Abstract
Caste theory predicts that social insect colonies are organized into stable groups of workers specialized on particular task sets. Alternative concepts of organization of work suggest that colonies are composed of extremely flexible workers able to perform any task as demand necessitates. I explored the flexibility of workers in temporal castes of the honey bee Apis mellifera by determining the ability of colonies to reorganize labor after a major demographic disturbance. I evaluated the flexibility of temporal castes by comparing the foraging rates of colonies having just lost their foragers with colonies having also lost their foragers but having been given a week to reorganize. The population sizes and contents of the colonies in each group were equalized and foraging rates were recorded for one week. Colonies given a week’s initial recovery time after the loss of their foragers were found to forage at significantly higher rates than those colonies given no initial recovery time. This result was consistent for nectar and pollen foraging. These results suggest that honeybee workers lack sufficient flexibility to reorganize labor without compromising foraging. This finding is consistent with the caste concept model of organization of work in insect societies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of the organization of work in social insect colonies typically focus on either of two areas: colony organization or task allocation. Students of colony organization tend to assume that the colony has evolved what Wilson thought of as “a strategic design” for the efficient performance of the colony’s tasks (reviewed in Wilson 1971; Oster and Wilson 1978; Hölldobler and Wilson 1990). According to this view, the need for maximizing the production of work at the colony level leads to a stable division of labor among the workers. The caste concept, according to which in many species the workers of a colony are organized into groups of specialists characterized by size or age, encapsulated this view (Wilson 1968, 1976, 1985; Sakagami 1953; Seeley 1982; Jeanne 1988). In contrast, studies of task allocation focus on understanding how a colony allocates labor among tasks in relation to changing labor needs within a colony (reviewed in Gordon 1996). Researchers in this area tend to assume that there is a strong trade-off between specialization in task performance, which leads to castes, and flexibility in task performance, which enables a rapid reorganization of workers in response to changing demands for work. Due to this trade off, caste is assumed to be of limited value for understanding task allocation (Bourke and Franks 1995).
Although the flexibility of workers in colonies under stress was well established (Rösch 1930; Lindauer 1952; Wilson 1984), subsequent studies showing large amounts of task switching in colonies not under great stress cast doubt on the relatively neat picture presented by earlier researchers (reviewed in Calabi 1988; Calabi and Rosengraus 1988; Calabi and Traniello 1989a, b; Gordon 1989a, b). Gordon suggested that the concept of caste was outdated and that a term such as ‘task group’ would be more appropriate since it does not imply a stable specialization on the part of a worker performing a task.
Although the study of task allocation was somewhat neglected in the past in favor of caste studies, current models of task allocation that ignore the existence of castes neglect many studies showing clear caste differences (Sakagami 1953; Wilson 1976, 1980a, b; Seeley 1982; Seeley and Kolmes 1991; Jeanne et al. 1988; Naug and Gadagkar 1998). One can make compatible the caste concept and the current views of task allocation stressing flexibility if one revises the caste concept such that it allows for increased flexibility in task allocation. In a previous paper, I report support for the hypothesis that the caste concept only applies to those tasks for which a physiological specialization is required for the task’s performance (Johnson 2003). Honeybee nurses, for example, have active hypopharyngeal glands that are used to produce food for the brood. Foragers have atrophied hypopharyngeal glands and thus cannot perform the task without undergoing a lengthy process of glandular regeneration. In addition to physiological differences, caste differences may also be based on morphological differences or on the differential learning of information necessary for efficient task performance.
In general, this revised caste concept stressing the importance of relatively inflexible differences between workers directly related to the performance of the tasks around which the caste system is organized is important for the study of task allocation because only a handful of tasks require an inflexible specialization for their performance, meaning most tasks can be performed by any worker as the need arises. Further, workers spend a considerable amount of time inactive; thus, there is always a ready supply of reserve labor under normal circumstances. Taken together, these findings suggest that a colony should have little difficulty reallocating labor within the confines of the caste system for the majority of their tasks. Studies showing frequent task switching are therefore not fatal criticisms of the caste concept (reviewed in Calabi 1988; Gordon 1989b). Left unsettled, however, is the question: what happens when labor is needed for those key tasks requiring specializations? The revised caste system, just briefly outlined, is still inflexible with respect to tasks requiring a specialization. The argument has been made that flexibility within the colony’s most critical (physiologically dependent) tasks would be adaptive, since a large portion of the foragers may be lost in catastrophes such as storms (Bourke and Franks 1995). That the caste concept cannot allow for this degree of reallocation of labor has led some authors to favor a method of organization dependent not on stable internal differences in workers, but on flexible rules of thumb, which allow any worker to quickly fill in for any other (Gordon 1989a, 1989b; Bourke and Franks 1995; Deneubourg and Goss 1989).
These arguments stressing the importance of flexibility in task allocation, however, are based on the largely untested assumption that colonies can quickly recover from catastrophic events that destroy most of the members of a particular caste. The impetus for this assumption lies in studies showing that colonies initiated with a single age cohort of workers can differentiate into fully functional colonies (Rösch 1930; Robinson et al. 1989, 1992). Studies have shown that within a day or two of the loss of a caste, some of the remaining workers begin to show changes in their titers of juvenile hormone, which correlates with the transition between castes (Robinson et al. 1989; Huang and Robinson 1996; reviewed in Huang and Robinson 1999). What these studies do not show, however, is what effect the demographic disturbance has at the colony level. These studies focused just on those individuals that accelerated or reversed their development, not on the level of work being performed at the colony level.
Determining whether honey bee colonies can quickly reorganize labor after a catastrophe eliminating most of the workers of one caste is the purpose of this study. That colonies can accomplish this feat has been suggested many times, and evidence suggesting that colonies in nature may withstand large periodic losses due to predation exists, but to date no study has recorded the performance of colonies having undergone a catastrophic loss of workers. It is thus possible that colonies have not evolved this ability and their degree of flexibility in task allocation has been exaggerated.
I experimentally explored this question by removing the foragers from two groups of colonies. One group was given one week to reorganize after the loss of their foragers, while the other group was given no initial recovery time. If castes do not exist, then the week separating treatments should have been unnecessary for the reorganization of the labor force and therefore of no consequence to the functioning of the colony. If, however, temporal castes do exist, then one would expect a decrease in the foraging rates of those colonies given less time to recover after the loss of their foragers. The ability of the colonies to reorganize after the loss of their foragers was assessed by recording their foraging rates for a week after the loss of the foragers in the second group of colonies. A week is an appropriate length of time for two reasons: workers in the honey bee only remain in each of their within-nest temporal castes for about one week, (Seeley 1982), and second, the honey bee economy is characterized by a boom and bust cycle. Weeks, or even months, of meager foraging can be followed by intense nectar flows (Seeley 1985). Colonies likely gain much of the weight they need to last through the winter during these short nectar flows. Colonies, therefore, need to be able to quickly, within days, maximize their intake of nectar. Thus, whatever system of organization the honey bee has evolved for reallocating labor must operate on the time scale of hours or days, not weeks.
Materials and methods
Bees and study site
This study was conducted in the summer of 2003 at the Liddell Field Station of Cornell University in Ithaca, NY. Colonies of bees were housed indoors in observation hives and were given access to the outdoors via tubes extending outside. Colonies were housed in two frame observation hives. Twelve colonies of Italian honey bees, Apis mellifera lingustica, were used in the study. Six colonies were assigned to each of the treatments.
Setting up colonies
The same procedure was used for setting up both groups of colonies. Each colony was established by taking two full-depth frames of comb from the brood nest of the second story of a two story Langstroth hive and placing them in observation hives. Caged queens were then added to each colony. Queens were released two days after their introduction. All queens used in the study were accepted by their colonies. Additional bees from the brood nests of the same source colonies were added to the observation hives because the broodnests of large colonies typically contain relatively low numbers of bees and taking bees from elsewhere would likely have contaminated the new colonies with foragers. Bees were added until colonies were equal in size. Combs were chosen such that they contained relatively equal amounts of open brood, pollen, and honey. Thus, all colonies contained sufficient stores of honey and pollen to prevent starvation. None of the colonies contained brood near eclosing at the time of set up, thus, colony size was stable throughout the two weeks of the experiment.
Description of the experiment
Two trials of the experiment were performed. Each trial of the experiment required six colonies. The first three colonies were set up according to the procedure outlined above then left undisturbed for seven to ten days. Seven days was the goal, but poor weather stretched it to ten in the second trial. After this window of time, the remaining three colonies were set up. No data were collected on the day following the set up of the second set of colonies in order to give those colonies time to reorient to their new location. Pilot studies showed that two days is sufficient for a normally functioning colony to reorient to a new location and begin foraging. Data collection thus began on the third day following the set up of the second set of colonies.
Data collection
Five minute counts of the number of foragers entering each colony were recorded hourly. Whether or not each forager was carrying pollen also was recorded. Those not carrying pollen were assumed to be carrying nectar. Counts were not made in the late afternoon when colonies were performing orientation flights. Four counts of foragers were made per colony per day for five days in each trial. Day four in the second trial was rained out. Two of the hourly counts, one in trial one and one in trial two were also rained out.
On day three of each trial’s data collection period, I performed a census of each colony. Over the glass on both sides of each hive was placed a 4×4 cm numbered grid. The number of bees in twenty percent of the grid cells was counted and that number was multiplied by five to estimate colony size. After each census the same grid was used to estimate the contents of the combs of each colony. I recorded whether the comb in each grid square contained brood, pollen, nectar, or empty cells. If a cell contained more than one substance an estimate was made of the fraction of each type of material.
Statistical analysis
Mixed model regression (SAS Institute 1998) was used to test for differences in foraging rates between the two treatments (proc-mixed module of SAS). In this analysis, colony and trial were defined as random effects. The Mann-Whitney U–Wilcoxon Rank-Sum W Test was used to test for differences in population size, nectar cells, pollen cells, brood cells, and empty cells.
Results
Colony censuses
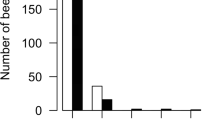
Population sizes for the two groups of colonies are shown in Fig. 1a. Although the colonies were set up a week apart, there was no difference in population size between the two groups (Wilcoxon Rank Sum: W=33.5, n1=6, n2=6, P=0.42). This can be attributed to the fact that approximately equal numbers of bees were introduced to all colonies and that care was made in choosing combs from which brood was unlikely to emerge during the week between the establishment of the two sets of colonies.
a Mean (± SE) population sizes for colonies given 7+ days recovery time and colonies given no initial recovery time. b Mean area in cm2 (±SE) of comb containing nectar cells, empty cells, brood cells, and pollen cells. The number of pollen cells was the only significant difference between the two groups (see text)
Colony contents
The amount of stored honey, stored pollen, number of empty cells, and number of cells containing brood for each group of colonies at the midpoint of the experiment is shown in Fig. 1b. Colonies given a week to recover from the loss of their foragers and colonies given no initial recovery time were not significantly different in their amounts of nectar (Wilcoxon Rank Sum: W=37.0, n1=6, n2=6, P=0.81), empty cells (Wilcoxon Rank Sum: W=36.0, n1=6, n2=6, P=0.69), or brood cells (Wilcoxon Rank Sum: W=47.0, n1=6, n2=6, P=0.23). Treatments did differ in their amounts of stored pollen (Wilcoxon Rank Sum: W=53.5, n1=6, n2=6, P=0.025). Pollen was the only colony variable I was unable to control by carefully choosing combs because the colonies were actively foraging during the week prior to the set up of the second group of colonies.
Studies of the effect of pollen on foraging have shown that adequate pollen stores retard foraging for pollen. In this experiment, those colonies given more recovery time had slightly more pollen that those colonies having just lost their foragers. However, the colonies given a week of recovery time also foraged more for pollen than did those colonies not given any initial recovery time (shown later). This means that although there was a slight difference in pollen stores between the two treatments, the difference was not large enough to retard pollen foraging in those colonies given a week to recover from the loss of their foragers. Both treatments were thus pollen deficient, but the extended recovery colonies were slightly less so.
Effect of recovery time on total foraging
The total foraging effort for the two groups of colonies is shown in Fig. 2. Colonies given a week to recover from the loss of their foragers foraged at significantly higher rates than did colonies not given any initial recovery time (F1,184=58.53, P<0.0001). A significant time effect was also found (F1,10=9.56, P=0.011) indicating that both groups of colonies varied in their rates of foraging over the course of the week. Colonies given a week to recover from the loss of their foragers appeared to fluctuate more in total foraging rate over the observation period, while the treatment receiving no initial recovery time showed a more steady increase, however, the interaction between response and time was not significant (F1,184=0.31, P=0.57).
Mean (±SE) number of total foragers entering the colony per day for colonies given 7+ days initial recovery time and colonies given no initial recovery time. a Trial 1, conducted beginning on 8 August 2003. b Trial 2, conducting beginning on 23 August 2003. Colonies given no initial recovery time foraged at significantly lower rates than those given seven days initial recovery time (see text)
Effect of recovery time on nectar foraging
The effect of recovery time on the rate of nectar foraging is shown is Fig. 3. Colonies given a week to recover from the loss of their foragers foraged significantly more than did those colonies given no initial recovery time (F1,184=30.95, P<0.0001). There was also a significant time effect (F1,10=8.53, P=0.015). The interaction between time and treatment, however, was not significant (F1,184=0.08, P=0.77) indicating that although the foraging rate changed in both groups the change was not different between groups. This finding, that both groups varied their foraging efforts significantly over the course of a few days, is in keeping with what is known about nectar foraging, specifically that it is very sensitive to changes in the amount of nectar available in the field.
Mean (±SE) number of nectar foragers entering the colony per day for colonies given 7+ days initial recovery time and colonies given no initial recovery time. a Trial 1, conducted beginning on 8 August 2003. b Trial 2, conducting beginning on 23 August 2003. Colonies given no initial recovery time foraged at significantly lower rates than those given seven days initial recovery time (see text)
Effect of recovery time on pollen foraging
The pollen foraging rates for the two groups are shown in Fig. 4. Colonies given a week’s initial recovery time foraged at a higher rate than the colonies that received no initial recovery time (F1,184=21.70, P<0.0001). There were no significant time (F1,10=2.13, P=0.175) or time by treatment effects (F1,184=2.28, P=0.13), indicating that the availability of pollen was not as sensitive to short term fluctuations in the environment as was the availability of nectar.
Mean (±SE) number of pollen foragers entering the colony per day for colonies given 7+ days initial recovery time and colonies given no initial recovery time. a Trial 1, conducted beginning on 8 August 2003. b Trial 2, conducting beginning on 23 August 2003. Colonies given no initial recovery time foraged at significantly lower rates than those given seven days initial recovery time (see text)
Discussion
The results of this study show that honey bee colonies do not quickly recover from the loss of their foragers. There were significant differences in all three responses (total foraging, nectar foraging, and pollen foraging) between the two groups of colonies. In fact, at seven days after the loss of their foragers, colonies were still ‘recovering’ as they were still foraging at a reduced rate relative to those colonies which had been given an additional week of recovery time (Fig. 2).
These results support the caste concept view of colony organization as opposed to the view that colonies are composed of extremely flexible workers. The primary thrust of the caste concept is that workers have limited task repertoires. This leads to the prediction that the complete loss of a caste should cause a large decrease in the amount of work performed within the lost caste’s task repertoire. This study showed just such a decrease after the loss of the forager caste.
Honey bees have not evolved the ability to rapidly reorganize after a major demographic disturbance, suggesting that this sort of event does not occur frequently enough to have generated the evolution of this trait. Although it has been suggested that colonies may lose most of their foragers to storms (Bourke and Franks 1995), this is very unlikely. Bees have a refined ability to sense a coming storm, which is known to every beekeeper who has witnessed thousands of foragers suddenly appearing at the hive entrance immediately prior to a storm (Frisch 1967). In any event, when this or other catastrophic losses do occur, survivorship of such colonies through the winter is likely low.
There are two proximate explanations for the lack of flexibility found in this study. Within-nest bees either did not make the switch to foraging, or they did but were unable to forage at a rate comparable to more experienced foragers. There is evidence suggesting that within-nest bees can, under certain circumstances, switch castes. Schulz et al. (1998) found that young bees can increase their rate of development in response to food shortage. None of the colonies in this study was food deprived, so one can only speculate as to how this may have affected the results. However, a rough synthesis of Schulz et al’s study and this one might be that bees have the physiological ability to switch quickly, within a few days if they are hungry, but minus hunger are either incapable of switching (hunger is the only cue allowing them to switch) or lack the information gathering ability to determine that an increased rate of development is necessary. Determining which of these two possibilities is correct would be a productive area of future research on this topic.
There is evidence that honey bee foragers become better at their task over time (Menzel et al. 1973; reviewed in Winston 1987). Not only do caste-specific changes occur in the physiology of workers related specifically to foraging (Harrison 1986), but foragers also learn methods for better locating and working particular types of flowers (reviewed in Winston 1987; Dukas and Visscher 1994). Dukas and Visscher found that a forager’s rate of nectar uptake increases with experience, peaking at about 7 days into their foraging career. This increase in efficiency was found to be due to increases in the size of nectar loads, not to decreases in the length of foraging trips, which they found did not change with experience. Thus, it is unlikely that the effects of differential experience between the foragers in the two treatments in this study led to the differences observed, since foraging rate was recorded, not foraging efficiency.
Previous studies of caste flexibility in honeybees have stressed that colonies can reorganize after a major disturbance (Rösch 1930; Huang and Robinson 1992; reviewed in Robinson 1992; Bourke and Franks 1995). The physiological nature of the acceleration of the development of young bees and the developmental reversion of older bees have both been explored in some detail (Huang and Robinson 1996; reviewed in Huang and Robinson 1999). At the colony level, these studies provide a qualitative understanding of flexibility in honey bee colonies. They show that colonies have some degree of flexibility in responding to the loss of large numbers of workers. What these studies do not provide is a quantitative description of the process of reorganization. The functioning of demographically altered colonies in comparison to normal colonies was never explored. The absence of a quantitative description of how quickly colonies recover from catastrophes has lead to serious misunderstandings. Specifically, some authors have exaggerated both the ability of colonies to adjust to the catastrophic loss of workers and the ability of workers to quickly perform any task as demand necessitates (Bourke and Franks 1995; Beshers and Fewell 2001).
An intuitively appealing synthesis of the results of this study and the results of earlier studies on flexibility in honey bees is that colonies contain a relatively small number of bees which are extremely flexible allowing them to function as a buffer against the loss of large numbers of workers or against large changes in the environment necessitating unusually large shifts in task allocation. It is possible that the underlying mechanism may simply be a fast development rate in these bees. In support of this are the findings from numerous temporal polyethism studies showing naturally precocious foragers, those that begin foraging as young as 8–10 days old, in colonies not under any sort of stress (Sakagami 1953; Seeley 1982; Seeley and Kolmes 1991). In a sense, these extremely flexible workers may be the oil that lubricates the temporal caste system in times of stress.
In summary, this study clarifies a significant misunderstanding concerning the flexibility of honey bee colonies and returns the discussion of division of labor in honey bee colonies to one of caste as an explanation for colony organization. However, caste is merely one of two equally important questions in the study of the social physiology of insect societies. Task allocation, the study of how individuals know what to do and when, is equally important. It is important to stress that these two approaches to the study of social physiology are not in competition with one another but are entirely complementary. A colony, like a metazoan body, has many levels at which one must explore the system.
References
Beshers SN, Fewell JF (2001) Models of division of labor in social Insects. Annu Rev Entomol 46:413–440
Bourke AFG, Franks NR (1995) Social evolution in ants. Princeton University Press, Princeton, NJ
Calabi P (1988) Behavioral flexibility in Hymenoptera: a re-examination of the concept of caste. In: Trage JC (ed) Advances in Myrmecology. Brill Press, Leiden
Calabi P, Rosengraus R (1988) Interindividual differences based on behavior transition probabilities in the ant Camponotus sericeiventris. In: Jeanne RL (ed) Interindividual behavioral variability in social insects. Westview Press, Boulder, Colorado, pp 61–90
Calabi P, Traniello JFA (1989a) Behavioral flexibility in age castes of the ant Pheidole dentata. J Insect Behavior 2:663-677
Calabi P, Traniello JFA (1989b) Social organization in the ant Pheidole dentata. Physical and temporal caste ratios lack ecological correlates. Behav Ecol Sociobiol 24:69–78
Deneubourg JL, Goss S (1989) Collective patterns and decisions-making. Ethol Ecol Evol 1:295–311
Dukas R, Visscher PK (1994) Lifetime learning for foraging honey bees. Anim Behav 48:1007–1012
von Frisch K (1967) The dance language and orientation of bees. Harvard University Press, Cambridge
Gordon DM (1989a) Dynamics of task switching in harvester ants. Anim Behav 38:194-204
Gordon DM (1989b) Caste and change in social insects. In: Harvey PH, Partridge L (eds) Oxford Surveys in Evolutionary Biology. Oxford University Press, Oxford, UK
Gordon DM (1996) The organization of work in social insect colonies. Nature 380:121-124
Harrison JM (1986) Caste-specific changes in honeybee flight capacity. Physiol Zool 59:175–187
Hepburn HR, Radloff SE (1998) Honeybees of Africa. Springer, Berlin, Heidelberg, New York
Hölldobler B, Wilson EO (1990) The ants. Harvard University Press, Cambridge
Huang ZY, Robinson GE (1992) Honeybee colony integration—worker-worker interactions mediate hormonally regulated plasticity I division of labor. Proc Natl Acad Sci USA 89:11726–11729
Huang ZY, Robinson GE (1996) Regulation of honey bee division of labor by colony age demography. Behav Ecol Sociobiol 39:147–158
Huang ZY, Robinson GE (1999) Social control of division of labor in honey bee colonies. In: Detrain C, Deneubourg JL, Pasteels JM (eds) Information processing in social insects. Birkhauser Press, Basel
Jeanne RL, Downing HA, Post DC (1988) Age polyethism and individual variation in Polybia occidentalis, an advanced eusocial wasp. In: Jeanne RL (ed) Interindividual behavioral variability in social insects. Westview, Boulder
Johnson BR (2003) Organization of work in the honeybee: a compromise between division of labor and behavioral flexibility. Proc R Soc Lond B 270:147–152
Lindauer M (1952) Ein Beitrag zur Frage der Arbeitsteilung im Bienenstaat. Z Vergl Physiol 34:299–345
Menzel R, Erber J, Masuhr T (1973) Learning and memory in the honey bee. In: Browne LB (ed) Experimental analysis of insect behavior. Springer, Berlin, Heidelber, New York
Naug D, Gadagkar R (1998) The role of age in temporal polyethism in a primitively eusocial wasp. Behav Ecol Sociobiol 42(1)37–47
Oster GF, Wilson EO (1978) Caste and ecology in the social insects. Princeton University Press, Princeton
Robinson GE (1992) Regulation of division of labor in insect societies. Annu Rev Entomol 37:637–665
Robinson GE, Page GE, Strambi C, Srambi A (1989) Hormonal and genetic control of behavioral integration in honey bee colonies. Science 246:109–112
Robinson GE, Page RE, Strambi C, Strambi A (1992) Colony integration in Honey bees: mechanisms of behavioral reversion. Ethology 90:336–348
Rösch GA (1930) Untersuchungen über die Arbeitsteilung im Bienenstaat, II. Z Vergl Physiol 12:1–71
Sakagami SF (1953) Untersuchungen über die Arbeitsteilung in einem Zwergvolk der Honigbiene. Beiträge zur Biologie des Bienenvolkes, Apis mellifica L. I. Jpn J Zool 11:117–185
SAS Institute (1998) Changes and enhancements to release 6.12 of SAS/STAT. SAS Institute, Cary, NC
Schulz DJ, Huang ZY, Robinson GE (1998) Effects of colony food shortage on behavioral development in honey bees. Behav Ecol Sociobiol 42:295–303
Seeley TD (1982) Adaptive significance of the age polyethism schedule in honeybee colonies. Behav Ecol Sociobiol 11:287–293
Seeley TD (1985) Honeybee ecology. Princeton University Press, Princeton
Seeley TD (1997) Honey bee colonies are group-level adaptive units. Am Nat 150:S22–S24
Seeley TD, Kolmes SA (1991) Age polyethism for hive duties in honey bees—illusion or reality? Ethology 87:287–297
Wilson EO (1968) The ergonomics of caste in the social insects. Am Nat 102:41–66
Wilson EO (1971) The insect societies. Harvard University Press, Cambridge
Wilson EO (1976) Behavioral discretization and number of castes in an ant species. Behav Ecol Sociobiol 1:141–154
Wilson EO (1980a) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). I. The overall pattern in A. sexdens. Behav Ecol Sociobiol 7:143–156
Wilson EO (1980b) Caste and division of labor in leaf-cutter ants (Hymenoptera: Formicidae: Atta). II. The ergonomic optimization of leaf cutting. Behav Ecol Sociobiol 7:157–165
Wilson EO (1984) The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera: Formicidae). Behav Ecol Sociobiol 16:89–98
Wilson EO (1985) The sociogenesis of insect colonies. Science 228:1489–1495
Winston ML (1987) The biology of the honey bee. Harvard University Press, Cambridge
Acknowledgements
I would like to thank Tom Seeley for helpful advice in the design of the experiment and for commenting on the manuscript. I would also like to thank Kern Reeve, Cole Gilbert, and Paul Sherman for providing comments on the manuscript. This work was funded by a National Science Foundation Graduate Research Fellowship
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Traniello
Rights and permissions
About this article
Cite this article
Johnson, B.R. Limited flexibility in the temporal caste system of the honey bee. Behav Ecol Sociobiol 58, 219–226 (2005). https://doi.org/10.1007/s00265-005-0949-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0949-z