Abstract
The concept of a suite of foraging behaviors was introduced as a set of traits showing associative directional change as a characterization of adaptive evolution. I report how naturally selected differential sucrose response thresholds directionally affected a suite of honey bee foraging behaviors. Africanized and European honey bees were tested for their proboscis extension response thresholds to ascending sucrose concentrations, reared in common European colonies and, captured returning from their earliest observed foraging flight. Race constrained sucrose response threshold such that Africanized bees had significantly lower sucrose response thresholds. A Cox proportional hazards regression model of honey bee race and sucrose response threshold indicated that Africanized bees were 29% (P<0.01) more at risk to forage over the 30-day experimental period. Sucrose response threshold organized age of first foraging such that each unit decrease in sucrose response threshold increased risk to forage by 14.3% (P<0.0001). Africanized bees were more likely to return as pollen and water foragers than European foragers. Africanized foragers returned with nectar that was significantly less concentrated than European foragers. A comparative analysis of artificial and naturally selected populations with differential sucrose response thresholds and the common suite of directional change in foraging behaviors is discussed. A suite of foraging behaviors changed with a change in sucrose response threshold that appeared as a product of functional ecological adaptation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal behavior is a product of evolution configured by environment. Genes exist in individuals and individuals comprise populations that are geographically distributed into habitats to which they become adapted over time. The interaction of genes and environment selects adaptations that emerge as patterns of ecological change (Reznick and Ghalambor 2001). Foraging behavior is relevant to adaptive evolution whereby different ecological habitats present different patterns, quantities, and qualities of resource availability. The honey bee has adapted to tropical and temperate ecological habitats that are highly divergent and therefore make a good study organism for patterns of change in quantitative foraging traits as a consequence of adaptive evolution.
Bees that evolve in different ecotypes differ in their forage choice behavior. Africanized foragers are more likely to return with loads of pollen when fostered at the same time in the same colony with temperate European bees (Danka et al. 1987; Fewell and Bertram 2002). The bias in pollen collection by Africanized bees is believed to be a consequence of a higher rate of colony-level reproduction by Africanized bees (Winston et al. 1983). Africanized honey bees (AHB) are known to reproduce at a rate significantly higher than European honey bee (EHB) colonies (Winston et al. 1983). Honey bee colonies reproduce by colony fission, thus the rate at which a colony increases its membership affects rate of reproduction. Pollen is the source of protein in a colony and used to produce young bees. Adult nurse bees convert pollen into proteinacious glandular secretions fed to larvae. The amount of incoming pollen affects the number of larvae raised and the growth rate of the colony (Danka et al. 1987). More pollen means more larvae are reared (Dreller and Tarpy 2000).
There appears to be a relationship between selection for increased pollen foraging and decreased age of first foraging. Africanized bees forage at significantly younger ages than European bees when fostered at the same time in Africanized colonies (Winston and Katz 1982). Strains of European bees selected to hoard high and low amounts of pollen also show different rates of foraging behavior development. Five different studies have been conducted on the age of first foraging using the high and low pollen hoarding strains, constituting 13 different trials under different treatment conditions (Calderone and Page 1988, 1991, 1996; Pankiw and Page 2000; Pankiw et al. 2002). In 11 cases the high strain foraged at significantly younger ages than the low strain.
Forage choice behavior and genotype are associated with sensitivity to sucrose. Honey bees respond reflexively by extending the proboscis when a sufficiently concentrated drop of sucrose solution is applied to the antennae. This is called the proboscis extension response to sucrose (PER). The response threshold of an individual can be estimated by the lowest sucrose concentration that elicits proboscis extension when bees are presented with an ascending concentration series of sucrose solutions (Page et al. 1998; Pankiw and Page 1999). Sucrose response thresholds of pre-foragers, measured 1–3 weeks before foraging, strongly correlate with their subsequent foraging behavior. Pankiw and Page (2000) demonstrated that bees with the lowest sucrose response threshold became water foragers, the next highest became pollen foragers, then pollen and nectar, and then nectar foragers; those with the highest response thresholds are most likely to return empty from foraging trips. Genotype affects sucrose response thresholds. The high pollen hoarding strain of bees has characteristically lower response thresholds than the low strain (Pankiw and Page 1999; Pankiw et al. 2001). Africanized bees also have characteristically low sucrose response thresholds (Pankiw and Rubink 2002) like the high pollen hoarding strain of bee.
Selection for pollen hoarding in European bees has changed choice and quality of liquids collected. Low pollen hoarding bees are more likely to collect nectar and high pollen hoarding bees are more likely to collect water (Page et al. 1998; Pankiw and Page 1999; Pankiw et al. 2001, 2002). Nectar foragers of the high strain return with significantly less concentrated nectar than low strain foragers. Forage choice and nectar quality are positively correlated with honey bee proboscis extension response threshold to sucrose (Pankiw and Page 2000). Collectively, the above studies suggest that foraging behaviors change with an associated change in the sucrose response threshold distribution of a population. Tested here was the hypothesis that bees naturally selected to have lower response thresholds to sucrose showed associated changes in a suite of foraging behaviors. Africanized bees were expected to have a greater probability to collect pollen and water, and return with loads of nectar that were less concentrated than that of European foragers. The relationship between increased pollen foraging and decreased age of first foraging suggested a functional relationship. Here I tested whether baseline sucrose response threshold and honey bee ecotype simultaneously produced differential age of first foraging patterns.
Methods
Source of bees
Three European colony sources and three Africanized colony sources were used to obtain newly emerged experimental bees. European queen sources originated from northern California. Africanized queen sources originated from the Rio Grande Valley of Texas, which has been Africanized since 1990 (Sugden and Williams 1990; Rubink et al. 1996). The Africanized honey bee genome is introgressed with an unknown amount of the EHB genome. African maternal lineage was confirmed using mitochondrial DNA analysis (Crozier and Crozier 1992). Racial defensive behavior phenotype was identified using the traditional "patch test" (Hunt et al. 1999). This assay consisted of a black suede leather patch (approx. 10 cm×8 cm) that was suspended from the end of a 100 cm piece of wood and rhythmically raised and lowered at the hive entrance. Time to first sting was recorded as well as number of stings per 60 s following the first sting. Colonies were tested on 3 successive days.
PER assay
The PER assay was used to test each bee's sensitivity to an ascending concentration series of sucrose solutions. Bees from the African and European sources were emerged from their combs in an incubator maintained at 32 °C and 55% RH. Every 30 min for 4 h newly emerged bees were removed from the combs, paint marked on the thorax to indicate racial source, and placed in a common cage with no food or water prior to testing. Cages were placed inside an incubator maintained at 24–27 °C and 80% RH. Individual sucrose response scores of 396 African and 397 European bees were measured within the first 6 h of adult life. Bees were secured with tape into modified Eppendorf tubes (2 ml) that restrained body movement but allowed free movement of the antennae and mouthparts (Bitterman et al. 1983). All bees were first tested for their response to water. Any bees responding to water were allowed to imbibe water until they no longer responded to water stimulation. In this way the confounding effect of thirst on sucrose sensitivity was controlled (Edgecomb et al. 1989; Pankiw et al. 2001). The PER assay sucrose solutions were based on a log10 series of −1.0, −0.5, 0.0, 0.5, 1.0, and 1.5 corresponding to the following sucrose concentrations; 0.1%, 0.3%, 1%, 3%, 10%, and 30% (wt/vol). Sucrose solutions were prepared using distilled and Milli-pore filtered water as the solvent for Sigma sucrose (99.5% purity). A droplet of solution was expressed from the tip of a 27-gauge needle and touched to each antenna. An inter-trial interval of 5 min was maintained to reduce sensitization (Menzel et al. 1999). Positive and negative proboscis extension response to each sucrose solution was recorded. Tested bees were individually identified with colored, plastic number tags glued to the thorax. An additional 300 African and 300 European newly emerged bees from the same sources, emerged at the same time, but not tested in the PER assay were Testors paint-marked on the thorax to distinguish race.
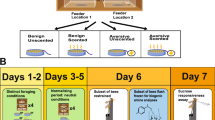
This experiment was repeated three times. Experimental bees of each race were randomly selected and equally divided for introduction to three common, unrelated European colonies at the same time. Approximately 130 Africanized and 130 European bees tested for their sucrose response thresholds were introduced to each foster colony. An additional 100 Africanized and 100 European paint marked bees were introduced to each colony at the same time. Open-mated, sister queens headed the three foster colonies. Each foster colony was comprised of approximately 10,000 adult workers. During the course of the experiment colony conditions were maintained to contain: four Langstroth deep frames of honey, three frames of brood that were approximately equal parts open and sealed, one frame of empty space and one frame of pollen. Colonies were manipulated every 6 days to maintain these conditions.
Foraging behavior measures
The foraging age of an individual was determined by the number of days from emergence to capture at the colony entrance as a returning forager. Colony entrances were observed for returning experimental bees for 20 min during the first 2 days after introduction. Beginning on the 3rd day, to the termination of the experiment, colony entrances were blocked with wire mesh for 15-min intervals separated by at least 30 min. The entrances were blocked from 0800 to 1700 hours for a total of 4 h per day. Foragers were individually captured in small cylindrical wire cages. Captured foragers were anesthetized with CO2. Gently squeezing the abdomen expelled crop contents into pre-weighed capillary tubes. The tubes were re-weighed and the subtotal recorded (Kimax-51, Catalog No. 34502; see Gary and Lorenzen 1976). The nectar loads were measured for sugar concentration using a hand-held Brix refractometer (Atago HRS-500: 0–90%±0.2%, Tokyo, Japan). Pollen load weight was calculated by doubling the value of one pollen pellet removed from the corbicula of a hind leg. Workers with trace amounts of pollen (<0.001 g) were assumed to have a total of 0.001 g of pollen. The wet weight of each empty forager was measured. All weight measures were made to the nearest 0.001 g using an AccuLab electronic balance (±1 mg, Huntington Valley, Pa., USA). Following the measurements above, foragers were euthanized by freezing.
A termination census was conducted on day 31 between 0500 and 0700 hours. The census consisted of collecting all experimental bees from the foster colonies. Bees were collected from combs with forceps and placed into a soap–water solution. The euthanasia solution did not remove plastic tags or Testors paint from the thorax (Pankiw and Page 2001). Bees were strained from the solution, recorded by number tag and color identity. Termination census data were used in the statistical analysis for age of first foraging.
Statistical analysis
Logistic regression analysis was used to analyze racial differences in proboscis extension response to ascending concentrations of sucrose (Pampel 2000). Following the first sucrose solution in the PER assay, individual responses were not independent. The repeated measures and dependent options were used in the PROC GENMOD procedure of SAS (Allison 1998; SAS 2000; Ben-Shahar and Robinson 2001; Hartz et al. 2001). PROC GENMOD produced generalized estimating equations (GEE) for the effect of race. PROC GENMOD also calculated a logistic regression line that best fitted each response curve that was used to produce a visual display.
For each individual that foraged a PER score was calculated by summing positive responses in the PER assay. Individuals that did not respond were assigned a score of zero (Pankiw and Page 2000). The PER score is directly related to the response threshold of the individual because most bees continue to respond to all increasing concentrations of sucrose following their initial response. PER scores were used in statistical analyses of the relationship between foraging behaviors and responsiveness to sucrose. Age of first foraging data were not normally distributed (Sokal and Rohlf 1995). Cox proportional hazards regression analysis was used to examine the effects of race and PER score on age of first foraging as well as to model these relationships and produce a visual display (PROC PHREG in Allison 1998; SAS 2000). Ties were handled using the EXACT option (PROC PHREG in SAS). Cox proportional hazards regression is a component of survival analysis statistical procedures that utilizes numbers of foragers and non-foragers as determined in the termination census, in the statistical model.
The effects of race and PER score on forage choice were analyzed using Kruskal-Wallis and Mann-Whitney U tests (Sokal and Rohlf 1995). Foragers were classified according to loads returned. "Pollen" foragers were those returning with no other resource except pollen. "Nectar" foragers were those returning with no other resource except nectar. Those returning with some nectar and pollen were classified as "both". Additionally, there were "water" foragers (≤5% sucrose concentration) and some were "empty" returning with no resources. Pollen, nectar, and bee weights were normally distributed. Analysis of variance (ANOVA) and covariance (ANCOVA) were used to analyze these variables. Nectar quality (% sucrose) data were analyzed using the Mann-Whitney U test (Sokal and Rohlf 1995). Means were reported with±standard errors.
Results
Source of bees
Workers from the three Africanized and the three European sources exhibited African and European mitochondrial DNA, respectively. Mean time to first stinging in the "patch test" defensive behavior assay was 8.5±0.7 s and 70.5±26.6 s for AHB and EHB colonies, respectively. Average number of stings in a 60 s period after the first sting was 64.8±6.1 and 4.4±0.7, AHB and EHB colonies, respectively.
Proboscis extension assay
Africanized bees were significantly more responsive to sucrose in the PER assay than European bees (GEE, χ 2=68.8, 1 df, P<0.0001; Fig. 1).
Age of first foraging
In a saturated model of Cox regression analysis including colony replicate, bee race and their interaction, colony replicate had no significant effect on age of first foraging (χ 2=0.1, P>0.05); therefore replicates were pooled for subsequent analysis and visual display of results (Allison 1998). Overall, Africanized bees foraged at a significantly younger mean age than European bees; Proportional Hazards χ 2=7.8, P<0.01, e β=0.8. Within the subset of bees that were tested in the PER assay, race had a significant effect on foraging age (χ 2=8.5, P<0.01, e β=0.71). The hazard ratio statistic, e β, was transformed to a more meaningful statistic that indicated Africanized bees were 29% more at risk to forage than European bees over the 30-day experimental period (Allison 1998). For each unit increase in PER score an individual was 14.3% more at risk to forage over the 30-day experimental period (χ 2=22.0, P<0.0001; Fig. 2).
Model of sucrose response scores as measured in newly emerged, inexperienced Africanized and European honey bees and their age of first foraging. Africanized bees were 29% (P<0.0001) more likely to forage than European bees. For each unit increase in proboscis extension response to sucrose score, an individual was 14.3% (P<0.0001) more likely to forage over the 30-day experimental period
The PER assay significantly affected age of first foraging (χ 2=25.1, P<0.0001, e β=1.5). To test for an interaction of the PER×race on age of first foraging, ANOVA was used. PER scores were log transformed to meet assumptions of analysis of variance (Sokal and Rohlf 1995). There was no interaction of PER×race on foraging age (F 1,347 =0.9, P>0.05). This means that the races were not differentially affected by the PER assay. Mean foraging age of PER-tested Africanized was 17.9±0.3 days and non-tested 20.0±0.4 days. For Europeans mean foraging age of PER-tested bees was 19.0±0.4 days and non-tested 21.4±0.2 days.
Measures of forage choice behavior
Division of foraging labor
Forager race and sucrose response threshold organized foraging division of labor. Sucrose response threshold distributions were not significantly different between colonies [saturated categorical model (CATMOD) χ 2=1.0, 2 df, P>0.05]. The sucrose response threshold distributions of the various foraging classes were not differentially affected by colony environment (saturated CATMOD replicate×forager class χ 2=1.9, 6 df, P>0.05). Division of foraging labor between races was significantly different such that Africanized bees were more likely to return with loads of pollen and water, whereas European bees were more likely to return with nectar or empty (race×forager class Contingency Table analysis; χ 2=19.3, 3 df, P<0.0001; see Fig. 3).
Sucrose response threshold predicted foraging division of labor for both races (AHB Kruskal-Wallis χ 2=34.0, 4 df, P<0.0001; EHB Kruskal-Wallis χ 2=19.0, 4 df, P<0.001). Race significantly constrained PER scores such that Africanized bees had lower PER scores in the following forager classes: nectar only U=251, P<0.05; pollen only U=1925, P<0.0001; both nectar and pollen U=282.5, P<0.05; water U=12, P<0.05 (Fig. 3). Empty forager PER scores for AHB and EHB were not different (P>0.05).
Resources returned
The effect of replicate was insignificant on amounts of nectar, nectar concentration (log transformed), and pollen (nectar F=1.0, 2 df, P>0.05; nectar concentration F=3.0, 2 df, P>0.05; pollen F=3.1 2 df, P>0.05). The sucrose concentration of nectar returned by Africanized bees was significantly lower than European bees (Nectar only, Mann-Whitney U 43,75 =869.5, P<0.0001; With nectar, Mann-Whitney U 108,132 =4273.0, P<0.0001; Fig. 4 ). Spearman rank correlation analysis showed a positive relationship between sucrose response threshold and sugar concentration of nectar returned among the races (ρ=0.4, P<0.01) and among the races (Africanized: ρ=0.4, P<0.01; European: ρ=0.34, P<0.05).
Race significantly affected bee wet-weight (ANOVA F 1,692=63.3, P<0.0001) such that the mean weight of Africanized bees (66.4±0.4 mg) was less than that of European bees (81.6±2.0 mg). Analyses of co-variance (ANCOVA) were performed on load weights adjusted for bee wet weight (Sokal and Rohlf 1995). ANCOVA diagnostic procedures for the assumption of homogeneity of variances were met (nectar: F 1,115=2.8, P>0.05; pollen: ANCOVA: F 1,309=3.1, P>0.05 ). European nectar foragers returned with significantly heavier loads than Africanized nectar foragers (ANCOVA: F 1,115=6.3, P<0.01; Fig. 5). There were no significant differences between the races for pollen load weights (ANCOVA; F 1,309=1.6, P>0.05; Fig. 5).
Discussion
Clearly a suite of foraging behaviors changed with adaptation to tropical and temperate habitats. Natural selection for low response thresholds to sucrose resulted in a greater probability to forage at an earlier age, to collect pollen and water, and to collect nectar with less concentrated sugar. Natural selection for higher response thresholds to sucrose resulted in a greater probability to collect nectar, return with nectar that was higher in sucrose concentration, return empty and, to forage at an older age. Change in one component of a suite of behaviors appeared to direct change in the entire suite (Table 1).
A similar directional change appears to occur with artificial selection. For example, bees artificially selected to hoard high and low amounts of pollen show repeatable and characteristically different sucrose response thresholds (Page et al. 1998; Pankiw and Page 1999, 2001; Pankiw et al. 2001, 2002; Scheiner et al. 2001a, 2001b). High-strain bees have lower response threshold to sucrose than low-strain bees among pre-foragers, foragers, queens and drones. High-strain foragers are more likely to return with loads of water compared to low-strain foragers. Low-strain foragers are more likely to return with loads of nectar. Low-strain nectar foragers collect nectar with significantly higher sucrose concentrations than high-strain nectar foragers. Alternatively, low-strain foragers are more likely to return empty than are high-strain foragers.
A change in sucrose response threshold by natural selection appeared to change the same suite of behaviors in the same direction in this study (Table 1). Africanized bees have lower response thresholds than European bees (Pankiw and Rubink 2002). Observed here and elsewhere Africans forage at younger ages and are more likely to collect pollen than Europeans when fostered in the same colony (Winston and Katz 1982; Fewell and Bertram 2002). Africanized bees hoard more pollen than European bees (Danka et al. 1987). Associated with a lower sucrose response threshold, I observed that Africanized bees were more likely to forage for pollen and water, and chose less concentrated nectar. It seems unlikely that change in one trait could result in a change of six traits in the same direction by chance alone in artificial and natural populations. It suggests that this suite of foraging behaviors is a physiological phenomenon directionally regulated at a proximate neurological level.
Age of first foraging of Africanized bees was significantly younger than in European bees. This cannot be explained by missing European bees because they were retrieved in the colony census at the termination of the experiment. There was a functional relationship between sucrose response threshold measured in newly emerged workers and their age of first foraging. Africanized bees had significantly lower sucrose response thresholds than Europeans. For every unit decrease in PER score, Africanized and European bees showed increased risk for foraging at a younger age. Additionally, African genes further increased an individual's risk to forage at an earlier age. This may be interpreted as Africanized bees having greater sensitivity to environmental cues that prime and release foraging behavior, ordering the rate of foraging ontogeny.
Bees tested for their sucrose response thresholds foraged at significantly younger ages than bees that were not tested. Africanized and European bees were both affected and in the same direction. This could be interpreted as an effect of handling stress. It is well known that chronic stressors affect the physiology and ontogeny of invertebrates and vertebrates (Hoffmann and Parsons 1994). Harris and Woodring (1992) stressed workers by grasping one leg with forceps. Octopamine levels peaked at 10 min and reduced to pre-stress levels by 20 min. Colonies of bees chronically dosed with octopamine showed significantly decreased ages of first foraging (Schulz and Robinson 2001; Barron et al. 2002). Bees tested for their sucrose response thresholds 15, 30, and 60 min after being secured in the holders, indicated there was a positive relationship between time after handling and sucrose response threshold (Pankiw and Page, unpublished data). The results of this experiment suggest there were ontogenetic effects to short-term handling stress associated with the PER test.
Sucrose response threshold was strongly correlated with forage choice behaviors. The association between sucrose response threshold and foraging choice behaviors measured here has been demonstrated in two previous independent studies (Pankiw and Page 2000) and is a robust neurosensory correlate of foraging behavior. Natural selection affected the sucrose response threshold distributions of Africanized and European bees, acting as a genotypic constraint on forage choice. The results from this study clearly show that sucrose response threshold should not be confused with a determinant of forage choice. Sucrose response threshold is associated with forage choice behavior and relative, such that it is constrained by genotype. The distribution of sucrose response thresholds changed in temperate and tropical evolved bees. Selection acted on genetic variability affecting response threshold distributions; consequently the pattern of foraging organization changed.
There was a positive correlation between sucrose response threshold and nectar quality choice in both races. The mean sucrose concentration of nectar returned by Africanized foragers was lower than that of European foragers. This is interpreted as a consequence of naturally selected differential sucrose response threshold distributions constraining nectar quality choice. Accordingly, European nectar foragers returned with significantly heavier loads than Africanized nectar foragers because more concentrated nectar is heavier than less concentrated nectar. There was no difference in pollen load weights, at least in this experiment. In a study of the spatial foraging patterns of Apis mellifera scutellata in Africa, Schneider and McNally (1993) concluded that during periods of patchy resource availability, African foragers may be required to accept any resources within flying distance to ensure survival. It is reasonable to hypothesize that lower sucrose response thresholds observed in Africanized bees may be an outcome of A. m. scutellata adaptation to patchy resource environments. Low sucrose response thresholds are associated with an increased probability of foraged rewards of relatively low or no sucrose quality.
A behavioral suite may be interpreted as a product of functional ecological adaptation. That the suite changed in the same direction with a single trait change in naturally and artificially selected populations suggests fundamental change at a proximate level having a cascade effect at higher levels of organization. The view of a set of traits engaged in coordinated change as a suite may serve to transform the complexity of foraging behavior into a relational unit to offer new insights in the evolution and organization of complex systems, and functional adaptation.
References
Allison PD (1998) Survival analysis using the SAS system. A practical guide. SAS Institute, Cary, N.C.
Barron AB, Schulz DJ, Robinson GE (2002) Octopamine modulates responsiveness to foraging-related stimuli in honey bees (Apis mellifera). J Comp Physiol A 188:603–610
Ben-Shahar Y, Robinson GE (2001) Satiation differentially affects performance in a learning assay by nurse and forager honey bees. J Comp Physiol A 187:891–899
Bitterman ME, Menzel R, Feitz A, Schäfer S (1983) Classical conditioning of proboscis extension in honeybees (Apis mellifera). J Comp Psychol 97:107–119
Calderone NW, Page RE (1988) Genotypic variability in age polyethism and task specialization in the honey bee, Apis mellifera (Hymenoptera: Apidae). Behav Ecol Sociobiol 22:17–25
Calderone NW, Page RE (1991) Evolutionary genetics of division of labor in colonies of the honey bee (Apis mellifera). Am Nat 138:69–92
Calderone NW, Page RE (1996) Temporal polyethism and behavioural canalization in the honey bee, Apis mellifera. Anim Behav 51:631–643
Crozier RH, Crozier YC (1992) The cytochrome b and ATPase genes of honeybee mitochondrial DNA. Mol Biol Evol 9:474–482
Danka RG, Hellmich II, Rinderer TE, Collins AM (1987) Diet-selection ecology of tropically and temperately adapted honey bees. Anim Behav 35:1858–1863
Dreller C, Tarpy DR (2000) Perception of the pollen need by foragers in a honeybee colony. Anim Behav 59:91–96
Edgecomb RS, Pyle AR, Murdock LL (1989) The role of water in tarsal thresholds to sugar in the blowfly Phormia regina. J Exp Biol 142:245–255
Fewell JH, Bertram SM (2002) Evidence for genetic variation in worker task performance by African and European honey bees. Behav Ecol Sociobiol 52:318–325
Gary NE, Lorenzen K (1976) A method for collecting the honey-sac contents from honeybees. J Apic Res 15:73–79
Harris JW, Woodring J (1992) Effects of stress, age, season, and source colony on levels of octopamine, dopamine, and serotonin in the honey bee (Apis mellifera L.) brain. J Insect Physiol 38:29–35
Hartz SM, Ben-Shahar Y, Tyler M (2001) Logistic growth curve analysis in associative learning data. Anim Cogn 4:185–189
Hoffmann AA, Parsons PA (1994) Evolutionary genetics and environmental stress. Oxford University Press, New York
Hunt GE, Collins AM, Rivera R, Page RE, Guzman-Novoa E (1999) Quantitative trait loci influencing honeybee alarm pheromone levels. J Hered 90:585–589
Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A (1999) Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci 113:744–754
Page RE, Erber J, Fondrk MK (1998) The effect of genotype on response thresholds to sucrose and foraging behavior of honey bees (Apis mellifera L.). J Comp Physiol A 182:489–500
Pampel FC (2000) Logistic regression. A primer. Sage, Thousand Island Oaks, Calif.
Pankiw T, Page RE (1999) The effects of genotype, age, and caste on response thresholds to sucrose and foraging behavior of honey bees. J Comp Physiol A 185:207–213
Pankiw T, Page RE (2000) Response thresholds to sucrose predict foraging division of labor in honey bees. Behav Ecol Sociobiol 47:265–267
Pankiw T, Page RE (2001) Genotype and colony environment affect honey bee (Apis mellifera L.) development and foraging behavior. Behav Ecol Sociobiol 51:87–94
Pankiw T, Rubink WL (2002) Pollen foraging response to brood pheromone by Africanized and European honey bees (Apis mellifera L.). Ann Entomol Soc Am 95:761–767
Pankiw T, Waddington. KD, Page RE (2001) Modulation of sucrose response thresholds in honey bees (Apis mellifera): influence of genotype, feeding and foraging experience. J Comp Physiol A 187:293–301
Pankiw T, Tarpy DR, Page RE (2002) Genotype and rearing environment affect honeybee perception and foraging behaviour. Anim Behav 64:663–672
Reznick DN, Ghalambor CK (2001) The population ecology of contemporary adaptations: what empirical studies reveal about the conditions that promote adaptive evolution. Genetica 112–113:183–198
Rubink WL, Luévano-Martínez P, Sugden EA, Wilson WT, Collins AM (1996) Subtropical Apis mellifera (Hymenoptera: Apidae) swarming in dynamics and Africanization in northeastern Mexico and southern Texas. Ann Entomol Soc Am 89:243–251
SAS (2000) The SAS system version 8.01. SAS Institute, Cary, N.C.
Scheiner R, Page RE, Erber J (2001a) The effects of genotype, foraging role and sucrose responsiveness on the tactile learning performance of honey bees (Apis mellifera L.). Neurobiol Learn Mem 76:138–150
Scheiner R, Page RE, Erber J (2001b) Responsiveness to sucrose affects tactile and olfactory learning in preforaging honey bees of two genetic strains. Behav Brain Res 120:67–73
Schneider SS, McNally LC (1993) Spatial foraging patterns and colony energy status in the African bee, Apis mellifera scutellata. J Insect Behav 6:195–210
Schulz DJ, Robinson GE (2001) Octopamine influences division of labor in honey bee colonies. J Comp Physiol A 187:53–61
Sokal RR, Rohlf FJ (1995) Biometry. The principles and practice of statistics in biological research, 3rd edn. Freeman, New York
Sugden EA, Williams KR (1990) October 15: the day the bee arrived. Glean Bee Cult 119:18–21
Winston ML, Katz SJ (1982) Foraging differences between cross-fostered honeybee workers (Apis mellifera) of European and Africanized races. Behav Ecol Sociobiol 1:125–129
Winston ML, Taylor O, Otis GW (1983) Some diffferences between temperate European and tropical African and South American honeybees. Bee World 64:12–21
Acknowledgements
Thanks are extended to W.L. Rubink for supplying Africanized queens, and two anonymous reviewers for constructive criticism that improved this manuscript. This project was supported by funds from the Texas Honey Bee Initiative and USDA 58-6204-8-093. Bees were handled in compliance with current laws of the United States of America.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.F.A. Moritz
Rights and permissions
About this article
Cite this article
Pankiw, T. Directional change in a suite of foraging behaviors in tropical and temperate evolved honey bees (Apis mellifera L.). Behav Ecol Sociobiol 54, 458–464 (2003). https://doi.org/10.1007/s00265-003-0640-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0640-1