Abstract
Simultaneous hermaphrodites are predicted to optimally divide resources between male and female function, which can result in both size-dependent mating behaviors and conflict between potential mates. Predicted strategies include size-assortative mating, conditional exchange of gametes, and mating patterns where relative size affects investment in each sexual role. This study investigated the effect of body size on the mating strategies of a hermaphroditic opisthobranch, Bulla gouldiana. Although individuals were spatially aggregated in the field with high levels of movement and size variation, there was little evidence for predictions. Laboratory experiments, however, revealed complicated effects of mass on the probability and duration of mating, as well as gender choice. Pairs were more likely to mate if they included at least one large animal, with the larger animal typically inseminating the smaller. When both individuals were large, they were more likely to each mate in both sexual roles by switching roles once. Although B. gouldiana did not usually alternate between sexual roles multiple times within mating events, paired individuals behaved similarly (neither or both mating as sperm donors) more often than expected by chance. This suggests some level of reciprocity, which is unlikely to be conditional given rates of unilateral mating. When the larger member of the mating pair inseminated the smaller, the duration of insemination increased with the size of the smaller sperm recipient. Copulations lasted longer in pairs that switched sexual roles than in those that did not switch roles. This study suggests that variation in body size can lead to size-dependent mating patterns, but only some of the patterns in B. gouldiana support theoretical predictions. We review other studies that have addressed similar issues, providing inconsistent mating patterns in sperm-storing hermaphrodites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much of our understanding of sexual selection and sexual conflict in animals stems from extensive research on species with separate sexes (Andersson 1994). Because sexual selection can also play an important role in simultaneous hermaphrodites (Charnov 1979, 1996; Arnold 1994; Morgan 1994; Michiels 1998), our understanding of this evolutionary force may be restricted to a subset of possibilities. Having both sexes combined in the same individual may limit sexual selection for traits involved in mate acquisition (Greeff and Michiels 1999a), but other features, such as multiple mating, internal fertilization and sperm storage in invertebrate hermaphrodites, lead to traits for sperm competition similar to those seen in dioecious counterparts (Michiels 1998). Features unique to hermaphrodites, such as flexible division of resources between male and female function and conflict between individuals over sexual roles, complicate sexual strategies in these species (Charnov 1982; Michiels 1998; Greeff and Michiels 1999a). There has been some success in explaining sex allocation and mating strategies of simultaneous hermaphrodites, but varying results from empirical studies point to the need for additional research (Jarne and Charlesworth 1996; Michiels 1998).
Body size is generally thought to be a key feature influencing reproductive behaviors and allocation strategies in hermaphrodites. Body size has been used to model the timing of sex change in sequential hermaphrodites (Ghiselin 1969; Charnov 1982; Warner et al. 1975). It has also been predicted to determine the allocation of resources to male versus female function in simultaneous hermaphrodites (Lloyd and Bawa 1984; Petersen and Fischer 1996; Klinkhamer et al. 1997; St. Mary 1994 Angeloni et al. 2002). In sperm-storing hermaphrodites, an individual's sex allocation strategy should depend on its own body size relative to the size of its current mate. Under many conditions, large animals with more reproductive resources should invest a greater proportion of resources in female function than small animals (Angeloni et al. 2002). When mating with a large animal, more resources should be diverted to sperm transfer than when mating with a small animal (Angeloni et al. 2002). Thus sex allocation in a simultaneous hermaphrodite is predicted to reflect a general strategy based on an individual's body size relative to the population, as well as a specific strategy during a given encounter based on the relative size of the mating partners. It may be possible to detect these predicted effects of body size on sex allocation by observing basic mating patterns, if the time invested in mating in each sexual role reflects underlying sex allocation. In that case, we would expect larger animals to invest more time in the female role than smaller animals, and time spent mating in the male role should increase with the size of the current mate.
Other mating patterns have been predicted for simultaneous hermaphrodites including size-assortative mating and conditional reciprocity, or gamete trading (Ridley 1983; Leonard and Lukowiak 1984, 1991; Fischer 1987; Leonard 1991; Michiels 1998). Size-assortative mating is predicted when large animals are favored mates (e.g., due to increased fecundity) and either insemination is reciprocal or large individuals have a competitive advantage in securing similarly large mates (Ridley 1983). Conditional exchange of gametes is a way to resolve conflict between incompatible interests of mating partners, for example when both partners want to mate in the same sexual role at the exclusion of the other role (Michiels 1998). Conditional reciprocity is difficult to demonstrate, although mating patterns consistent with conditional reciprocity are seen when individuals repeatedly alternate sexual roles and trade gametes evenly (Fischer 1980; Leonard and Lukowiak 1984, 1985, 1991; Sella 1985, 1988; Sella et al. 1997; Michiels 1998; Vreys and Michiels 1998).
In this study, we examined the effect of body size on sexual strategies in the opisthobranch, Bulla gouldiana, including data from both the laboratory and a wild population. This bubble snail is ideal for investigating predicted effects of body size on mating strategies of sperm-storing hermaphrodites for several reasons. B. gouldiana has internal fertilization, a sperm storage organ (Robles 1975), and mates unilaterally with one individual donating sperm to another at a given time, a trait that allows for the detection of size-based asymmetries in mating roles. B. gouldiana is common in bays and intertidal coastal areas throughout southern California (Morris et al. 1980) and has a distinct mating season (Farfan and Ramirez 1988). B. gouldiana is found in large groups, or herds, and individuals within a group vary greatly in size (Paine 1965; Graham 1973) allowing for the possibility of frequent encounters with conspecifics of varying body size, and therefore the opportunity for size-based mate choice. B. gouldiana is estimated to live up to three years (Graham 1973), and mates multiply during its lifetime (personal observation).
Using a combination of field and laboratory techniques we asked: (1) Does body size, either absolute or relative, affect the mating behavior of B. gouldiana? If so, (2) how specifically does body size affect an individual's mating strategy (i.e., duration of mating, sexual role)? And finally, (3) how do these results compare to existing models of hermaphroditic sexual strategies and conflict?
Methods
Field population
We studied a population of B. gouldiana in tide pools of the Scripps Coastal Reserve, University of California Natural Reserve System in La Jolla, California (N32°51′36″, W117°15′4″) from November 10, 1996, through May 31, 1997, to measure spatial dispersion and mating patterns in the field. This study period covered the entire growth, breeding season, and decline of the B. gouldiana population. Algal cover dominated the rocky bottom of the intertidal study site. We divided the square (8.9 m×8.9 m) site into a 13×13 grid (169 quadrats) and conducted a census of B. gouldiana approximately every 14 days during low tides (maximum tidal height: 5 cm). During each census we recorded the quadrat location of each B. gouldiana within the site and weighed each individual to the nearest 0.1 g using a spring scale with a mesh basket to allow excess water to drain. We noted the mass and sexual role of each mating individual, ‘sperm donor’ or ‘sperm recipient’, based on body position and penis intromission. To determine if individuals were spatially aggregated by size, we categorized animals as small, medium or large to create approximately equal proportions for a given census, and evaluated their distribution across four even sections of the study site (northwest, northeast, southwest, southeast) with a contingency table analysis.
On four separate occasions we marked a random sample of B. gouldiana within the study site by gluing plastic numbers to shells to determine movement rates and individual turnover (total N=23). This method of tagging did not result in adverse effects or tag loss in 40 control B. gouldiana maintained in a laboratory tank for one month (see below for conditions). We recaptured marked individuals on each of the three days following tagging and at each subsequent census. We searched for marked individuals in the study site and within 10 m around the site. All marked individuals were mapped within the tide pools by using a Sokkia C32™ surveyor to obtain distance and angle measurements from a fixed point.
Mating experiments
We collected B. gouldiana for laboratory mating experiments from a dense population at Mariner's Cove, Mission Bay, California (N32°45′36″ W117°15′4″). We conducted mating experiments from March through May, 1997 and January through May, 1998. Over the course of the study we collected individuals at random to obtain a mass distribution of the wild population, but only isolated animals greater than 3.5 g for mating experiments to ensure sexual maturity (Farfan and Ramirez 1988). Thus the mean mass of individuals used in the experiments (11.0±0.29 g; range=3.7–27.0 g; n=254) was greater than that of the population from which they were collected (6.2±0.20 g; range=0.5–27.0 g; n=672). One week prior to being used in a mating experiment, each B. gouldiana was isolated in a separate container. All isolated individuals were kept at the Scripps Institution of Oceanography on 12l/12d cycle in a 25°C running seawater tank to stimulate reproductive receptivity (Farfan and Ramirez 1988). All animals were provided with ample Zostera marina as food while in isolation and were returned to an area near the collection site following experiments.
We conducted a total of 127 mating experiments. All mating experiments included only a single pair observed for two hours. We selected individuals for a bout based on pretrial mass (nearest 0.1 g) so that experiments included a range of body sizes and size differences between paired animals. Trials were conducted in a small mating arena (20 cm×20 cm×7 cm) filled with 25°C seawater.
We recorded the duration and sequence of all behaviors during experiments including movements, contacts, and mating. A sperm donor typically mounts a mate, extrudes its penis and inserts it into the recipient's genital opening. A pair was ‘mating’ while one individual maintained intromission of its penis. Pairs that were still mating at the end of the 2-h session were allowed to continue until they physically separated of their own accord. Although we did not measure actual sperm transfer, an individual was considered a ‘sperm donor’ while its penis was inserted into its mate, and ‘sperm recipient’ while the mate's penis was inserted. At the end of the experiment we categorized each B. gouldiana as ‘sperm donor,’ ‘sperm recipient,’ ‘both’ if it mated as both a sperm donor and recipient, or ‘unmated’ if it did not mate.
There were three possible outcomes of our experiments: no mating, mating in one direction, or alternating roles. To test whether pairs were more likely to alternate roles than expected by chance, we compared the frequencies of these three events to random expectations using a binomial probability of mating as a sperm donor (y) or not mating as a sperm donor (n), calculated from observed mating rates (following Michiels and Bakovski 2000). The expected frequencies of the three mating outcomes (no mating: n 2; mating in one direction: ny; alternating roles: y 2) were used to calculate the expected number of pairs mating each way assuming an individual's decision to mate as a sperm donor is independent of its partner's decision.
Preliminary analyses demonstrated that the date of the experiment did not affect mating patterns (probability of mating or switching roles); thus it was not included in any further analyses. We categorized each individual within a pair as ‘larger’ or ‘smaller’ based on relative body mass (excluding two pairs with animals of equal mass, as measured to the nearest 0.1 g), and analyzed whether relative or absolute body masses of paired animals affected their mating patterns. We used multiple logistic regression to determine whether relative or absolute body masses of either the larger or smaller individual affected the probability of mating, the sexual role adopted in any given mating and the probability of switching roles. To ensure independence between predictors, we first conducted logistic regression analyses that included size difference and either the absolute size of the larger animal or the absolute size of the smaller animal. When size difference was not a significant predictor, a second analysis was performed including the absolute sizes of both large and small animals as predictors in the model. We used multiple linear regression to determine whether absolute body masses of smaller and larger animals affected the duration of copulations. In both logistic and linear regression models, variables were removed by backward elimination if their associated P>0.15 (Tabachnick and Fidell 1996).
Results
Field population
We found between 1 and 116 individuals within the study site on 14 sample days (54±8.8), representing seasonal variation in the population size. B. gouldiana were significantly spatially aggregated compared to random (Poisson) expectations on 10 of the 14 days even after removing from the analysis 51 quadrats that never contained B. gouldiana and were thus considered uninhabitable (chi-square tests excluding 51 uninhabitable quadrats: all df=2–4, dependent on the day, χ 2>9.5, P<0.01). The location of aggregations was stable over time, as certain quadrats consistently hosted the highest proportions of animals (Kendall's coefficient of concordance: W c=0.11, χ 2 117=421.5, P<0.01); the same 10% of quadrats held an average 28% of the animals on any census and 20% of quadrats harbored 46% of the population.
Bulla gouldiana were not aggregated by mass; small, medium and large individuals were randomly distributed across four broad sections of the study site (contingency table: all χ26<5.8, all P>0.40) on all days except for January 10, when large animals were concentrated in the northeast portion of the study site (contingency table: χ26=23.9, P<0.001).
Tagged individuals varied in rates of movement, traveling between 2.9 and 9.9 m in one day (more than the full width of the study area) and an average of 5.4±0.6 m (n=12). These are conservative measures because they assume linear movements between each point, and do not include individuals that traveled too far to be recaptured in or near the study site. Some marked B. gouldiana were absent from the study site for up to one month before being recaptured. All of these findings suggest high levels of mixing and movement by individual animals over time.
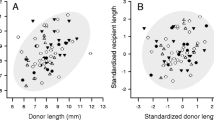
We found 43 pairs of mating B. gouldiana on two days at the beginning of May, 1997. Gender choice was random with respect to body size; sperm recipients were not significantly different in mass from their sperm donors (paired t-test: t 42=1.2, P=0.23), and neither sperm donors nor sperm recipients were different in mass from the mean of the population (one-sample t-tests: sperm donors: 6.0±0.2 g, t 42=−1.1, P=0.28; sperm recipients: 6.4±0.2 g, t 42=0.7, P=0.49; population: 6.2±0.3 g). There was no evidence for size-assortative mating; sperm donor and recipient masses were not correlated (N=41, r=0.082, P=0.60).
Mating experiments
Did body size affect the probability of mating?
Only 59 (46%) of the 127 pairs mated, even though a total of 108 pairs made contact with each other during experiments. The mass difference between paired animals was not a significant predictor of the probability of mating, and thus was removed from stepwise logistic regression models that included either the masses of the smaller or larger members of pairs, providing no evidence for size-assortative mating. In a logistic regression model only the absolute mass of the larger individual (not the smaller individual) was a significant predictor of the probability of mating (Table 1; Fig. 1). A pair had greater than 50% probability of mating if the larger animal was larger than 14.2 g.
Did body size affect the sexual roles adopted?
Twenty-four (41%) of the 59 mating pairs switched sexual roles after the initial mating bout. Changing roles in a second mating occurred more often than expected by chance based on the overall frequency of sperm donation (N=24, expected=14), as did the frequency of not mating at all (N=68, expected=57), while unilateral mating was less common than expected (N=35, expected=56; \(x_1^2 = 17.73 \), P<0.001). In only five cases where individuals switched roles did the initial sperm donor mate as a male a second time, and in no cases did these individuals switch roles again.
Relative body size could affect the way that a pair mates. However, we found that the difference in body mass between mating partners did not affect the probability of switching roles (vs. mating unilaterally; logistic regression: \(\chi _1^2 = 0.07 \), P=0.79), or the sexual role that each individual adopted. Among pairs that mated without switching sexual roles, we found no consistency in the roles adopted by the smaller or larger animal (larger sperm donor: 19 pairs; smaller sperm donor: 15 pairs; binomial test: P=0.61). Similarly, among pairs that alternated roles, there was no consistency in which individual acted as a sperm donor first (larger first: 14 pairs; smaller first: 9 pairs; binomial test: P=0.31), but the animal that initiated the first contact was the one that mated in the male role first (\(x_1^2 = 5.4 \), P<0.05).
Absolute body masses of both members of mating pairs had an effect on mating roles and role alternation (Table 1), but this complicated effect was driven primarily by the mass of the smaller animal, which had a greater effect than the mass of the larger animal (Table 1). Looking at the effect of the size of the smaller animal alone, we found that when it was very small the smaller animal typically mated as a sperm recipient and ceased mating after that copulation. However, as the smaller animal increased in mass the probability that it would inseminate the larger animal increased (Table 1; Fig. 2). This increase in sperm donation by the smaller individual at larger masses was accompanied by an increased likelihood that the pair would switch sexual roles (Table 1; Fig. 2).
The mass of the smaller member of laboratory mating pairs was a significant predictor of the way the pair mated. The space between the x-axis and the lower curve represents the probability that the larger animal (L) inseminated the smaller animal (S) without reciprocation. The space between the two curves represents the probability that both animals mated in both sexual roles. The space between the upper curve and the top of the graph represents the probability that S inseminated L without reciprocation
Did body size affect copulation duration?
Body mass was related to the variation seen in copulation duration. An individual inseminated its mate between 4 and 63 min (26.1±1.6 min). When the larger individual inseminated the smaller, the duration of copulation did not depend on the mass of the larger donor, but increased with the mass of the smaller sperm recipient (Table 1; Fig. 3). When the smaller individual inseminated the larger, the duration of copulation did not depend on the mass of either mating partner (Fig. 3; regression: F 2,35=0.07, R 2=0.003, P=0.80). Smaller animals inseminated larger animals longer than vice versa, but this result was not significant (t-test: t 78=1.7, P=0.09, mean diff.=5.2 min). The mean copulation duration within pairs that switched roles was longer than the copulation duration in pairs that did not switch roles (t-test: t 57=2.9, P<0.01, mean diff.= 9 min) (Fig. 4). However, insemination durations were not correlated within those pairs that switched roles (r 22=0.22, P=0.29), providing no evidence for conditional exchange of sperm, if copulation duration reflects the amount of sperm transferred.
a In laboratory pairs where the larger animal inseminated the smaller, the duration of copulation increased with the mass of the smaller sperm recipient (Y=5.8+1.9×). b When the smaller animal inseminated the larger, the duration of copulation did not depend on the mass of the larger sperm recipient (Y=26.6+0.1)
Mean duration of copulations within laboratory pairs where the larger animal (L) inseminated the smaller (S), and where S inseminated L. Circles represent pairs in which only one individual transferred sperm to the other without reciprocation. Squares represent pairs that switched sexual roles. Error bars are standard errors around the mean
Discussion
Certain conditions may be necessary for simultaneous hermaphrodites to demonstrate reciprocity and size-dependent mating patterns that are predicted by theoretical models of sexual conflict and sex allocation. B. gouldiana appears to meet several of those conditions. Individuals are found in large aggregations, and are highly mobile, giving them opportunity to select particular mates and mate with a number of partners. The presence of a sperm storage organ, with possible sperm digestion capabilities (Robles 1975) increases both the conflict between partners as well as the competition between current and future mates. B. gouldiana observed mating within a population demonstrate tremendous body size variation (4–27 g), which is predicted to influence the available resources for gamete production, and thus reproductive strategies. Courtship in this species involves extensive contact, providing opportunity for each individual to assess relative body size using tactile and chemical cues prior to copulation. All inseminations are unilateral at a given point in time, making size-dependent mating patterns and active reciprocity easy to detect. Based on these characteristics, we predicted that we would be able to detect size-dependent mating patterns in this species.
However, both our field studies and laboratory experiments provided little or no support for predictions of size-assortative mating, size-dependent role assignment, or conditional reciprocity models. We did find that switching sexual roles or not mating at all were more common than expected, suggesting that individuals were more likely to mate as a sperm donor when they had also mated as a sperm recipient. However, this reciprocity is probably not conditional, given the frequency of unilateral mating (28% of all pairs, and 59% of mating pairs).
Experiments revealed complicated effects of absolute body mass on mating parameters, with evidence for an increase in the probability and duration of mating with body size, which would lead us to predict that mating individuals sampled in the field would be larger than the average mass in the population. However, this was not true for our field study; mating individuals did not differ in mass from the population mean. This difference between lab and field results could be a consequence of the different body sizes in the two populations we studied; experimental animals came from a population with a larger mean mass and mass range than the population studied in the field, which only ranged from 1.0 to 9.2 g, and perhaps did not vary enough to see the same body size effects. For example, experimental pairs were more than 50% likely to mate if they included an individual larger than 14.2 g, a mass outside the range seen in the field population. These body size effects may only be evident in populations with considerable size variation.
We compiled a table reviewing studies on the effects of body size on sex allocation and mating patterns in simultaneously hermaphroditic animals with internal fertilization to determine whether our results are general or unusual (Table 2). We selected only studies with data sufficient to evaluate at least one of the three categories of mating strategies we investigated in B. gouldiana: size based reproductive patterns, size-assortative mating, and mating patterns suggestive of conditional reciprocity. We identified 24 different species in which these questions have been investigated (including this study), which fell within three phyla (Annelida, Platyhelminthes, Mollusca). We did not include in our survey a number of other studies on mating strategies of hermaphrodites with external fertilization and the effects of density or prior mating history on mating strategies. We discuss some of the issues raised by this summary and our findings below.
Size-dependent gender choice
Size-dependent resource variation may lead to size-dependent sex allocation, with several models predicting increases in female function with size in simultaneous hermaphrodites (Lloyd and Bawa 1984; Petersen and Fischer 1996; Angeloni et al. 2002). In sperm-storing hermaphrodites, relatively small individuals are predicted to have a smaller pool of resources than larger individuals, and thus must invest a greater fraction of those resources in sperm to achieve a competitive level of sperm displacement in a mate's storage organ. Relatively large animals have a larger pool of resources, and can invest only a small fraction in sperm to achieve the same level of sperm displacement, leaving a larger fraction of resources for investment in female function (Angeloni et al. 2002). If mating patterns reflect sex allocation strategies, we predicted that larger animals would invest more time mating in the female role and smaller animals would invest more time mating in the male role. This prediction was not supported for B. gouldiana in the field, where sperm donors and sperm recipients did not differ in mass. Nor was it supported in the lab; smaller members of mating pairs were not more likely to inseminate larger members than vice versa.
Several recent studies provide evidence that investment in female function increases with body size in sperm-storing hermaphrodites, while others have not found this size-dependent gender pattern (Table 2). This difference in how size affects gender shows no clear association with broad taxonomic affiliation nor with sperm transfer mode (Table 2). The fact that we did not detect size-dependent gender choice suggests that either sex allocation strategies are affected by additional factors not considered in theoretical treatments of the issue (e.g., the interaction between body size and sperm digestion) so that sex allocation does not directly vary with size in B. gouldiana, or that the allocation strategy is not expressed through mating duration in each sexual role. It is possible that our measures of ‘allocation’ to mating in each sexual role, based on duration of copulation, do not directly reflect actual sex allocation and investment to gamete production. If this were the case, further investigation would be needed to explain why mating duration in each sexual role reflects predicted size-dependent sex allocation strategies in some species but not others (e.g., Otsuka et al. 1980; DeWitt 1996; Yusa 1996; Angeloni and Bradbury 1999; Angeloni 2003; Angeloni et al. 2003; see Table 2). Perhaps this is related to the average cost (e.g., time, risk, energy) of copulation in each species; if the time invested in each mating event represents a great cost, the duration spent in each sexual role may be more likely to reflect underlying sex allocation strategies. For example, size-dependent mating patterns have supported predictions in other opisthobranchs of the genus Aplysia, which have long mating durations (up to 24 h), and in Alderia modesta, which mates by hypodermic injection of sperm anywhere on the body (Table 2). These forms of mating might involve a greater cost than the investment made by mating B. gouldiana. At this point there are no clear explanations for inconsistent results among studies and species. A comparative study incorporating phylogeny could relate size-dependent mating patterns with physical, behavioral, and ecological traits to explain differences in findings among species. Directly measuring relative levels of sperm and egg production would provide more accurate measures of sex allocation and perhaps more consistent support for predictions. It would also be useful to experimentally manipulate body size and resource levels to determine how each of those parameters independently affects sex allocation strategies.
Support for predictions may be found inconsistently among sperm-storing hermaphrodites (Table 2) because assumptions of theoretical models, such as perfect size assessment, frequent mating rates, equal mating opportunities independent of body size, and constant reproductive resources for each mating event (Angeloni et al. 2002) may not always reflect real conditions. We do not know how well these assumptions apply to B. gouldiana or whether they apply only inconsistently to most hermaphroditic species; for most species, including this one, we do not currently have complete information on field mating rates, size assessment abilities, how resources might vary depending on individual history, or how mating history and levels of sperm previously received might affect future mating strategies. Also, predictions for optimal sex allocation are strongly determined by the shape of male and female gain curves, and it is usually assumed that female fitness increases linearly with investment in eggs while male fitness diminishes with investment in sperm transfer to a limiting sperm storage organ (Charnov 1996; Angeloni et al. 2002). Predicted sex allocation strategies change dramatically with the shape of gain curves, for example if egg survival is limited by clutch size, if sperm is digested or if a large investment in sperm is required before any male fitness is gained (Greeff and Michiels 1999b; Pen and Weissing 1999; Greeff and Parker 2000; Angeloni et al. 2002). A rigorous study of fitness gains with investment in sperm and eggs in B. gouldiana would provide more specific, and perhaps different, predictions for this species.
Sperm-storing hermaphrodites capable of assessing body size are predicted to invest more in sperm transfer when mating with large animals than with small animals (Angeloni et al. 2002). In our laboratory experiments, smaller members of pairs mated as sperm donors for a longer duration than larger animals; however, this relationship was not significant. If this had been a stronger relationship, we also would have expected to see a size-based mating pattern in the field, because we would have been more likely to sample pairs with sperm recipients larger than sperm donors, given their longer copulation durations. In B. gouldiana larger individuals modulated the duration of copulation with the body size of their smaller sperm recipients, supporting predictions if duration of copulation reflects investment in sperm transfer, as was shown in some other opishobranchs (Longley and Longley 1984; Yusa 1994b). However, smaller animals did not alter copulation duration with the body size of their larger recipients, limiting the strength of any conclusions. These results are similar to the findings of Peters and Michiels (1996) for the flatworm Schmidtea polychroa; large animals copulated for a shorter period of time when the partner was smaller, but small individuals did not copulate for a longer period of time when their partner was larger. In both species the causes and cues that result in one partner ending a copulation are unclear; perhaps small animals run out of sperm during transfer to large animals and are thus unable to increase the duration of sperm transfer with an increase in the size of the larger partner. Interpreting underlying mating strategies from the resulting mating behavior can be challenging when it is unclear which individual is controlling the interaction.
It is possible that differences in insemination duration reflect differences in sperm transfer rates rather than differences in the absolute amount of sperm transferred or the underlying investment in sperm production. True size-based allocation decisions would be better measured by relating body size to the number of eggs produced, volume of sperm produced and transferred, and histology of the gonads. While some studies have measured relative investment in ovaries versus testes in hermaphrodites (e.g., Petersen 1991; St. Mary 1994; Trouvé et al. 1999; Schärer et al. 2001), it would be useful to translate these investment patterns into sperm and egg production and eventual fertilization success.
Size-assortative mating
Because fecundity increases with size in most gastropods, including other opisthobranchs (e.g., Kandel and Capo 1979; Switzer-Dunlap and Hadfield 1979; Baur 1988; Tomiyama and Miyashita 1992; Erlandsson and Johannesson 1994; Yusa 1994a; Karlsson and Haase 2002), large animals are predicted to be preferred sperm recipients. Furthermore, if animals insist on mates of similar size (or fecundity), or large animals have a competitive advantage at securing similarly large sperm recipients, encounters will result in size-assortative mating (Ridley 1983). We found no evidence for size-assortative mating in B. gouldiana; masses of mating animals in the field were not correlated, and the relative masses of experimental animals was not a significant predictor of their probability of mating.
Our results differ from the size-assortative mating patterns described for several other hermaphrodites (Table 2). Indeed, most studies that have looked for size-assortative mating patterns in simultaneous hermaphrodites have found evidence for it. Two other studies, on the terrestrial snail Arianta arbustorum (Baur 1992) and the freshwater flatworm Schmidtea polychroa (Peters and Michiels 1996), did not detect size-assortative mating in wild populations of simultaneous hermaphrodites. Why do B. gouldiana not choose mates of similar size despite having access to potential mates of a variety of sizes? We agree with Peters and Michiels (1996) that costly mate searching, a possible explanation for random mating in A. arbustorum (Baur 1992), is unlikely to explain this pattern in B. gouldiana. One alternative is that large size does not increase the competitive ability of an individual to secure a similarly large mate. For large individuals to have increased access to other large mates, they would need the ability to displace smaller competitors or to reject smaller mates in favor of large individuals. Our field observations are too short to determine if individuals displace other individuals, and our lab pairings never involved more than two individuals. Furthermore, rejection of a sperm donor may be extremely unlikely if unwanted sperm can be digested in the copulatory bursa of B. gouldiana (Robles 1975). Alternatively, larger mates may not actually be more desirable as sperm recipients, because of excessive sperm digestion or more intense sperm competition. Information on the fate of sperm after it is transferred would be required to evaluate these possibilities.
Conditional reciprocity
Conditional reciprocity or gamete trading, where copulating hermaphrodites evenly transfer gametes, has been proposed as a way to resolve conflict over mating in a preferred sexual role (Leonard and Lukowiak 1984, 1991; Fischer 1987; Michiels 1998). Mating B. gouldiana do not simultaneously exchange sperm nor do they consistently mate with repeated alternations of sexual roles as seen in some other hermaphrodites (Table 2; for examples of simultaneous sperm exchange see: Reise 1995; Michiels 1998; Michiels and Streng 1998; Vreys and Michiels 1998; Karlsson and Haase 2002; Angeloni 2003; Anthes and Michiels 2005; for examples of repeated alternation see: Fischer 1980, 1984, 1987; Leonard and Lukowiak 1984, 1985, 1991; Michiels et al. 2003; Sella 1985, 1988; Petersen 1995; Sella et al. 1997; Michiels 1998).
Paired individuals were more likely to behave similarly (neither mate as sperm donor, or both mate as sperm donors) than expected by chance, suggesting some level of cooperation (Michiels and Bakovski 2000; Koene and ter Maat 2005). Copulations also lasted longer for pairs that switched roles than for those that did not. However, the high rate of unilateral mating and the finding that copulation durations were not correlated within reciprocating pairs indicate that this reciprocity is probably not conditional. Conflict over sexual roles is assumed to be widespread between mating hermaphrodites (Leonard 1991; Michiels 1998), but B. gouldiana may represent one hermaphrodite where the interests of mating partners are compatible, or perhaps are reconciled by some unknown mechanism. It is also possible that our estimates of the frequency of role switching are affected by our experimental methods. If our two-hour mating experiments were too short to detect repeated alternation of sexual roles that might occur over a longer time period, we would have underestimated the frequency of role switching. Alternatively, the role switching that we did observe could be an effect of restricted access to mates in laboratory pairs that would rarely occur in the wild, causing us to overestimate the frequency of role switching, as has been suggested for Lymnaea stagnalis (van Duivenboden and ter Maat 1988). These effects of lab mating trials are also a potential concern in other studies in our review (Table 2). Extended field observations would reveal the true rate that mating pairs exchange sperm and alternate roles.
Conclusions
Aside from increases in the probability of mating with body size and some evidence for modulation of copulation duration with the mate's size, B. gouldiana does not demonstrate size-dependent mating strategies commonly predicted and observed in some other simultaneous hermaphrodites (Table 2). Controlled laboratory observations of basic mating behavior do provide valuable information on hermaphroditic strategies, which is needed to test the many predictions from a growing body of theoretical work. However, also needed are careful measures of actual fitness gains with investment in each sexual role to accurately formulate predictions and precise measures of investment in male and female function to adequately test predictions. Most previous studies of sperm-storing hermaphrodites have found behavior suggestive of some kind of size-dependent mating strategy, either size-assortative mating or size-dependent gender choice (Table 2). However, our review underscores the diversity in size-dependent mating strategies among simultaneous hermaphrodites representing many different taxonomic groups, sperm transfer modes, and ecologies. An analysis incorporating phylogeny and more detailed information on behavior and ecology (e.g., mating costs, encounter rates) may help to explain differences in mating patterns across species. To facilitate such an analysis, there must be greater consistency in the types of data collected to evaluate the predicted strategies presented here, as well as an attempt to gather field data on the ecology of these species. We hope that our review will stimulate such efforts in field work as well as rigorous comparative studies.
References
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Angeloni L (2003) Sexual selection in a simultaneous hermaphrodite with hypodermic insemination: body size, allocation to sexual roles and paternity. Anim Behav 66:417–426
Angeloni L, Bradbury J (1999) Body size influences mating strategies in a simultaneously hermaphroditic sea slug, Aplysia vaccaria. Ethol Ecol Evol 11:187–195
Angeloni L, Bradbury JW, Burton RS (2003) Multiple mating, paternity and body size in a simultaneous hermaphrodite, Aplysia californica. Behav Ecol 14:554–560
Angeloni L, Bradbury JW, Charnov EL (2002) Body size and sex allocation in simultaneously hermaphroditic animals. Behav Ecol 13:419–426
Anthes, N, Michiels NK (2005) Do “sperm trading” simultaneous hermaphrodites always trade sperm? Behav Ecol 16:188–195
Arnold SJ (1994) Bateman's principles and the measurements of sexual selection in plants and animals. Am Nat 144:S127–S149
Baur B (1988) Population regulation in the land snail Arianta arbustorum: density effects on adult size, clutch size and incidence of egg cannibalism. Oecologia 77:390–394
Baur B (1992) Random mating by size in the simultaneously hermaphroditic land snail Arianta arbustorum: experiments and an explanation. Anim Behav 43:511–518
Baur B, Locher R, Baur A (1998) Sperm allocation in the simultaneously hermaphroditic land snail Arianta arbustorum. Anim Behav 56:839–845
Charnov EL (1979) Simultaneous hermaphroditism and sexual selection. Proc Natl Acad Sci USA 76:2480–2484
Charnov EL (1982) The theory of sex allocation. Princeton University Press, Princeton
Charnov EL (1996) Sperm competition and sex allocation in simultaneous hermaphrodites. Evol Ecol 10:457–462
Crozier WJ (1917) Evidence of assortative mating in a nudibranch. Proc Natl Acad Sci USA 3:519–522
Crozier WJ (1918) Assortative mating in a nudibranch, Chromodoris zebra Heilprin. J Exp Zool 47:247–292
DeWitt TJ (1996) Gender contests in a simultaneous hermaphrodite snail: a size advantage model for behavior. Anim Behav 51:345–351
Erlandsson J, Johannesson K (1994) Sexual selection on female size in a marine snail, Littorina littorea (L.). J Exp Marine Biol Ecol 181:145–157
Farfan BC, Ramirez LFB (1988) Spawning and ontogeny of Bulla gouldiana (Gastropoda: Opisthobranchia: Cephalaspidea). Veliger 31:114–119
Fischer EA (1980) The relationship between mating system and simultaneous hermaphroditism in the coral reef fish, Hypoplectrus nigricans (Serranidae). Anim Behav 28:620–633
Fischer EA (1984) Egg trading in the chalk bass, Serranus tortugarum, a simultaneous hermaphrodite. Z Tierpsychol 66:143–151
Fischer EA (1987) Mating behavior in the black hamlet – gamete trading or egg trading? Environ Biol Fishes 18:143–148
Ghiselin MT (1969) The evolution of hermaphroditism among animals. Quart Rev Biol 44:189–208
Gianguzza P, Badalamenti F, Jensen KR, Chemello R, Cannicci S, Riggio S (2004) Body size and mating strategies in the simultaneous hermaphrodite Oxynoe olivacea (Mollusca, Opisthobranchia, Sacoglossa). Funct Ecol 18:899–906
Graham J (1973) Population interaction between Navanax inermis and Bulla gouldiana. Dissertation, California State University, San Diego
Greeff JM, Michiels NK (1999a) Low potential for sexual selection in simultaneously hermaphroditic animals. Proc Roy Soc Lond B 266:1671–1676
Greeff JM, Michiels NK (1999b) Sperm digestion and reciprocal sperm transfer can drive hermaphroditic sex allocation to equality. Am Nat 153:421–430
Greeff JM, Parker GA (2000) Spermicide by females: what should males do? Proc Roy Soc Lond B 267:1759–1763
Jarne P, Charlesworth D (1996) Hermes meets Aphrodite: an animal perspective. Trends Ecol Evol 11:105–107
Kandel P, Capo TR (1979) The packaging of ova in the egg cases of Aplysia californica. Veliger 22:194–198
Karlsson A, Haase M (2002) The enigmatic mating behaviour and reproduction of a simultaneous hermaphrodite, the nudibranch Aeolidiella glauca (Gastropoda, Opisthobranchia. Can J Zool 80:260–270
Klinkhamer PGL, de Jong TJ, Metz H (1997) Sex and size in cosexual plants. Trends Ecol Evol 12:260–265
Koene JM, ter Maat A (2005) Sex role alternation in the simultaneously hermaphroditic pond snail Lymnaea stagnalis is determined by the availability of seminal fluid. Anim Behav 69:845–850
Leonard JL (1991) Sexual conflict and the mating systems of simultaneously heramphroditic gastropods. Am Malacol Bull 9:45–58
Leonard JL, Lukowiak K (1984) Male–female conflict in a simultaneous hermaphrodite resolved by sperm trading. Am Nat 124:282–286
Leonard JL, Lukowiak K (1985) Courtship, copulation, and sperm trading in the sea slug, Navanax inermis (Opisthobranchia: Cephalaspidea). Can J Zool 63:2719–2729
Leonard JL, Lukowiak K (1991) Sex and the simultaneous hermaphrodite: testing models of male-female conflict in a sea slug, Navanax inermis (Opisthobranchia). Anim Behav 41:255–266
Lloyd DG, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. Evol Biol 17:255–338
Longley AJ, Longley RD (1984) Mating in the gastropod mollusk Aeolidia papillosa: behavior and anatomy. Can J Zool 62:8–14
Lüscher A, Wedekind C (2002) Size-dependent discrimination of mating partners in the simultaneous hermaphroditic cestode Schistocephalus solidus. Behav Ecol 13:254–259
Michiels NK (1998) Mating conflicts and sperm competition in simultaneous hermaphrodites. In: Birkhead TR, Moller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, pp 219–254
Michiels NK, Bakovski B (2000) Sperm trading in a hermaphroditic flatworm: reluctant fathers and sexy mothers. Anim Behav 59:319–325
Michiels NK, Newman LJ (1998) Sex and violence in hermaphrodites. Nature 391:647
Michiels NK, Streng A (1998) Sperm exchange in a simultaneous hermaphrodite. Behav Ecol Sociobiol 42:171–178
Michiels NK, Hohner A, Vorndran IC (2001) Precopulatory mate assessment in relation to body size in the earthworm Lumbricus terrestris: avoidance of dangerous liaisons? Behav Ecol 12:612–618
Michiels NK, Raven-Yoo-Heufes A, Brockmann KK (2003) Sperm trading and sex roles in the hermaphroditic opisthobranch sea slug Navanax inermis: eager females or opportunistic males? Biol J Linnean Soc 78:105–116
Morgan MT (1994) Models of sexual selection in hermaphrodites, especially plants. Am Nat 144:S100–S125
Morris RH, Abbot DP, Haderlie EC (1980) Intertidal invertebrates of California. Stanford University Press, Stanford
Ohbayashi-Hodoki K, Ishihama F, Shimada M (2004) Body size-dependent gender role in a simultaneous hermaphrodite freshwater snail, Physa acuta. Behav Ecol 15:976–981
Otsuka C, Rouger Y, Tobach E (1980) A possible relationship between size and reproductive behavior in a population of Aplysia punctata Cuvier, 1803. Veliger 23:159–162
Paine RT (1965) Natural history, limiting factors and energetics of the opisthobranch Navanax inermis. Ecology 46:603–619
Pen I, Weissing FJ (1999) Sperm competition and sex allocation in simultaneous hermaphrodites: a new look at Charnov's invariance principle. Evol Ecol Res 1:517–525
Pennings SC (1991) Reproductive behavior of Aplysia californica Cooper: diel patterns, sexual roles and mating aggregations. J Exp Marine Biol Ecol 149:249–266
Peters A, Michiels NK (1996) Do simultaneous hermaphrodites choose their mates? Effects of body size in a planarian flatworm. Fresh Biol 36:623–630
Petersen CW (1991) Sex allocation in hermaphroditic sea basses. Am Nat 138:650–667
Petersen CW (1995) Reproductive behavior, egg trading, and correlates of male mating success in the simultaneous hermaphrodite, Serranus tabacarius. Environ Biol Fish 43:351–361
Petersen CW, Fischer EA (1996) Intraspecific variation in sex allocation in a simultaneous hermaphrodite: the effect of individual size. Evolution 50:636–645
Reise H (1995) Mating behaviour of Deroceras rodnae Grossu & Lupu, 1965 and D. praecox Wiktor, 1966 (Pulmonata: Agriolimacidae). J Moll Stud 61:325–330
Ridley M (1983) The explanation of organic diversity: the comparative method and adaptations for mating. Clarendon Press, Oxford
Robles LJ (1975) The anatomy and functional morphology of the reproductive system of Bulla gouldiana. Veliger 17:278–291
Schärer L, Karlsson LM, Christen M, Wedekind C (2001) Size-dependent sex allocation in a simultaneous hermaphrodite parasite. J Evol Biol 14:55–67
Sella G (1985) Reciprocal egg trading and brood care in a hermaphroditic polychaete worm. Anim Behav 33:938–944
Sella G (1988) Reciprocation, reproductive success, and safeguards against cheating in a hermaphroditic polychaete worm, Ophryotrocha diadema Akesson 1976. Biol Bull 175:212–217
Sella G, Premoli MC, Turri F (1997) Egg trading in the simultaneously hermaphroditic polychaete worm, Ophryotrocha gracilis (Huth). Behav Ecol 8:83–86
St. Mary CM (1994) Sex allocation in a simultaneous hermaphrodite, the blue-banded goby (Lythrypnus dalli): the effects of body size and behavioral gender and the consequences for reproduction. Behav Ecol 5:304–313
Switzer-Dunlap M, Hadfield MG (1979) Reproductive patterns of Hawaiian aplysiid gastropods. In: Stancyk SE (ed) Reproductive ecology of marine invertebrates. University of South Carolina Press, Columbia, pp 199–210
Switzer-Dunlap M, Meyers-Schulte K, Gardner EA (1984) The effect of size, age, and recent egg laying on copulatory choice of the hermaphroditic mollusc Aplysia juliana. Int J Invert Reprod Dev 7:217–225
Tabachnick BG, Fidell LS (1996) Using Multivariate Statistics, 3rd edn. HarperCollins College Publishers, New York
Tomiyama K (1996) Mate-choice criteria in a protandrous simultaneously hermaphroditic land snail Achatina fulica (Ferrusac) (Stylommatophora: Achatinidae). J Moll Stud 62:101–111
Tomiyama K, Miyashita K (1992) Variation of egg clutches in the giant African snail, Achatina fulica (Ferrusac) (Stylommatophora: Achatinidae) in Ogasawara Islands. Venus 51:293–301
Trouvé S, Jourdane J, Renaud F, Durand P, Morand S (1999) Adaptive sex allocation in a simultaneous hermaphrodite. Evolution 53:1599–1604
van Duivenboden YA, ter Maat A (1988) Mating behavior of Lymnaea stagnalis. Malacologia 28:53–64
Vreys C, Michiels NK (1997) Flatworms flatten to size up each other. Proc Roy Soc Lond B 264:1559–1564
Vreys C, Michiels NK (1998) Sperm trading by volume in a hermaphrodite flatworm with mutual penis intromission. Anim Behav 56:777–785
Warner RR, Robertson DR, Leigh EG Jr (1975) Sex change and sexual selection. Science 190:135–139
Warner RR (1984) Mating behavior and hermaphroditism in coral reef fishes. Am Sci 72:128–136
Yusa Y (1994a) Size-related egg production in a simultaneous hermaphrodite, the sea hare Aplysia kurodai Baba (Mollusca: Opisthobranchia). Publ Seto Marine Biol Lab 36:249–254
Yusa Y (1994b) Factors regulating sperm transfer in an hermaphroditic sea hare, Aplysia parvula Morch, 1863 (Gastropoda: Opisthobranchia). J Exp Marine Biol Ecol 181:213–221
Yusa Y (1996) The effects of body size on mating features in a field population of the hermaphroditic sea hare Aplysia kurodai Baba 1937 (Gastropoda: Opisthobranchia). J Moll Stud 62:381–386
Acknowledgements
We are grateful to Jack Bradbury for guidance throughout the study. For help in the field and lab we thank Bryan Glogowski, Elizabeth Kitazono, Peter Marcovici, Chris Nagy, Dinh Nguyen, Kim Owens, Amy Ritter and Alex Trujillo. Isabelle Kay allowed access to the Scripps Coastal Reserve. Colette St. Mary, Bruce Lyon and three anonymous reviewers provided valuable suggestions for improving the manuscript. Lisa Angeloni was supported by the National Science Foundation under Grant No. DBI-0310305 during preparation of the manuscript. The experiments in this study comply with the current laws of the USA
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. St. Mary
Rights and permissions
About this article
Cite this article
Chaine, A., Angeloni, L. Size-dependent mating and gender choice in a simultaneous hermaphrodite, Bulla gouldiana . Behav Ecol Sociobiol 59, 58–68 (2005). https://doi.org/10.1007/s00265-005-0009-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-005-0009-8