of preferred males obtained more copulations than eschewed males in POP, but the results did not reach significance (T =2, N=9, P=0.07; Fig. 4b). For all females, on average, 75.2% of all copulations in POP were with preferred males based on resistance (N=8, SD=0.222; 51.3% based on proceptivity N=9, SD=0.299). The remainder was divided among all other males.
During non-POP, preferred males (based on proceptivity) obtained more copulations than eschewed males (T=3, N=11, P=0.02; Fig. 5a), but this was not the case for preferred males based on resistance rates (T =4, N=9, P=0.67; Fig. 5b).
How do females influence male mating success?
Preferred and eschewed males differ significantly in the number of copulations that they obtain. This difference is influenced by two female factors: (1) females use proceptive and resistance behaviors to exhibit preferences for or against particular males and (2) females are successful at implementing their preferences. Female preferences are evidenced by the fact that females were more proceptive to preferred males than to eschewed males [78.5 vs 21.5%, respectively (see Fig. 6, i; T =0, N=9, P=0.004). Females similarly resisted 83.6% of solicitations by eschewed males but resisted only 16% of solicitations by preferred males (T =0, N=8, P=0.004; Fig. 6, ii). The effectiveness of these behaviors is measured by the percentage of mating attempts in which female proceptivity or resistance succeeded. It is notable that both female proceptivity and resistance were generally very effective in POP [with both preferred and eschewed males (Fig. 6, ii), proceptivity, average 95.2% success with all males; resistance, average 69.2% success with all males]. Male solicitation success rates were significantly negatively correlated with female resistance rates in both phases (POP, r=−0.78, z=−4.51, N=13, n=31, P=0.0001 (Fig. 7); non-POP, r=−0.794, z=−4.93, N=14, n=40, P=0.0001), suggesting that female resistance effectively deterred unwanted solicitations.
Initiation of sexual behavior and success of female solicitations in preferred (top) and eschewed (bottom) males. Preferred and eschewed males do not differ statistically in solicitation count, but both initiate more sexual interactions than females. Female solicitations are almost always successful (i.e., lead to copulation) with both preferred and eschewed males. Female response to male solicitations differs substantially among males. Female resistance is relatively successful regardless of whether a male is eschewed or preferred. Variations in (i) accepted female solicitations, (ii) cooperative matings, and (iii) unsuccessful resistance to male solicitations influence male mating success, such that there is substantial variation in POP mating success between preferred and eschewed males (based on nine females’ preferred and eschewed males); n=average for each female
Female success in attaining their mate preferences in POP helps to explain why female preference for preferred vs eschewed males is reflected in POP male mating success (i.e., copulation counts) (T= 0, N=7, P=0.016; Fig. 6, iv). If females were unsuccessful in attaining their preferences, the copulation counts would likely reflect male behavior (e.g., male solicitation rates).
The results for non-POP show a similar pattern in female behavior toward preferred and eschewed males as seen during POP. During non-POP, 91.2% of female proceptivity was toward preferred males, compared to 8.8% for eschewed males (T =0, N=11, P=0.001). Females resisted 45.6% of eschewed male solicitations, compared to only 6.2% of preferred males’ (T=1, N=12, P=0.029), which is significant; but the range of resistance was greater in POP. Female resistance was effective 69.6% of the time toward eschewed males, compared to 61.9% for preferred males (T=2, N=6, P=0.625). Preferred males obtained 60.3% of the copulations in non-POP, compared to 39.7% for eschewed males (preferred by proceptivity, T=3, N=11, P=0.024; preferred by resistance, T= 3, N=8, P=0.547; average 17.9 cops/preferred males and 9.2 cops/eschewed males).
We tested the possibility that preferred males had higher mating success than eschewed males simply because they solicited more. However, pairwise comparisons showed no significant differences between preferred and eschewed male solicitation counts (POP, T=3, N=9, P=0.512; non-POP: (T=3, N=11, P=0.089). The solicitation rates by preferred and eschewed males also showed no significant differences in either phase (POP, T=4, N=8, P=0.641; non-POP, T=5, N=11, P=0.577).
Though female resistance is effective, we tested whether differences between preferred and eschewed male mating success could be a result of preferred males potentially having greater success in overriding female resistance. However, no significant differences were found between the efficacy of female resistance to preferred or eschewed male solicitations in either phase (POP, T=2, N=6, P=0.688 (Fig. 6, iii); non-POP, T =2, N=6, P=0.625). Thus, the significantly higher female resistance toward eschewed males and general effectiveness of resistance may largely explain the differences between preferred and eschewed male mating success.
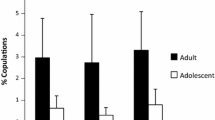
Females may also attempt to influence paternity through copulation durations. Taï females terminated the majority of copulations (93%). For each female, copulation durations were significantly higher for preferred than eschewed males based on both proceptivity and resistance (all mating days, proceptivity T=4, N=12, P=0.05; resistance T=3, N=11, P=0.02; Fig. 8a,b).
The results of the first part of this study indicate that during POP, but not in non-POP, individual female choice was effective. Females are able to implement their mate preferences through variation in proceptivity and resistance and the efficacy of both strategies.
What factors affect the efficacy of female proceptivity and resistance?
In the mixed-model multiple regression, the correlation values between age and rank in both sexes were high, though not significant, potentially due to small sample sizes (male rank and age, r S=0.594, N=7, P=0.16; female rank and age, r S=0.509, N=14, P=0.06). Thus, age or rank was used to determine the best model.
Rank and age were key predictors of the efficacy of female mate choice (Table 4), thus supporting our predictions. For proceptivity, both female rank and male rank were important factors, as higher-ranked females had greater proceptivity success than lower-ranked females did, and female proceptivity in general was more successful toward higher-ranked males. The interaction between female and male rank was of particular importance, as for lower-ranked females proceptivity was more successful with higher-ranked males, whereas higher-ranked females were more successful with lower-ranked males. Group was also an influential factor for proceptivity success, as female proceptivity was less successful in the North group than in the South group.
The influence of male age and the interaction between male and female age on resistance approached statistical significance; females were less successful at resisting older males. Older females were more effective at resisting younger males, while younger females resisted older males more effectively than older females did. Female ability to resist unwanted male solicitations successfully did not differ in POP or non-POP or between groups.
Highest-ranking females obtained a greater proportion of copulations in POP with preferred males than other females did, though the results were not significant, potentially due to the small sample size (r s=0.641, N=9, P=0.06; Fig. 9). There was no significant correlation between proportion of copulations with preferred males in POP and female age (r s=−0.143, N=9, P=0.71).
Social influences on the efficacy of female choice
There was no significant difference in female resistance success toward aggressive males in POP or non-POP (POP T=2, N=9, P=0.09; non-POP T=4, N=10, P=0.374). There was no significant difference in female ability to influence the outcome (combined proceptivity and resistance) when in a sexual interaction involving affiliative behavior (grooming, support, and food sharing) than when there was no affiliative behavior, in either phase (non-POP, T=2, N=11, P=0.232; POP, T=1, N=5, P=1.0).
The presence of a dominant male could influence the outcome of sexual interactions. Females showed a greater ability to resist male solicitations during POP when the dyad was in the presence of a dominant male than when not (T=1, N=7, P=0.047), though no difference was found in non-POP (T=2, N=8, P=0.313). Females showed no differences in proceptivity success when a more dominant male was present in POP (T=1, N=5, P=0.500). However, in non-POP, proceptivity was more successful when no dominant male was present (T =0, N=10, P=0.016).
Female estrous synchrony did not affect the efficacy of female choice. Females showed no difference in their ability to successfully resist males when other females were simultaneously in estrus than when not in either phase (non-POP, T=4, N=11, P=0.311; POP, T=1, N=3, P=0.500). Females also showed no difference in proceptivity success when other females were simultaneously in estrus than when not (non-POP, T=4, N=13, P=0.496; POP, T=0, N=5, P=0.500). Target females occasionally unsuccessfully presented to males who were soliciting or courting other females. During these times, no aggression was seen among estrous females actively competing for males.
Discussion
Although females are expected to maximize their reproductive success by being selective of their mates (Darwin 1871), the effectiveness of female choice in light of male counter-strategies has received little attention in primates and other mammals. Results from Taï indicate that, particularly during POP, males that are preferred by females (i.e., less resisted) obtain higher mating success compared to other males. This suggests that female choice is effective among Taï chimpanzees and an important component of sexual selection in chimpanzees. Male and female rank, group, and phase were all important influences on proceptivity success. Male and female ages were both important factors determining the success of female resistance to male solicitations.
Taï female mate choice appears to be effective through the following mechanisms: females altering their responses to particular males by either (a) increasing or decreasing female proceptivity or (b) increasing or decreasing resistance to a male’s solicitations, and (c) the overall effectiveness of female proceptivity and resistance (Fig. 6). Female proceptivity is negatively correlated with resistance in POP, thus high female proceptivity and lower resistance to particular males work in tandem to influence the desired mating outcome. It is unlikely that high female resistance rates toward some males were due to these males having solicited more, as preferred and eschewed males do not differ significantly in solicitation counts or rates, and we found no significant correlation between male solicitation rates and female resistance (see also Stumpf and Boesch 2005).
As Stumpf and Boesch (2005) have indicated, females use a mixed strategy to obtain their reproductive goals by being resistant to eschewed males during POP and by being more proceptive to all males in non-POP. This result is supported in the current study. While the majority of females had more copulations with preferred than eschewed males based on proceptivity, pairwise results did not reach significance, likely because females are generally not very proceptive in POP (Stumpf and Boesch 2005) and, instead, females appear to rely on a strategy in POP of resistance toward eschewed males to influence paternity. During non-POP, male mating success reflected female preferences based on proceptivity but not on resistance. The lack of a relationship between resistance and male mating success in non-POP is not due to males overriding female resistance in this phase. Because female resistance toward males is lower in non-POP compared to POP (see Stumpf and Boesch 2005), during non-POP, some eschewed males obtain higher mating success than more preferred males. This is not surprising in the non-POP phase, when conception is unlikely and other strategies such as paternity confusion are expected to be important (van Schaik et al. 2000; Stumpf and Boesch 2005). The relationship between preferred and eschewed preference categories is consequently less distinct in non-POP compared to POP, resulting in a less clear relationship between female preference and male copulation counts in this phase.
One might expect that preferred males have higher mating success because they are high ranking, with alpha male monopolization of females explaining their greater mating success. However females preferred both dominant and particular low-ranked males (see Stumpf and Boesch 2005; Fig. 5; Stumpf 2004: Appendix b), and of these preferred males obtained higher mating success than eschewed males. These results indicate that variation in male mating success is not simply the product of alpha male monopolization of females. In comparing these results to paternity data for the same community (mostly different individuals), Boesch et al. (2006) found that 38 to 50% of offspring were sired by the dominant male, while the remainder were sired by other males. It remains unclear whether these sirings detailed by Boesch et al. (2006) were the result of female or male counterstrategies. However the fact that females can effectively reduce the number of copulations with eschewed males and increase the number of copulations with high or loa-ranked preferred males suggests that female startegies can influence the likelihood of particular males siring their offspring.
Inter- and intra-specific variation in the efficacy of female choice
Among primates and other mammals with vastly different mating systems, patterns of dominance, and sexual dimorphism, the efficacy of female mating strategies may vary substantially. Orangutan female resistance is rarely successful (Rodman and Mitani 1987) and preliminary results suggest this is also the case for gorillas (Sicotte 2001). Species in which females are dominant or co-dominant to males (e.g. bonobos, lemuroids, and hyenas) are likely more able to influence male mating success (see Pereira and Weiss 1991; see also Richard 1992). Female choice also may be more effective in species with simultaneously sexually receptive females (e.g., seasonal breeders; see Soltis et al. 2001), in species with extended estrous periods (i.e., humans, chimpanzees; this study), and in species occupying habitats with plentiful resources (such as woolly spider monkeys which are characterized by little male–male aggression and direct competition for females; Milton 1985; Strier 1992).
Demographic and/or habitat differences across sites may also influence intra-specific variation in female choice. Female proceptivity in Taï chimpanzees was much more effective (77%) than the 40% success rate recorded by Wallis (1992) in Gombe (Pan troglodytes schweinfurthii). However, male solicitation success rates in the Wallis (1992) study were similar to the findings from this study (76 and 74%, respectively). Both contrast with Mahale (also P. t. schweinfurthii), where only 41.6% of mature male solicitations were successful (Nishida 1997). These differences may reflect differing demographic influences, in particular, a potentially greater likelihood of interruption by higher-ranking males in Mahale compared to the likelihood of interruption in Taï, where fewer males were present. In addition, the differences in the efficacy of female choice in the different chimpanzee sites may be influenced by variation in socio-ecological factors across the sites. In more seasonal chimpanzee habitats (such as Kibale, Mahale, and Gombe), the females may be less prone to use prolonged resistance to male solicitations compared with Taï because the energetic and time costs are too high. In addition, Taï females spend less time alone and more time in both all-female and mixed parties than females at other sites do (Boesch and Boesch-Achermann 2000; Lehmann and Boesch 2003). Subtle demographic and environmental differences may consequently permit more social and, in turn, sexual influence among Taï females than females in other chimpanzee communities. Female chimpanzees in East Africa appear to experience intense sexual coercion (Tutin 1979; Wrangham and Smuts 1980; Goodall 1986; Matsumoto-Oda 1998), whereas while present, sexual coercion does not appear to occur at similar rates in Taï. In addition, lengthier and more numerous estrous cycles of Taï chimpanzees compared to eastern subspecies (e.g., Wrangham 2002) lead to the prediction that female choice may be more effective in this subspecies.
Male coercion
Despite the finding that female choice is effective in chimpanzees, male counter-strategies such as coercion are evident. Forced copulation is known, but very rare in chimpanzees of any rank (Goodall 1986) and was observed just once during this study (it was unsuccessful, as the female simply moved away before ejaculation). However, males in this study were observed to herd, harass, and aggress against females. For females, both resistance and copulation have their associated costs (Chapman et al. 1993; Rice 1996; Clutton-Brock and Parker 1995; Watson et al. 1998), and the costs of harassment or an individual’s nutritional need, rather than choice, may influence a female’s decision to resist or accept a solicitation (Watson et al. 1998). Despite the costs, Taï females often resisted particular males at high rates. As a result, many females were chased and beaten, often resulting in injury. However, male aggression did diminish the efficacy of female resistance (see also Soltis et al. 1997; Enomoto 1981; but see Nishida 1997). Females still risked the costs of resistance and were generally effective, suggesting that, despite clear male dominance and coercion in chimpanzees, females can choose not to mate. As conceptions are thought to result from copulations that occurred during a very limited time window within POP (Gomendio and Roldan 1993), by successfully delaying mating with eschewed males for a few hours when conception likelihood is highest, a female can conceivably reduce the likelihood that these males sire her offspring. The results from other studies suggest that females in a variety of species are also able to successfully reject male mounting attempts (Huffman 1987, 1991; see also LeBoeuf 1978; Soltis et al. 1999; East et al. 2003). Influencing copulation duration may be another way that females minimize the influence of male coercion. As there is a minimum time likely needed for ejaculation to occur, by limiting copulation duration, females may end unwanted copulations early and decrease a male’s likelihood of insemination when a female is most likely to conceive.
It has been argued that male coercion functions to intimidate females long before they come into estrus (cf., Goodall 1986). Coercion may then subtly affect female behavior in estrus and increase the likelihood of mating with the coercive male (Clutton Brock and Parker 1995). While this may occur, it does not appear to positively affect mating success as males who were generally more aggressive toward females did not obtain higher mating success with any female in this study (see also Soltis et al. 1999). Manson (1992) and Soltis et al. (1997) also found no evidence that male aggression inhibits female responses such as proximity maintenance and leaving. Evidence from other species suggests that female resistance to particular males does not decrease over time and persistent males do not obtain more POP copulations than other males (see Cunningham 2003; Fox 1998, 2002). This also contradicts the argument that female resistance is a strategy to increase indirect benefits by selecting for persistent males (e.g. Eberhard 1996; see also Chapman et al. 2003).
In light of male chimpanzees’ greater size and strength, which would allow them to overpower females, the question arises as why female resistance is so effective. Among orangutans, forced copulations are common (Galdikas 1985; Schürmann and van Hooff 1986), comprising up to 90% of unflanged male copulations (Galdikas 1985; Mitani 1985), and these males are reproductively successful (Utami et al. 2002). The long orangutan inter-birth intervals and short estrus periods may increase the value of each copulation compared to that of chimpanzees, for whom most mating is non-conceptive. Thus, forced copulation may be an evolutionarily advantageous male strategy in orangutans, but not in chimpanzees. Another explanation for the differences between orangutans and chimpanzees in forced copulation frequency may be that orangutan females are often solitary and isolated, thus lacking potential aid from other individuals against unwanted mating. There is some support for this as forced copulation is two to four times more prevalent in Borneo, where females are much more often solitary, than in Sumatra (Galdikas 1985; Fox 1998; Mitani 1985; Schürmann and van Hooff 1986). However, for chimpanzees, even during consorts (in which a chimpanzee male and female travel apart from the rest of the community for days or weeks), forced copulations do not occur (R. Stumpf, unpublished data). Social rules may explain the rarity of forced copulations in chimpanzees. The high cohesiveness of chimpanzee communities leads to substantial sociality. In addition, the females are thought to influence male social status (Boesch and Boesch-Achermann 2000). Thus, chimpanzee males who force mating may risk loss of both female and male support. In contrast, orangutan male rank may not be influenced by females, and forced copulation in this genus may have few social costs.
What factors influence the success of female strategies?
Female proceptivity was not always successful. As direct female competition was not apparent, ineffective female proceptivity was likely due to male mate preference. Female proceptive behavior was less successful in the North group than in the South, perhaps because the male-to-female sex ratio was lower in the North group, permitting greater male mate choice and less male–male competition. Proceptivity tended to be more successful during POP than non-POP. This result suggests that males will not forgo opportunities to sire offspring despite higher risks of aggression from other males. Female success at resisting male solicitations was high regardless of estrous phase. In POP, male–male competition is expected to be high, and females may be influenced by male behavior. The fact that females were able to successfully resist males, despite expected high male interest in mating, may be due to the greater presence of high-ranking males who may suppress low-ranking males’ continued solicitations.
Females were less successful at resisting older male solicitations, potentially because older males are more experienced and may also invest extensive energy in trying to mate. Older females may more successfully resist younger males because they are less intimidated, while younger female resistance may be more effective with older males because younger females are not as attractive to chimpanzee males (Tutin 1979; Goodall 1986). Male affiliation was not an influential factor affecting the outcome of sexual interactions. This mirrors findings by Hemelrijk et al. (1999) on captive chimpanzees, in that no relationship was found between paternity and prior male affiliative behavior toward females. As predicted, higher-ranked females had proportionally higher mating success with preferred males than with other males in POP compared to lower-ranked females. This appears to be largely influenced by the finding that higher-ranked females were more effective at resisting eschewed males than lower-ranked females.
In conclusion, Taï females were effective in obtaining their mate choice during the periovulatory period, suggesting that female choice influences paternity. This is particularly important because female chimpanzees are subordinate to males and mate promiscuously. Having evolutionarily disengaged sex from conception may have increased female chimpanzee capacity to implement female choice. While female promiscuity suggests a lack of selectivity, upon closer examination, females appear to pursue a more complex strategy of paternity concentration and confusion (van Schaik et al. 2000; Nunn 1999; Stumpf and Boesch 2005) and this strategy appears to be successful. Additional studies with larger sample sizes are needed to further assess male and female stratigies and their influence on paternity.
Notes
We use preference to define with whom a female would like to mate, whereas choice is the implementation of that preference (see Halliday 1983).
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Bercovitch F (1991) Mate selection, consortship formation, and reproductive tactics in adult female savanna baboons. Primates 32:437–452
Birky WA (2002) Mating patterns and social structure in a wild group of Formosan macaques. Ph.D. thesis, Department of Anthropology, Rutgers University
Boesch C, Boesch-Achermann H (2000) The chimpanzees of the Taï Forest: behavioral ecology and evolution. Oxford University Press, Oxford
Boesch C, Kohou G, Nene H, Vigilant L (2006) Male competition and paternity in wild chimpanzees of the Taï forest. A J Phys Anthropol 130(1):103–15
Boinski S (1987) Mating patterns in squirrel monkeys (Saimiri oerstedii). Behav Ecol Sociobiol 21:13–21
Brown WD (1997) Female remating and the intensity of female choice in black-horned tree crickets, Oecanthus nigricornis. Behav Ecol 8:66–74
Buck MR (1998) Female mate choice in sooty mangabeys: social constraints on mating behaviour. Ph.D. thesis, Department of Anthropology, Emory University
Burley NT, Parker PG, Lundy K (1996) Sexual selection and extra-pair fertilization in a socially monogamous passerine, the zebra finch (Taeniopygia guttata). Behav Ecol 7:218–226
Bygott JD (1979) Agonistic behavior, ‘dominance’, and social structure in wild chimpanzees of the Gombe National Park. In: Hamburg B, McCown E (eds) The great apes. Benjamin/Cummings, Menlo Park, CA
Chapman T, Hutchings J, Partridge L (1993) No reduction in the cost of mating Drosophila melanogaster females with spermless males. Proc R Soc Lond 153:211–217
Chapman T, Arnqvist G, Bangham J, Rowe L (2003) Sexual conflict. Trends Ecol Evol 18:41–47
Clutton-Brock TH, Parker GA (1995) Sexual coercion in animal societies. Anim Behav 49:1345–1365
Cords M, Mitchell BJ, Tsingalia HM, Rowell TA (1986) Promiscuous mating amongst blue monkeys in the Kakamega forest, Kenya. Ethology 72:214–226
Cowlishaw G, Dunbar RIM (1991) Dominance rank and mating success in male primates. Anim Behav 41:1045–1056
Cunningham M (1986) Measuring the physical in physical attractiveness: quasi-experiments on the sociobiology of female facial beauty. J Pers Soc Psychol 50:925–935
Cunningham EJA (2003) Female mate preferences and subsequent resistance to copulation in the mallard. Behav Ecol 14:326–333
Cunningham EJA, Russell AF (2000) Egg investment is influenced by male attractiveness in the mallard. Nature 404:74–77
Czekala NM, Mitchell WR, Lasley BL (1987) Direct measurement of urinary estrone conjugates during the normal menstrual cycle of the gorilla (Gorilla gorilla). Am J Primatol 12:223–229
Darwin C (1871) The descent of man, and selection in relation to sex. Murray, London
de Waal FMB (1982) Chimpanzee politics: power and sex among apes. Allen & Unwin, London
Deschner T, Heistermann M, Hodges K, Boesch C (2003) Timing and probability of ovulation in relation to sex skin swelling in wild West African chimpanzees, Pan troglodytes verus. Anim Behav 66:551–560
Drickamer LC, Gowaty PA, Holmes CM (2000) Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav 59:71–378
Drickamer LC, Gowaty PA, Wagner DM (2003) Free mutual mate preferences in house mice affect reproductive success and offspring performance. Anim Behav 65:105–114
Dunbar RIM (1984) Reproductive decisions: an economic analysis of gelada baboon social strategies. Princeton University Press, Princeton, NJ
East ML, Burke T, Wilhelm K, Greig C, Hofer H (2003) Sexual conflicts in spotted hyenas: male and female mating tactics and their reproductive outcome with respect to age, social status and tenure. Proc R Soc Lond Ser B Biol Sci 270:1247–1254
Eberhard WG (1996) Female control: sexual selection by cryptic female choice. Princeton University Press, Princeton, NJ
Elder J, Yerkes R (1936) The sexual cycle of the chimpanzee. Anat Rec 67:119–143
Enomoto T (1981) Male aggression and the sexual behavior of Japanese monkeys. Primates 1:15–23
Fox EA (1998) The function of female mate choice in the Sumatran orangutan (Pongo pygmaeus abelii). Ph.D. thesis, Department of Biological Anthropology and Anatomy, Duke University
Fox EA (2002) Female tactics to reduce sexual harassment in the Sumatran orangutan (Pongo pygmaeus abelii). Behav Ecol Sociobiol 52:93–101
France JT (1981) Overview of the biological aspects of the fertile period. Int J Fertil 26:143–152
France JT, Graham FM, Gosling L, Hair P, Knox BS (1992) Characteristics of natural conceptual cycles occurring in a prospective study of sex preselection—fertility awareness symptoms, hormone levels, sperm survival, and pregnancy outcome. Int J Fertil 37:244–255
Galdikas B (1985) Subadult male orangutan sociality and reproductive behavior at Tanjung Puting. Am J Primatol 8:87–99
Gomendio M, Roldan ERS (1993) Mechanisms of sperm competition: linking physiology and behavioral ecology. Trends Ecol Evol 8:95–100
Goodall J (1986) The chimpanzees of Gombe: patterns of behavior. Harvard University Press, Cambridge, MA
Gowaty PA, Steinichen R, Anderson WW (2002) Mutual interest between the sexes and reproductive success in Drosophila pseudoobscura. Evolution 56:2537–2540
Halliday TR (1983) The study of mate choice. In: Bateson P (ed) Mate choice. Cambridge University Press, Cambridge, pp 3–22
Harcourt AH (1989) Environment, competition, and reproductive performance of female monkeys. Trends Ecol Evol 4:101–105
Hasegawa T, Hiraiwa-Hasegawa M (1990) Sperm competition and mating behavior. In: Nishida T (ed) The chimpanzees of the Mahale mountains: sexual and life history strategies. University of Tokyo Press, Tokyo, pp 115–132
Hemelrijk CK, Meier C, Martin RD (1999) ‘Friendship’ for fitness in chimpanzees? Anim Behav 58:1223–1229
Hine E, Lachish S, Higgie M, Blows MW (2002) Positive genetic correlation between female preference and offspring fitness. Proc R Soc Lond Ser B Biol Sci 269:2215–2219
Howard RD, Moorman RS, Whiteman HH (1997) Differential effects of mate competition and mate choice on eastern tiger salamanders. Anim Behav 53:1345–1356
Hrdy SB (1979) Infanticide among animals: a review, classification, and examination of the implications for the reproductive strategies of females. Ethol Sociobiol 1:13–40
Huffman MA (1987) Consort intrusion and female mate choice in Japanese macaques. Ethology 75:221–234
Huffman MA (1991) Mate selection and partner preference in female Japanese macaques. In: Fedigan LM, Asquith PJ (eds) The monkeys of Arashiyama: thirty-five years of study in the East and the West. State University of New York Press, New York, pp 101–122
Janson CH (1984) Female choice and mating system of the brown capuchin monkey, Cebus apella (Primates: Cebidae). Z Tierpsychol 65:177–200
Keddy AC (1986) Female mate choice in vervet monkeys (Cercopithecus aethiops). Am J Primatol 10:125–143
Knott CD (1997) Field collection and preservation of urine in orangutans and chimpanzees. Trop Biodivers 4:95–102
Kolm N (2001) Females produce larger eggs for large males in a paternal mouthbrooding fish. Proc R Soc Lond B 268:2229–2234
Lehmann J, Boesch C (2003) Social influences on ranging patterns among chimpanzees (Pan troglodytes verus) in the Taï National Park, Côte d’Ivoire. Behav Ecol 14(5):642–649
LeBoeuf B (1978) Sex and evolution. In: McGill T, Dewsbury D, Sachs B (eds) Sex and behavior. Plenum, New York
Manly BFJ (1997) Randomization, bootstrap, and Monte Carlo methods in biology. London: Chapman & Hall
Manson JH (1992) Measuring female mate choice in Cayo Santiago rhesus macaques. Anim Behav 44:405–416
Matsumoto-Oda A (1998) Injuries to the sexual skin of female chimpanzees at Mahale and their effect on behaviour. Folia Primatol 69:400–404
Matsumoto-Oda A (1999) Female choice in the opportunistic mating of wild chimpanzees (Pan troglodytes schweinfurthii) at Mahale. Behav Ecol Sociobiol 46:258–266
Milton K (1985) Mating patterns of Woolly spider monkeys, Brachyteles arachnoides: implications for female choice. Behav Ecol Sociobiol 17:53–59
Mitani J (1985) Mating behavior of male orangutans in the Kutai reserve. Anim Behav 33:392–402
Nishida T (1997) Sexual behavior of adult male chimpanzees of the Mahale Mountains National Park, Tanzania. Primates 38:379–398
Nishida T, Hiraiwa-Hasegawa M, Hasegawa T, Takahata Y (1985) Group extinction and female transfer in wild chimpanzees in the Mahale Mountains. Z Tierpsychol 67:284–301
Nishida T, Takasaki H, Takahata Y (1990) Demography and reproductive profiles. In: Nishida T (ed) The chimpanzees of the Mahale Mountains. University of Tokyo Press, Tokyo, pp 63–98
Norris K (1993) Heritable variation in a plumage indicator of viability in male great tits, Parus major. Nature 362:537–539
Nunn CL (1999) The evolution of exaggerated sexual swellings in primates and the graded-signal hypothesis. Anim Behav 58:229–246
Parker G (1979) Sexual selection and sexual conflict. In: Blum MS, Blum NA (eds) Sexual selection and reproductive competition in insects. Academic, New York, pp 123–166
Partridge L (1980) Mate choice increases a component of offspring fitness in fruit flies. Nature 283:290–291
Partridge L, Hurst LD (1998) Sex and conflict. Science 281:2003–2008
Paul A (2002) Sexual selection and mate choice. Int J Primatol 23:877–904
Pereira ME, Weiss ML (1991) Female mate choice, male migration, and the threat of infanticide in ringtailed lemurs. Behav Ecol Sociobiol 28:141–152
Perloe SI (1992) Male mating competition, female choice and dominance in a free ranging troop of Japanese macaques. Primates 33:289–304
Petrie M (1994) Improved growth and survival of offspring of peacocks with more elaborate trains. Nature 371:598–599
Promislow DEL, Smith EA, Pearse L (1998) Adult fitness consequences of sexual selection in Drosophila melanogaster. Proc Natl Acad Sci USA 95:10687–10692
Pusey A (1980) Inbreeding avoidance in chimpanzees. Anim Behav 28:543–582
Pusey A, Williams J, Goodall J (1997) The influence of dominance rank on the reproductive success of female chimpanzees. Science 277:828–831
Rabe-Hasketh S, Everitt B (2004) A handbook of statistical analysis using Stata, 3rd edn. Chapman & Hall/CRC Boca Raton, USA
Reynolds JD, Gross MR (1992) Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc R Soc Lond Ser B Biol Sci 250:57–62
Richard A (1992) Aggressive competition between males, female-controlled polygyny and sexual monomorphism in a Malagasy primate, Propithecus verreauxi. J Hum Evol 22:395–406
Rice W (1996) Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature 381:232–243
Rodman P, Mitani J (1987) Orangutans: sexual dimorphism in a solitary species. In: Smuts B, Cheney D, Seyfarth R, Wrangham R, TT Struhsacker (eds) Primate societies. University of Chicago Press, Chicago, pp 146–54
Schürmann CL, van Hooff JARAM (1986) Reproductive strategies of the orang-utan: new data and a consideration of existing socio-sexual models. Int J Primatol 7:265–287
Sicotte P (2001) Female mate choice in mountain gorillas. In: Robbins MM, Sicotte P, Stewart KJ (eds) Mountain gorillas: three decades of research at Karisoke. Cambridge University Press, Cambridge
Setchell JM, Kappleler PM (2003) Selection in relation to sex in primates. Adv Study Behav 33:87–173
Siegel S, Castellan NJ Jr (1988) Non-parametric statistics for the behavioral sciences. McGraw-Hill, New York
Small MJ (1989) Female choice in non-human primates. Yearb Phys Anthropol 32:103–127
Small MF (1990) Promiscuity in Barbary macaques (Macaca sylvanus). Am J Primatol 20:267–282
Smuts BB, Smuts RW (1993) Male aggression and sexual coercion of females in non-human primates and other mammals: evidence and theoretical implications. Adv Study Behav 22:1–63
Soltis J, Mitsunaga F, Shimizu K, Yanagihara Y, Nozaki M (1997) Sexual selection in Japanese macaques I: female mate choice or male sexual coercion? Anim Behav 54:725–736
Soltis J, Mitsunaga F, Shimizu K, Yanagihara Y, Nozaki M (1999) Female mating strategy in an enclosed group of Japanese macaques. Am J Primatol 47(4):263–278
Soltis J, Thomsen R, Takenaka O (2001) The interaction of male and female reproductive strategies and paternity in wild Japanese macaques, Macaca fuscata. Anim Behav 62:485–494
Strier KB (1992) Causes and consequences of nonaggression in the woolly spider monkey, or muriqui (Brachyteles arachnoides). In: Silverberg J, Gray JP (eds) Aggression and peacefulness in humans and other primates. Oxford University Press, New York, pp 100–116
Strier K (1997) Mate preference of wild muriqui monkeys (Brachyteles arachnoides): reproductive and social correlates. Folia Primatol 68:120–133
Stumpf RM (2004) Female reproductive strategies in chimpanzees of the Taï National Park, Cote d’Ivoire. Ph.D. thesis, Stony Brook University, Stony Brook, NY
Stumpf RM, Boesch C (2005) Does promiscuous mating preclude female choice? Female sexual strategies in chimpanzees of the Taï Forest, Côte d’Ivoire. Behav Ecol Sociobiol 57:511–524
Tabachnik BG, Fidell LS (2001) Using multivariate statistics. Allyn and Bacon
Takahata Y, Ihobe H, Idani G (1996) Comparing copulations of chimpanzees and bonobos: do females exhibit proceptivity or receptivity? In: McGrew WC, Marchant LF, Nishida T (eds) Great ape societies. Cambridge University Press, Cambridge, pp 146–155
Takasaki H (1985) Female life history and mating patterns among the M group chimpanzees in Mahale National Park, Tanzania. Primates 26:121–129
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine, Chicago, pp 136–179
Tutin CEG (1979) Mating patterns and reproductive strategies in a community of wild chimpanzees (Pan troglodytes schweinfurthii). Behav Ecol Sociobiol 6:29–38
Tutin CEG (1980) Reproductive behaviour of wild chimpanzees in the Gombe National Park, Tanzania. J Reprod Fertil Suppl Suppl 18:43–57
Tutin CEG, McGinnis PR (1981) Chimpanzee reproduction in the wild. In: Graham CE (ed) Reproductive biology of the great apes. Academic, New York, pp 239–264
Utami SS, Goossens B, Bruford MW, de Ruiter JR, van Hooff JARAM (2002) Male bimaturism and reproductive success in sumatran orang-utans. Behav Ecol 13:643–652
Van Schaik CP, Hodges JK, Nunn CL (2000) Paternity confusion and the ovarian cycles of female primates. In: Van Schaik CP, Janson CH (eds) Infanticide by males and its implications. Cambridge University Press, Cambridge, pp 361–387
Van Schaik CP, Pradhan GR, van Noordwijk MA (2004) Mating conflict in primates: infanticide, sexual harassment and female sexuality. In: Kappeler PM, van Schaik CP (eds) Sexual selection in primates: new and comparative perspectives. pp 131–150
Wallen K, Winston LA (1984) Social complexity and hormonal influences on sexual behavior in rhesus monkeys (Macaca mulatta). Physiol Behav 32:629–637
Wallis J (1992) Chimpanzee genital swelling and its role in the pattern of sociosexual behavior. Am J Primatol 28:101–113
Watson PJ, Arnqvist G, Stallman RR (1998) Sexual conflict and the energetic costs of mating and mate choice in water striders. The Am Nat 151:46–58
Wilcox A, Weinberg C, Baird D (1995) Timing of sexual intercourse in relation to ovulation. New Engl J Med 333:1517–1521
Wiley RH, Poston J (1996) Indirect mate choice, competition for mates, and coevolution of the sexes. Evolution 50:1371–1381
Wrangham R (2002) The cost of sexual attraction: is there a trade-off in female Pan between sex appeal and received coercion? In: Hohmann G, Boesch C, Marchant L (eds) Behavioural diversity in chimpanzees and bonobos. Cambridge University Press, Cambridge
Wrangham RW, Smuts BB (1980) Sex difference in the behavioral ecology of chimpanzees in the Gombe national park, Tanzania. J Reprod Fertil Suppl Suppl 28:13–31
Acknowledgements
The authors wish to thank the Centre Suisse de la Recherche Scientifique, the Ministèere de la Recherche Scientifique in Côte d’Ivoire, the Ministèere de l’Agriculture et des Resources Animales, Côte d’Ivoire, and the direction of Taï National Park for permission to conduct this research. R.M.S would particularly like to thank Diane Doran-Sheehy, John Fleagle, Patricia Wright, Charlie Janson, John Polk, Daniel Stahl, Charles Roseman, Germán Bollero, all members of the Taï Chimpanzee Project, particularly Valentin Gagnon, Camille Bolé, Nicaise Oulaï Daurid, Cathy Crockford, Tobias Deschner, Ilka Herbinger, and Roman Wittig, as well as helpful comments from two anonymous reviewers. Quidel Corporation generously donated ovulation and pregnancy test kits. Funding was provided by a National Science Foundation doctoral dissertation grant to R.M.S. and the Max Planck Society for the Advancement of Science. This study complies with Côte d’Ivoire regulations.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by D. Watts
Rights and permissions
About this article
Cite this article
Stumpf, R.M., Boesch, C. The efficacy of female choice in chimpanzees of the Taï Forest, Côte d’Ivoire. Behav Ecol Sociobiol 60, 749–765 (2006). https://doi.org/10.1007/s00265-006-0219-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0219-8