Abstract
Sperm competition models on the evolution of sperm size assume associations with another sperm quality trait, sperm longevity. Sperm length can also provide an indication of possible mechanisms affecting motility and thus fertilization success. Despite their importance, however, detailed mechanisms of sperm competition at the gamete level are poorly understood. In simultaneously hermaphroditic land snails, sperm traits and cryptic female choice are assumed to be crucial in determining fertilization success. We examined the variation in sperm length and number among individuals from four natural populations of the land snail Arianta arbustorum, a species with multiple mating and long-term sperm storage. We also assessed variation in velocity, motility and longevity of sperm in snails from two of the four populations. Independent of shell size, sperm length differed among populations and, to a minor extent, even among individuals within populations. Mean sperm length of a snail was not correlated with the number of sperm delivered in a spermatophore. The mean sperm velocity (=VCL) did not differ between snails from two populations. However, VCL varied among snails. Percentage motility and longevity of sperm differed between snails from the two populations. No correlations were found between length, velocity, percentage motility and longevity of sperm. To conclude, individual snails differed in sperm quality, and this variation may partly explain the differential fertilization success between A. arbustorum snails. Moreover, our findings did not support the positive association between sperm length and longevity assumed by sperm competition models for internally fertilizing species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variation in sperm quality, such as sperm size and measures of motility (velocity, percentage motility, and longevity), is widespread among animals and has been observed both between and within species (reviewed in Ward 1998 and Snook 2005). The term “quality” has been used to distinguish these traits from the effect of sperm number on male fertilization success (see Snook 2005). Variation in sperm quality among individuals is relevant for the evolution of sperm characteristics through postcopulatory sexual selection (sperm competition and cryptic female choice). In promiscuous species, sperm function as an integrated unit to ensure both fertility and to maximize the paternity of a male by outcompeting sperm from other males (Birkhead and Møller 1998) or by manipulating female reproduction (Peng et al. 2005). Therefore, phenotypic and/or genetic covariation can be expected between either different measures of sperm quality or sperm quality and sperm number (e.g., Moore et al. 2004; Birkhead et al. 2005).
Investment in sperm quality traits and in sperm number must be considered when examining the evolution of sperm characteristics through sperm competition. Theoretical models predict that the numerical superiority of sperm is an adaptive strategy to increase male fertilization success (Parker 1993; Parker and Begon 1993). Indeed, in several promiscuous species, a common male response to sperm competition is to increase the number of sperm delivered (e.g., Schulte-Hostedde and Millar 2004). Sperm competition models predict that sperm size can confer a fertilization advantage, but only under the assumption of a functional relationship between sperm quality traits. Sperm length might not change or increase or decrease with sperm longevity depending on the mode of reproduction (Parker 1998). Sperm size can increase under conditions of high sperm competition (e.g., the nematode Caenorhabditis elegans, LaMunyon and Ward 2002). However, decreased sperm competition did not consistently alter sperm length in flies (Hosken et al. 2001; Pitnick et al. 2001). Studies showed that males producing larger sperm might enhance their fertilization success (e.g., the bulb mite, Radwan 1996; the prosobranch snail Viviparus ater, Oppliger et al. 2003). More recently, other relevant ejaculate characteristics such as sperm velocity, motility, and longevity and the interaction among these traits on fertilization success have received increasing attention (e.g., Burness et al. 2004; Gage et al. 2004). Theory for internally fertilizing species predicts that enhanced sperm competition risk would favor increased sperm length when larger sperm enjoy higher survival and could be stored until fertilization (Parker 1998). Though studies so far indicate that sperm longevity decreases with sperm size in externally fertilizing fishes (Stockley et al. 1997; Gage et al. 2002), there is no information available on this relationship for internal fertilizers (Simmons 2001; Snook 2005).
Sperm morphology can provide an indication of possible mechanisms by which motility is affected. Theory predicts that longer sperm generate greater flagellar forces, and swim faster (Katz and Drobnis 1990). However, recent studies suggest that longer sperm are not faster than shorter sperm (Atlantic salmon, Gage et al. 2002; zebra finch, Birkhead et al. 2005). Sperm length and sperm motility are likely both important in determining male fertilization success in internally fertilizing species with sperm storage (e.g., Froman 2003; Snook 2005). Sperm motility predicts fertilization success in competitive situations for many species (e.g., Birkhead et al. 1999; Kupriyanova and Havenhand 2002). Moreover, Rogers and Chase (2002) suggested that in the snail Helix aspersa, the beating of longer sperm should generate resistance to incoming sperm in the sperm storage organ.
Sperm characteristics have so far been examined exclusively in gonochoristic species, with the exception of the hermaphroditic nematode C. elegans (LaMunyon and Ward 2002). Sexual selection is also likely to shape sperm characteristics in simultaneous hermaphrodites, even though this may conflict with the sperm receiver’s interests (Michiels 1998). The sperm donor must persuade the sperm receiver to use its sperm to fertilize eggs, and/or to avoid the postcopulatory control mechanisms (Michiels 1998). In gastropods, the interspecific variation in sperm morphology has been studied and is used as a taxonomical character (e.g., Healy 1996), while the intraspecific variation in sperm traits has not yet been analyzed quantitatively. Spermatozoa of terrestrial gastropods are among the longest within molluscs (e.g., 850 μm in Helix pomatia, and 1,140–1,400 μm in Hedleyella falconeri; Thompson 1973). The present study focuses on intraspecific variation in sperm characteristics in the simultaneously hermaphroditic land snail Arianta arbustorum. In this species, sperm are monomorphic and ca. 800 μm long (Bojat et al. 2001). There is experimental evidence for sperm competition in A. arbustorum: multiple mating is common (Baur 1994), viable sperm from different males can be stored for long periods (Baur 1988a), and multiple paternity and differential male fertilization success have been recorded (Baur 1998). A. arbustorum does not adjust the number of sperm delivered to the actual risk of sperm competition (Locher and Baur 2000), and its female role has some control over the fertilization of the eggs by selective sperm use (Baur 1994; Bojat and Haase 2002).
Sperm competition is one of the principal determinants of male fitness in species in which females mate promiscuously, but the selective pressures it causes are only partly understood, especially with respect to sperm characteristics favored under sperm competition. To investigate the underlying causes of differential fertilization success between snails, we assessed among- and within-population variation in sperm length and number of sperm transferred during copulation in A. arbustorum from four natural populations. To test the assumptions of sperm competition models on the evolution of sperm size (Parker 1998), we measured the velocity, motility, and longevity of sperm, and we assessed their relationship with sperm length in two of the examined populations. We observed that the sperm of A. arbustorum leave the spermatophore in bundles to reach the storage organ. Therefore, we also measured the velocity of sperm swimming in bundles. In pulmonate gastropods, sperm bundles have so far been observed in H. pomatia (Meisenheimer 1912).
Materials and methods
Study organism
A. arbustorum is common in moist habitats of northwestern and central Europe (Kerney and Cameron 1979). The snail has determinate growth (adult shell width 16–24 mm; Baur 1984). Individuals become sexually mature at 2–4 years, and adults live another 3–4 years (Baur and Raboud 1988). Mating in A. arbustorum includes elaborate courtship behavior, which lasts 2–18 h (Baur and Baur 1992). Copulation is reciprocal; after intromission, each snail transfers simultaneously one spermatophore, which is formed and filled with sperm during copulation (Hofmann 1923; Baminger and Haase 2001). In the field, A. arbustorum mates repeatedly in the course of a reproductive season, and fertile sperm can be stored for more than 1 year (Baur 1988a). Snails deposit one to three egg batches consisting of 20–50 eggs (Baur 1990). Outcrossing is the dominant mode of reproduction in A. arbustorum (Chen and Baur 1993). Breeding experiments showed that 27% of virgin snails prevented from mating produced a few hatchlings by self-fertilization in the second and third year of isolation (Chen and Baur 1993). However, the reproductive success of selfing individuals was less than 2% of that of outcrossing snails.
General method
Virgin snails were obtained by collecting subadult individuals of A. arbustorum from four localities in Switzerland. Snails that had not yet completed shell growth were sampled in a coniferous forest near Gurnigelbad (20 km south of Bern; 46°45′N, 7°28′E, 1,230 m a.s.l.), in deciduous forests near Thun (26 km SSE of Bern; 46°44′ N, 7°35′E, at 580 m) and Nuglar (12 km SE of Basel; 47°29′N, 7°42′E, at 430 m), and in an oak–hornbeam forest near Allschwil (4 km SW of Basel; 47°32′N, 7°31′E, at 335 m). Snails from the four localities are, hereafter, referred to as Gurnigel, Thun, Nuglar, and Allschwil populations.
Snails were kept singly in transparent plastic beakers (8 cm deep, 6.5 cm in diameter) lined with moist soil (approximately 4 cm) at 19.5°C with a light–dark cycle of 18:6 h. We cleaned the beakers twice per week and provided fresh lettuce ad libitum as food. Within 4 weeks, subadult individuals reached sexual maturity as indicated by the formation of a flanged lip at the shell aperture.
To examine sperm characteristics, randomly chosen snails (hereafter focal snails) were allowed to copulate with a mating partner (hereafter receiver) from the same population in a transparent plastic container (14×10×7 cm) lined with moist paper towel. The spermatophore produced by the focal snail was obtained by killing the receiver after copulation, and the delivered spermatophore was removed by dissecting the receiver female reproductive tract. We measured the shell width to the nearest 0.1 mm using a vernier calliper to examine possible effects of the size of the focal snail on sperm traits.
We counted the number of sperm and measured their length in snails from all four populations of A. arbustorum collected in summer 2001 and 2002 (samples size in Table 1). In each spermatophore, we measured the length (L) of the sperm-containing part and its diameter at both ends (D 1 and D 2) to the nearest 0.1 mm. Spermatophore volume was approximated assuming a truncated-cone volume \({\left[ {V = 1/12\pi L{\left( {D^{{\text{2}}}_{{\text{1}}} + D_{{\text{1}}} D_{{\text{2}}} + D^{{\text{2}}}_{{\text{2}}} } \right)}} \right]}\). We also examined possible relationships between the male reproductive output (sperm length or sperm number) on the female reproductive allocation (estimated by albumen gland dry weight) in this simultaneous hermaphrodite. The albumen gland was dissected out of the female reproductive tract of the focal snail. We determined the dry weight of the albumen gland to the nearest 0.1 mg. We also examined the relationships between sperm number, spermatophore volume (cubic millimeters), and copulation duration (minutes).
We measured sperm length and their motility in snails from two (Gurnigel and Nuglar) of the four populations collected in summer 2004 (samples size in Table 3). Snails of these populations showed large differences in sperm length (see Table 1).
Sperm length
Sperm characteristics were examined in spermatozoa from spermatophores, which were dissected out of the receiver approximately 3 h after copulation. Sperm of A. arbustorum are densely packed in the spermatophore (Baminger and Haase 2001). We developed a technique to isolate a single sperm from the spermatophore without damaging their 800- to 950-μm long tails. Using insect needles, we carefully broke off the wall of the sperm-containing part along the longitudinal axis of the spermatophore. To activate sperm, we placed the spermatophore for 12 h in 500 μl Ringer solution (3.5 g NaCl, 0.05 g KCl, 0.20 g NaHCO3, 0.10 g CaCl2 in 1,000 ml; pH 8.2; cf. Romeis 1989). The suspension with active sperm was used to measure the length of single sperm. For this purpose, aliquots of 16-μl sperm suspension were placed on three microscopic slides. The samples were covered with a coverslip and air-dried. The remaining sperm suspension was concentrated (at 30°C for 60 min in an Eppendorf 5301 concentrator) and added to the spermatophore sample, which was preserved at 4°C for sperm counting (see below).
We digitized randomly chosen sperm using a camera (SONY CCD-Iris) mounted on a compound microscope (Leica DMLD, magnification 200×) connected to a Macintosh computer. From these images, we measured the total sperm length (head and tail) of 25–29 sperm from each spermatophore using the public domain NIH-Image software (version 1.63; http://rsb.info.nih.gov/nih-image).
We assessed the reliability of multiple length measurements (n=6) on the same sperm (n=9) by calculating the repeatability following Lessells and Boag (1987). Repeatability of sperm length measurements was 0.92, indicating the technique was accurate. Broken tails of sperm could bias our measurements of sperm length. We found ten outliers (i.e., 0.53% of a total of 1,875 sperm measured from 72 snails; Grubbs’ test, public domain http://www.graphpad.com/articles/grubbs.htm). However, mean sperm length including these outliers did not differ from mean values in which outliers were excluded (ANOVA: F 2,68=0.32, P=0.58).
Sperm number
The procedure for sperm counting is described in detail in Locher and Baur (1997). Briefly, the sperm suspension obtained from the mechanically disrupted spermatophore was stained with a gallocyanin–chromium complex (a DNA marker). Two subsamples of known volume of the sperm suspension were transferred to a Bürk–Türk counting chamber. We counted all sperm heads in randomly chosen cells until the total number of sperm heads exceeded 400, and used the average of the two subsamples to calculate the total number of sperm transferred in a spermatophore.
To assess the proportion of sperm removed for sperm length measurements, we counted the number of sperm of three subsamples from each six spermatophores. A total of 1,542–3,271 (mean 2,352.1±264.3) sperm was removed for sperm length measurements. This corresponds to 0.045–0.096% (mean 0.068%) of the total number of sperm in a spermatophore. Consequently, we corrected our estimate of sperm number by multiplying the value with a correction factor of 1.00068.
Sperm motility: velocity, percentage motility, longevity
Sperm characteristics were examined in sperm from spermatophores, which were dissected from the receiver 1.5 h after copulation. Single spermatophores were placed into the aperture of a 20-μm deep standard microscopic chamber (CASA 2x, Leja, NL) filled with 10 μl Ringer solution. Motile sperm were video-recorded at room temperature (23.7±0.2°C; n=8 days) for 10 min using a differential interference contrast microscope (Polyvar, Reichert-Jung, Vienna, Austria; magnification 100×; with the neutral filter N3 and a light transmission of 12.5% to enhance image contrast). The microscopic chamber was checked top-down to minimize the risk of sampling a sperm more than once. “Edge effects” may increase sperm velocity, but this systematic error was the same for all measurements. The spermatophore with the remaining sperm was preserved in 200 μl Ringer solution, and kept at room temperature to assess percentage motility (see below).
Sperm velocity was examined using a computer assisted IVOS-semen analysis system (CASA version 12.1, Hamilton Thorne Research, University Hospital Basel). CASA uses a nearest-neighbor algorithm to select the closest motile object. We adjusted the procedure to A. arbustorum sperm by taking 50 frames per second, setting cell detection to a minimum contrast of 50 and minimum cell size to 3 pixels. We measured the average velocity (micrometers per second) of single sperm considering both the actual track velocity (VCL, average velocity, which includes deviations of sperm head movement) and the straight-line velocity (VSL, average velocity measured in a straight line). Sperm velocity (VCL and VSL) was recorded twice (90 and 120 min after sperm activation, i.e., 180 and 210 min after copulation) in 10–12 sperm from each spermatophore.
Velocity of sperm leaving the spermatophore in bundles was measured manually because sperm bundles could not be automatically identified by CASA. We measured VSL of 4–12 sperm bundles twice: when they were leaving the spermatophore and after 10 min in a microscopic chamber. In each case, five successive frames were taken at an interval of 1 s (using the software BioPictViewer+; Zschokke 2003). In four spermatophores, VSL of sperm bundles leaving the spermatophore (82.0±16.7 μm/s) was not significantly different from the VSL of those that moved freely in the microscopic chamber (50.0±9.9 μm/s) coming from the same spermatophore (paired t test: t=1.95, df=3, P=0.15). Some of the sperm bundles disentangled after leaving the spermatophore. This allowed us to compare VSL of sperm in bundles and VSL of singly swimming sperm of the same spermatophore.
We assessed percentage motility (number of active sperm out of 100 randomly chosen sperm) at 3 h intervals beginning 12 h after sperm activation. We determined percentage motility in 10-μl sperm suspension using a light microscope (Leica DMLD, magnification 100×). Repeated measurements of percentage motility also allow an estimate of sperm longevity, which is defined as time elapsed since activation until 95% of the sperm are inactive.
Data analysis
Statistical analyses were performed using SPSS 11.0.2 for Macintosh. Differences in coefficient of variation (CV, corrected for different sample sizes) were examined using Levene’s test. Differences in reproductive traits (log-transformed data) were examined using one-way analysis of covariance (ANCOVA) with population as fixed factor and shell width as covariate. Differences in percentage motility were examined using one-way repeated measures analysis of covariance. In all cases, the nonsignificant interaction was dropped from the model (Scheiner and Gurevitch 1993). Differences between populations were investigated using the Hochberg’s GT2 post hoc test for unequal sample sizes. Variance components for sperm length were partitioned among sources (i.e., among populations, among snails within population, and among sperm within snails) by using nested analysis of variance. Relationships between sperm traits were analyzed with the Pearson correlation test (for small sample sizes).
Results
Sperm length
Among-population variation
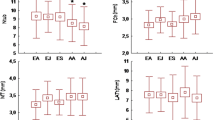
Snails differed in mean shell width (Table 1; Fig. 1). However, independent of shell width, mean sperm length of A. arbustorum differed among populations (Table 1; Fig. 1). Sperm length ranged from 878±3 μm in the Gurnigel population to 939±4 μm in the Nuglar population. The difference in mean sperm length in the four populations examined accounted for 6.7% of the average sperm length (grand mean: 904 μm).
Within-population variation
Different individuals produced sperm of different length (one-way ANCOVA: Gurnigel: F 57,1429=18.34, P<0.0001; Thun: F 13,356=7.67, P<0.0001; Nuglar: F 28,692=15.77, P<0.0001; Allschwil: F 16,439=15.00, P<0.0001; Fig. 1). Sperm length was not correlated with shell width (in all populations P≥0.08; Fig. 1). The interindividual variation in mean sperm length (CV) was similar for all populations: Gurnigel 2.5% (n=58), Thun 3.1% (n=14), Nuglar 2.5% (n=29), and Allschwil 2.3% (n=17) (Levene’s test: df 1=3, df 2=114, P=0.31). Mean sperm length of individuals fitted a normal distribution in each population (Shapiro–Wilk test: in all cases P≥0.62). Similarly, at the individual level, sperm length was normally distributed in all 118 snails examined, indicating that sperm length is unimodal in A. arbustorum. Forty-nine percent of the variation in sperm length can be attributed to differences among populations, 17.3% to differences among snails within population, and 33.7% to differences within individuals (Table 2). Thus, snails differed in sperm length both among and—to a minor extent—within populations.
Among-year variation
Snails from different populations produced sperm of different length; this difference was consistent among years (one-way ANCOVA: shell size F 1,82=0.11, P=0.74; year: F 1,1.06=1.46, P=0.43; population: F 1,2.90=20.01, P=0.02; year×population: F 2,82=5.60, P=0.02). The significant interaction term indicates that not all populations showed the same pattern. In fact, in the Nuglar population, sperm length differed between years (two-way ANCOVA: shell size F 1,26=0.01, P=0.93; year: F 1,26=11.80, P<0.002), probably due to a sampling effect (data from 2001: n=16 snails; from 2002: n=13 snails).
Sperm number
Among-population variation
Independent of shell width, the snails from the four populations transferred different numbers of sperm in their spermatophores (Table 1; Fig. 2). The mean number of sperm delivered ranged from 2.3×106 in the Gurnigel population to 5.3×106 in the Allschwil population (Table 1). Populations differed also in spermatophore volume, but not in copulation duration (Table 1). Spermatophore volume was positively correlated with sperm number (pooled data after removing the effect of shell size: r=0.34, n=111, P<0.0001). As observed in previous studies for A. arbustorum (e.g., Locher and Baur 1999), sperm number was not influenced by copulation duration (pooled data after removing the effect of shell size: r=0.18, n=65, P=0.15).
Within-population variation
The number of sperm transferred during copulation did not correlate with shell width (in all populations P≥0.57). The interindividual variation in sperm number within the populations examined (expressed as CV) was high: Gurnigel 43.6% (n=57), Thun 21.5% (n=14), Nuglar 37.7% (n=24), and Allschwil 38.3% (n=16).
Relationship between sperm length and number and female reproductive allocation
Considering all snails from the four populations, mean sperm length was positively correlated with the number of sperm transferred during copulation (log-transformed data: r=0.43, n=111, P<0.001; Fig. 2). However, this correlation was no longer significant after removing the effect of shell size (residuals from sperm length–shell width and sperm number–shell width relationships: r=0.02, n=111, P=0.81). Similarly, considering snails from each population separately, mean sperm length was not correlated with the number of sperm delivered (in all populations P≥0.10; Fig. 2). Thus, sperm length and the number of sperm transferred during copulation are independent reproductive traits in A. arbustorum.
In simultaneous hermaphrodites, reproductive allocation to the male function (i.e., sperm production) is not independent on reproductive allocation to the female function (egg production). We used albumen gland dry weight as an estimate for female reproductive allocation (Table 1). However, neither sperm length nor sperm number were correlated with albumen gland weight (pooled data after removing the effect of shell size: albumen gland weight–sperm length: r=0.11, n=71, P>0.35; albumen gland weight–sperm number: r=0.03, n=70, P>0.83).
Sperm motility
Variation in sperm velocity
VCL, the mean velocity of the actual sperm track, was recorded twice for each spermatophore at 120 and 190 min after activation; mean VCL of the two samples did not differ (paired t test: t=1.03, df=17, P=0.32). Independent of shell width, mean VCL did not differ between snails from the Gurnigel and Nuglar populations (one-way ANCOVA: shell width: F 1,20=0.01, P=0.92; population: F 1,20=0.42, P=0.52; Fig. 3). Thus, for further analyses we pooled the data on sperm velocity from snails of both populations.
Mean VCL (79.8±3.3 μm/s, n=23) differed among individual snails (one-way ANOVA: F 22,333=5.70, P<0.001). VCL ranged from 52 to 112 μm/s and showed an interindividual variation (expressed as CV) of 33% (n=23; Fig. 3). The distribution of mean VCL values did not differ from a normal distribution (Shapiro–Wilk test: df=23, P=0.47; Fig. 3). Furthermore, VCL values of all sperm measured in each spermatophore fitted a normal distribution (Shapiro–Wilk test: for all 23 snails P≥0.08). Similar results were obtained when sperm velocity was measured as straight-line velocity (VSL: 20.2±1.1 μm/s, n=23; data not shown).
Velocity of sperm in bundles
Sperm were observed to leave the spermatophore in bundles, which consisted of 50–100 sperm oriented in the same direction with wrapped flagella that beat synchronously. Mean VSL was calculated from 4–12 bundles per spermatophore. In the microscopic chamber, VSL of sperm bundles (53.0±5.1 μm/s, n=9) did not differ from VSL of singly swimming sperm (54.8±4.2 μm/s, n=9; paired t test: t=0.29, df=8, P=0.78).
Percentage motility and longevity
Percentage motility (the number of active sperm out of 100 sperm observed) was not affected by shell width; populations differed in percentage motility (one-way repeated measures ANCOVA: shell width: F 1,14=3.82, P=0.07; population: F 1,14=34.24, P<0.0001; time: F 2,28=2.63, P=0.09; Fig. 4). No sperm were motile after 21 h in snails from the Gurnigel population and after 39 h in snails from the Nuglar population (Fig. 4). Sperm longevity (time elapsed since activation until 95% of the sperm were inactive) differed between populations (Gurnigel 18.9±1.5 h, n=7; Nuglar 35.5±1.2 h, n=9), but was not affected by shell width (one-way ANCOVA: shell width: F 1,13=0.01, P=0.93; population: F 1,13=53.6, P<0.0001).
Relationships between sperm length and motility
No correlations between sperm length and motility parameters were found within populations (Table 3). Sperm length and sperm velocity were not correlated in pooled data from both populations (residuals from sperm length–shell width and sperm number–shell width relationships: r=0.21, n=23, P=0.34). Sperm in the Nuglar population were, on average, 61 μm longer than sperm in the Gurnigel population (Table 1) but they did not swim faster (Fig. 3). Furthermore, no correlations were found between sperm motility parameters (for all comparisons: Gurnigel P≥0.19; Nuglar P≥0.19). Thus, the sperm quality traits examined varied independently.
Discussion
The present study showed significant differences in sperm length, both among- and within-populations of A. arbustorum. To our knowledge, we present for the first time quantitative evidence for intraspecific sperm length variation in a simultaneously hermaphroditic gastropod. Different processes of postcopulatory sexual selection may result in sperm size differences (sperm competition, e.g., LaMunyon and Ward 2002; cryptic female choice, e.g., Pitnick et al. 2003), which in turn may lead to different paternity success (e.g., Oppliger et al. 2003). Therefore, interindividual differences in sperm length could account for the unexplained variance in fertilization success in A. arbustorum (Baur 1998).
Intraspecific variation in sperm length is widespread in gonochoristic animals (reviewed in Ward 1998 and Snook 2005). However, most of the previous studies focused on differences in sperm length among individuals. We found also considerable differences in sperm length among A. arbustorum populations, which could not be explained simply by differences in snail size. This is interesting, as most reproductive traits (e.g., egg size, clutch size) are size-related in A. arbustorum (Baur 1988b, 1990; Baur and Raboud 1988; Baur et al. 1998). The difference in sperm length observed between populations could be a result of (1) genetic differences between populations, (2) a bias introduced by sampling, and/or (3) different phenotypically plastic responses by animals sampled under different field conditions. These explanations are not mutually exclusive. Parent–offspring and sib comparisons revealed a significant heritability of sperm length in A. arbustorum, indicating a genetic basis (Minoretti et al., in preparation). Sample size effects might be small, as we examined sperm length in 58, 29, 16, and 14 individuals from the four populations. Sperm length was also significantly different between the two populations with the largest sample sizes (Gurnigel: n=58; Nuglar: n=29). In these two populations, sperm length was measured in 2 years (2001 and 2002). Sperm length did not differ among years in the Gurnigel population with relatively large sample sizes (30 and 28 snails in the 2 years). In the Nuglar population, however, sperm length differed between snails examined in 2001 and 2002. This difference could be due to relatively small sample sizes (16 and 13 snails).
We measured total sperm length because the borders between head, midpiece, and tail are indistinct in helicid spermatozoa (Dohmen 1983). The sperm head of A. arbustorum measures approximately 8 μm (1% of the total sperm length; Bojat et al. 2001). It is therefore unlikely that sperm head size accounted for the interindividual differences in sperm length. Thus, A. arbustorum produces monomorphic spermatozoa (Bojat et al. 2001), which vary in length. Individuals of A. arbustorum showed consistent sperm length in two successive matings (Minoretti et al., in preparation). Furthermore, sperm length was not affected by snail size and the weight of the albumen gland (a measure for female reproductive allocation). This suggests that sperm length in A. arbustorum is not dependent on body conditions, confirming similar studies on gonochoristic animals (e.g., Hellriegel and Blanckenhorn 2002; Schulte-Hostedde and Millar 2004). It remains to be determined whether snails with long sperm actually enjoy an increased fertilization success in multiply mated snails.
An intriguing aspect related to variation in sperm characteristics is that for quantitative traits like sperm size and sperm velocity, different “groups” of sperm within ejaculates may exist (e.g., Thurston et al. 1999, 2001). Although differences in sperm length are continuous within individuals of A. arbustorum, our results indicate that sperm length within the same snail is highly variable (the within-individual variation explained 33.7% of total sperm length). The presence of specific “groups” within ejaculates may provide a selective fertility advantage (e.g., Thurston et al. 1999, Baer et al. 2003). Bet-hedging for variable sperm may be selected for when it allows either differential ejaculation of a certain length fraction after recognition of the type of partner, or differential storage of a certain length fraction by partners of specific types (Baer et al. 2003). At least in some sperm-dimorphic species, females seem to be able to discriminate between groups of morphologically distinct sperm. For example, only short sperm were found in the spermatheca of Drosophila subobscura (Bressac and Hauschteck-Jungen 1996) and the cicada Graptopsaltria nigrofuscata (Kubo-Irie et al. 2003). In contrast, only long sperm enter the external layer of eggs in six Drosophila species (Karr and Pitnick 1996). In A. arbustorum, the female role may have some control over the fertilization of the eggs by selective sperm use (Baur 1994; Bojat and Haase 2002). Therefore, sperm length variation could also result from selective sperm storage by females (e.g., Miller and Pitnick 2002).
Independent of snail size differences, A. arbustorum individuals transferred different amounts of sperm during copulation. Sperm number was correlated neither with shell width nor with albumen gland weight. In A. arbustorum, sperm production seems to be less constrained by energy and nutrient uptake than egg production (Locher and Baur 2002). When sperm competition is fundamentally analogous to a raffle, the relative number of sperm will be the primary determinant of fertilization success (Parker 1993; Parker and Begon 1993). However, A. arbustorum does not adjust the number of sperm delivered to the actual risk of sperm competition (Locher and Baur 2000). In our study, sperm length did not trade-off with sperm number; as predicted by theory, these traits should vary independently under sperm competition (Parker 1998). The smaller coefficient of variation of sperm length compared to sperm number could indicate that sperm size is under stronger selection. Thus, sperm length may be a better predictor of fertilization success than the number of sperm transferred during copulation in A. arbustorum.
Interindividual differences in sperm motility could result in differential fertilization success (sperm velocity, e.g., Kupriyanova and Havenhand 2002; percentage motility, e.g., Froman et al. 2002). Snails differed in sperm motility parameters; the effect of sperm motility on fertilization success has not yet been examined in A. arbustorum. After copulation, sperm leaving the spermatophore should avoid the bursa copulatrix, where they are immobilized rapidly and digested (Lind 1973); only 0.1% of the sperm delivered reach the storage organ (spermatheca). Thus, interindividual differences in sperm velocity and percentage motility could enhance snail fertilization success by increasing the number of sperm that escape the bursa copulatrix and reach the spermatheca. Sperm swimming in bundles did not swim faster than singly swimming sperm, but sperm bundles could likely generate higher trusting forces and move along the female tract (e.g., Hayashi 1998). Thus, the transfer of sperm in bundles could be also an adaptation to overcome the “sperm trap” bursa copulatrix. Furthermore, there is some evidence that sperm motility may influence sperm storage (e.g., Rogers and Chase 2002; Froman 2003).
Longer sperm may be advantageous under conditions of sperm competition if they swim faster than shorter sperm as predicted by theory (Katz and Drobnis 1990). There is empirical evidence supporting this fact from species comparisons (mammals, Gomendio and Roldan 1991; Drosophila sp., Joly et al. 1991). However, there is no evidence of a relationship between sperm size and velocity within species, for either external (Atlantic salmon, Gage et al. 2002) or internal (zebra finch, Birkhead et al. 2005) fertilizers. We did not find any direct association between sperm length and sperm velocity in A. arbustorum, confirming these previous studies. Although snails from the two populations examined differed in mean sperm length, the swimming velocity of sperm did not differ. Sperm length could also contribute to A. arbustorum fertilization success if longer sperm generate greater flagellar forces as predicted by theory (Katz and Drobnis 1990). Sperm thrusting force is likely to be an important trait for gamete competence in internal fertilizers, increasing the probability of the sperm reaching the storage organ.
In species with internal fertilization and long-term sperm storage, theory predicts that sperm length can confer a fertilization advantage provided there is a positive association between sperm length and longevity (Parker 1998). So far, no study has examined this relationship in simultaneous hermaphrodites, nor in other internally fertilizing species with long-term sperm storage (see Snook 2005). In the Atlantic salmon, sperm length was not correlated with sperm velocity, but sperm length and longevity were negatively associated (Gage et al. 2002). In A. arbustorum, viable sperm could be stored in the spermatheca up to 1 year after copulation (Baur 1988a). There is no indication that sperm are nourished through the epithelium of the female reproductive tract in A. arbustorum (Bojat et al. 2001). In helicid snails, the spermatozoa are richly supplied with glycogen granules as an energy supply for sperm motility (Anderson and Personne 1976). In A. arbustorum, the glycogen granules are arranged in a helical structure along the axoneme of the sperm tail to the end of the flagellum (Bojat et al. 2001). Thus, longer flagella might contain more resources, resulting in large sperm with a higher energy supply for motility and/or longevity than smaller ones. Indeed, the longer sperm of snails from the Nuglar population survived longer than the shorter sperm of snails from the Gurnigel population. Within populations, however, no relationship between sperm length and longevity was found. Moreover, sperm velocity was not correlated with sperm longevity. Further work is needed to assess the energy supply of A. arbustorum sperm (ATP and/or glycogen content). For example, in domestic fowl, longer sperm have increased motility because of their higher ATP/mitochondria content (Froman et al. 1999, 2002).
Our data did not reveal any association between sperm size (total length) and sperm function (velocity, motility and longevity). Our findings do not support the assumptions of sperm competition models on the evolution of sperm size for internally fertilizing species like A. arbustorum (Parker 1998). Several of these comparisons could be nonsignificant due to low power in the statistical tests. To reach a power of 80% (α=0.05), sample sizes of >190 snails are required. However, the use of phenotypic measures to assign relationships between ejaculate traits might not be conclusive (Moore et al. 2004; Snook 2005). Future research should examine genetic correlations between sperm traits to elucidate the constraints on the evolution of sperm morphology and function (e.g., Moore et al. 2004).
Our results are relevant for understanding the mechanisms underlying fertilization success in simultaneous hermaphrodites. We found interindividual differences in sperm quality that can explain the differential paternity success of A. arbustorum individuals (Baur 1998). Considerable variation was also detected within individuals and among populations of A. arbustorum. Thus, the intraspecific variation in sperm traits in nature is likely to be even greater than reported here and in similar studies (reviewed in Ward 1998 and Snook 2005). Models of sexual selection for the evolution of sperm morphology and function should take into account different processes of postcopulatory sexual selection (e.g., Snook 2005). In this study, we showed how the interindividual differences in the sperm quality traits found in A. arbustorum could be a response to sperm competition risk in interaction with cryptic female choice.
References
Anderson WA, Personne P (1976) The molluscan spermatozoon: dynamic aspects of its structure and function. Am Zool 16:292–313
Baer B, Schmid-Hempel P, Høeg JT, Boomsma JJ (2003) Sperm length, sperm storage and mating system characteristics in bumblebees. Insectes Soc 50:101–108
Baminger H, Haase M (2001) Spermatophore formation in the simultaneously hermaphroditic land snail Arianta arbustorum (Pulmonata: Stylommatophora: Helicidae). Neth J Zool 51:347–360
Baur B (1984) Shell size and growth rate differences for alpine populations of Arianta arbustorum (L.) (Pulmonata: Helicidae). Rev Suisse Zool 91:37–46
Baur B (1988a) Repeated mating and female fecundity in the simultaneously hermaphroditic land snail Arianta arbustorum. Invertebr Reprod Dev 14:197–204
Baur B (1988b) Population regulation in the land snail Arianta arbustorum: density effects on adult size, clutch size and incidence of egg cannibalism. Oecologia 77:390–394
Baur B (1990) Seasonal changes in clutch size, egg size and mode of oviposition in Arianta arbustorum L. (Gastropoda) from alpine populations. Zool Anz 225:253–264
Baur B (1994) Multiple paternity and individual variation in sperm precedence in the simultaneously hermaphroditic land snail Arianta arbustorum. Behav Ecol Sociobiol 35:413–421
Baur B (1998) Sperm competition in molluscs. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic, London, pp 255–305
Baur B, Baur A (1992) Effect of courtship and repeated copulation on egg production in the simultaneously hermaphroditic land snail Arianta arbustorum. Invertebr Reprod Dev 21:201–206
Baur B, Raboud C (1988) Life history of the land snail Arianta arbustorum along an altitudinal gradient. J Anim Ecol 57:71–87
Baur B, Locher R, Baur A (1998) Sperm allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Anim Behav 56:839–845
Birkhead TR, Møller AP (1998) Sperm competition and sexual selection. Academic, London
Birkhead TR, Martinez JG, Burke T, Froman DP (1999) Sperm mobility determines the outcome of sperm competition in the domestic fowl. Proc R Soc Lond B 266:1759–1764
Birkhead TR, Pellatt EJ, Brekke P, Yeates R, Castillo-Juarez H (2005) Genetic effects on sperm design in the zebra finch. Nature 434:383–387
Bojat NC, Haase M (2002) Sperm storage in the simultaneously hermaphroditic land snail Arianta arbustorum. J Zool 258:497–503
Bojat NC, Sauder U, Haase M (2001) The spermathecal epithelium, sperm and their interactions in the hermaphroditic land snail Arianta arbustorum (Pulmonata, Stylommatophora). Zoomorphology 120:149–157
Bressac C, Hauschteck-Jungen E (1996) Drosophila subobscura females preferentially select long sperm for storage and use. J Insect Physiol 42:323–328
Burness G, Casselman SJ, Schulte-Hostedde AI, Moyes CD, Montgomerie R (2004) Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus). Behav Ecol Sociobiol 56:65–70
Chen XF, Baur B (1993) The effect of multiple mating on female reproductive success in the simultaneously hermaphroditic land snail Arianta arbustorum. Can J Zool 71:2431–2436
Dohmen MR (1983) Gametogenesis. In: Wilbur KM (ed) The Mollusca, vol 3. Academic, Orlando, pp 1–48
Froman D (2003) Deduction of a model for sperm storage in the oviduct of the domestic fowl (Gallus domesticus). Biol Reprod 69:248–253
Froman DP, Feltmann AJ, Rhoads ML, Kirby JD (1999) Sperm mobility: a primary determinant of fertility in the domestic fowl (Gallus domesticus). Biol Reprod 61:400–405
Froman DP, Pizzari T, Feltmann AJ, Castillo-Juarez H, Birkhead TR (2002) Sperm mobility: mechanisms of fertilizing efficiency, genetic variation and phenotypic relationship with male status in the domestic fowl, Gallus gallus domesticus. Proc R Soc Lond B 269:607–612
Gage MJG, Macfarlane C, Yeates S, Shackleton R, Parker GA (2002) Relationships between sperm morphometry and sperm motility in the Atlantic salmon. J Fish Biol 61:1528–1539
Gage MJG, Macfarlane CP, Yeates S, Ward RG, Searle JB, Parker GA (2004) Spermatozoal traits and sperm competition in Atlantic salmon: relative sperm velocity is the primary determinant of fertilization success. Curr Biol 14:44–47
Gomendio M, Roldan ERS (1991) Sperm competition influences sperm size in mammals. Proc R Soc Lond B 243:181–185
Hayashi F (1998) Sperm co-operation in the fishfly, Parachauliodes japonicus. Funct Ecol 12:347–350
Healy JM (1996) Molluscan sperm ultrastructure: correlation with taxonomic units within the Gastropoda, Cephalopoda and Bivalvia. In: Taylor J (ed) Origin and evolutionary radiation of the Mollusca. Oxford Univ Press, Oxford, pp 99–113
Hellriegel B, Blanckenhorn WU (2002) Environmental influences on the gametic investment of yellow dung fly males. Evol Ecol 16:505–522
Hofmann E (1923) Über den Begattungsvorgang von Arianta arbustorum (L.). Jena Z Med Naturwiss 59:363–400
Hosken DJ, Garner TWJ, Ward PI (2001) Sexual conflict selects for male and female reproductive characters. Curr Biol 11:489–493
Joly D, Cariou ML, Lachaise D (1991) Can sperm competition explain sperm polymorphism in Drosophila teissieri? Evol Biol 5:25–44
Karr TL, Pitnick S (1996) The ins and outs of fertilization. Nature 379:405–406
Katz DF, Drobnis EZ (1990) Analysis and interpretation of the forces generated by spermatozoa. In: Bavister BD, Cummins J, Roldan ERS (eds) Fertilization in mammals. Serono Symposia, Norwell, MA, pp 125–137
Kerney MP, Cameron RAD (1979) A field guide to the land snails of Britain and northwest Europe. Collins, London
Kubo-Irie M, Irie M, Nakazawa T, Mohri H (2003) Ultrastructure and function of long and short sperm in Cicadidae (Hemiptera). J Insect Physiol 49:983–991
Kupriyanova E, Havenhand JN (2002) Variation in sperm swimming behaviour and its effect on fertilization success in the serpulid polychaete Galeolaria caespitosa. Invertebr Reprod Dev 41:21–26
LaMunyon CW, Ward S (2002) Evolution of larger sperm in response to experimentally increased sperm competition in Caenorhabditis elegans. Proc R Soc Lond B 269:1125–1128
Lessells CM, Boag PT (1987) Unrepeatable repeatabilities: a common mistake. Auk 104:116–121
Lind H (1973) The functional significance of the spermatophore and the fate of spermatozoa in the genital tract of Helix pomatia (Gastropoda: Stylommatophora). J Zool 169:39–64
Locher R, Baur B (1997) A new technique to assess the number of spermatozoa in spermatophores of stylommatophoran gastropods. J Molluscan Stud 63:555–556
Locher R, Baur B (1999) Effects of intermating interval on spermatophore size and sperm number in the simultaneously hermaphroditic land snail Arianta arbustorum. Ethology 105:839–849
Locher R, Baur B (2000) Sperm delivery and egg production of the simultaneously hermaphroditic land snail Arianta arbustorum exposed to an increased sperm competition risk. Invertebr Reprod Dev 38:53–60
Locher R, Baur B (2002) Nutritional stress changes sex-specific reproductive allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Funct Ecol 16:623-632
Meisenheimer J (1912) Die Weinbergschnecke: Helix pomatia L. Klinkhardt LW, Leipzig
Michiels NK (1998) Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic, London, pp 219-254
Miller GT, Pitnick S (2002) Sperm–female coevolution in Drosophila. Science 298:1230–1233
Moore PJ, Harris WE, Montrose VT, Levin D, Moore AJ (2004) Constraints on evolution and postcopulatory sexual selection: trade-offs among ejaculate characteristics. Evolution 58:1773–1780
Oppliger A, Naciri-Graven Y, Ribi G, Hosken DJ (2003) Sperm length influences fertilization success during sperm competition in the snail Viviparus ater. Mol Ecol 12:485–492
Parker GA (1993) Sperm competition games: sperm size and sperm number under adult control. Proc R Soc Lond B 253:245–254
Parker GA (1998) Sperm competition and the evolution of ejaculates: towards a theory base. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic, London, pp 3–54
Parker GA, Begon ME (1993) Sperm competition games: sperm size and number under gametic control. Proc R Soc Lond B 253:255–262
Peng J, Chen S, Büsser S, Liu HF, Honegger T, Kubli E (2005) Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol 15:207–213
Pitnick S, Miller GT, Reagan J, Holland B (2001) Males’ evolutionary responses to experimental removal of sexual selection. Proc R Soc Lond B 268:1071–1080
Pitnick S, Miller GT, Schneider K, Markow TA (2003) Ejaculate-female coevolution in Drosophila mojavensis. Proc R Soc Lond B 270:1507–1512
Radwan J (1996) Intraspecific variation in sperm competition success in the bulb mite: a role for sperm size. Proc R Soc Lond B 263:855–859
Rogers DW, Chase R (2002) Determinants of paternity in the garden snail Helix aspersa. Behav Ecol Sociobiol 52:289–295
Romeis B (1989) Mikroskopische Technik. 17 edn. Urban und Schwarzenberg, München
Scheiner SM, Gurevitch J (1993) Design and analysis of ecological experiments. Chapman & Hall, New York
Schulte-Hostedde AI, Millar JS (2004) Intraspecific variation of testis size and sperm length in the yellow-pine chipmunk (Tamias amoenus): implications for sperm competition and reproductive success. Behav Ecol Sociobiol 55:272–277
Simmons LW (2001) Sperm competition and its evolutionary consequences in the insects. Princeton Univ. Press, Princeton, NJ
Snook RR (2005) Sperm in competition: not playing by the numbers. Trends Ecol Evol 20:46–53
Stockley P, Gage MJG, Parker GA, Møller AP (1997) Sperm competition in fishes: the evolution of testis size and ejaculate characteristics. Am Nat 149:933–954
Thompson TE (1973) Euthyneuran and other molluscan spermatozoa. Malacologia 14:167–206
Thurston LM, Watson PF, Holt WV (1999) Sources of variation in the morphological characteristics of sperm subpopulations assessed objectively by a novel automated sperm morphology analysis system. J Reprod Fertil 117:271–280
Thurston LM, Watson PF, Mileham AJ, Holt WV (2001) Morphologically distinct sperm subpopulations defined by Fourier shape descriptors in fresh ejaculates correlate with variation in boar semen quality following cryopreservation. J Androl 22:382–394
Ward PI (1998) Intraspecific variation in sperm size characters. Heredity 80:655–659
Zschokke S (2003) BioPictViewer+, v1.10 for Macintosh, University of Basel
Acknowledgements
We thank K. Beese, M. Dürrenberger (Microscope Centre, University of Basel), M. Haase, R. Locher, A. Wacker, and S. Zschokke for advice and help. We are grateful to C. De Geyter (Univ. Hospital Basel) for providing access to the IVOS-semen analysis system, and M. Fusco (Bauman Medical AG) for technical support. G. A. Armbruster, A. Baur, K. Beese, J. Mevi-Schütz, P. Stoll, S. Zschokke and an anonymous referee provided valuable comments on the manuscript. Financial support was received from the Swiss National Science Foundation. We declare that our study is in accordance with the current Swiss laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Moore
Rights and permissions
About this article
Cite this article
Minoretti, N., Baur, B. Among- and within-population variation in sperm quality in the simultaneously hermaphroditic land snail Arianta arbustorum . Behav Ecol Sociobiol 60, 270–280 (2006). https://doi.org/10.1007/s00265-006-0165-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-006-0165-5