Abstract
In some bird species, mothers can advantage the offspring of one sex either by elevating them in the laying order to promote earlier hatching or by allocating greater resources to eggs of the preferred sex. In size dimorphic species, the predictions as to which sex should benefit most from such pre-laying adjustments are ambiguous. The smaller sex would benefit from an initial size advantage to help compensate for the faster growth rate of the larger sex. However, an early advantage to offspring of the larger sex might have a greater effect on their lifetime reproductive success than an equivalent advantage to offspring of the smaller sex. We investigated these hypotheses in the polygynous brown songlark, Cinclorhamphus cruralis, which is one of the most sexually size dimorphic birds known. We conducted within-clutch comparisons and found that females hatched from larger eggs and were initially heavier (but not structurally larger) than their brothers. This may afford females an early competitive advantage, as egg volume remained correlated with chick mass until at least 5 days of age. Similarly, we found that hatch order was still positively associated with nestling mass and size when the brood was 10 days of age, but there was no clear relationship between offspring sex and hatching order. During this study, food was plentiful and there were few obvious cases of nestling starvation. When food is limited, we suggest that the greater nutrient reserves of female hatchlings could not only help compensate for their slower growth, but could also give them a survival advantage over their brothers early in the nestling period. Consequently, egg size dimorphism may be an adaptation that facilitates an early shift in brood sex-ratio towards cheaper daughters in conditions of low food availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In many sexually reproducing organisms, parents are expected to benefit from being able to adjust their relative investment in sons and daughters (Trivers and Willard 1973; Charnov 1982; Frank 1990; Sheldon 1998). Among birds, recent studies show that some species can skew the laying order of the sexes within a clutch (Clotfelter 1996; Kilner 1998; Krackow 1999; Nager et al. 1999; Legge et al. 2001; Badyaev et al. 2002; Krebs et al. 2002), while others appear to provision eggs differentially depending on the sex of the embryo (Mead et al. 1987; Anderson et al. 1997; Cordero et al. 2000; 2001). Both of these tactics have the potential to influence the overall allocation of parental resources to the sexes by affecting competitive disparities between male and female siblings. Eggs produced early in the laying order typically hatch early in the hatching sequence and, if hatching is asynchronous, these nestlings will have first access to food and grow larger by the time their younger siblings hatch (Howe 1976; Bortolotti 1986; Bednarz and Hayden 1991). Such an early competitive advantage may be maintained throughout the nestling period, and if brood reduction occurs it is usually the smaller, late hatching chicks that die (Lack 1968; Bednarz and Hayden 1991; Stoleson and Beissinger 1995). Consequently, promoting one sex earlier in the laying order should provide this sex with an advantage both in size and survival prospects (Stamps 1990; Badyaev et al. 2002).
Similarly, if the chicks of one sex are provisioned with larger eggs, these chicks should have a competitive advantage over chicks of the other sex (Anderson et al. 1997; Cordero et al. 2000, 2001). A size advantage to hatching from larger eggs may persist well into the nestling period of some altricial species (Magrath 1992; Nilsson and Svensson 1993; reviewed in Williams 1994; Smith et al. 1995), and in the case of the house wren, Troglodytes aedon, even when the chicks have achieved asymptotic mass (Styrsky et al. 1999, 2000). Consequently, mothers could use either of these two pre-laying mechanisms to manipulate the overall pattern of parental investment in the sexes.
In species with sexually size dimorphic young, members of the larger sex typically consume more parental resources than those of the smaller sex (e.g. Teather and Weatherhead 1988; Krijgsveld et al. 1998; reviewed in Anderson et al. 1993). However, predictions concerning sex allocation strategies prior to hatching remain ambiguous. On the one hand, chicks of the smaller sex may be at a considerable size disadvantage when competing for food with their faster growing siblings (Stamps 1990; Oddie 2000). Mothers may seek to temporarily offset this disadvantage or even skew the brood sex-ratio toward the smaller, cheaper sex either by promoting the members of this sex up the laying order or by provisioning their eggs better (Bednarz and Hayden 1991; Cordero et al. 2000). For example, in the Harris's hawk, Parabuteo unicinctus, and laughing kookaburra, Dacelo novaeguineae, males are more likely to hatch first in the clutch than their larger sisters, and this has been interpreted as a mechanism to avoid maladaptive brood-reduction (Bednarz and Hayden 1991; Legge et al. 2001). On the other hand, many size dimorphic birds are also polygynous, with the larger males typically having greater variance in lifetime reproductive success than the smaller females. In such species, body size or condition at fledging may have a greater influence on the lifetime reproductive success of sons than daughters, so parents may benefit by investing more in their sons when conditions are favourable (Trivers and Willard 1973; Albrecht 2000; Cordero et al. 2000). For example, Cordero et al. (2000) speculate that male house sparrows, Passer domesticus, hatch from larger eggs because males exhibit greater variance in condition-dependent reproductive success than females. Consequently, mothers of polygynous species should benefit by either producing sons earlier in the clutch or by better provisioning male eggs.
In this study, we investigate the relationship between laying order, egg size and offspring sex in the brown songlark, Cinclorhamphus cruralis, which is to our knowledge, the most sexually size dimorphic of all passerine birds (Andersson 1994). In this Australian endemic, adult males are 2.3 times heavier than adult females [74.8±4.0 g (n=21) vs 32.4±1.9 g (n=141); mean±SE], and males are already more than 50% heavier than their sisters when they fledge (Magrath et al., unpublished data). Brown songlarks are known both as migrants and nomads and, in some inland regions, nest opportunistically after rainfall has promoted the growth of grasses and shrubs that are required for breeding (Blakers et al. 1984). Breeding males contribute very little to parental care and defend territories on which as many as ten females may nest concurrently, indicating that the species is strongly polygynous (Magrath et al., unpublished data). Consequently, it may be argued that sons should benefit more from the potential advantages of hatching from larger eggs or from being laid earlier in the laying sequence. Alternatively, because male chicks are typically heavier and larger than their sisters by 5 days of age (Magrath et al., unpublished data), an initial size advantage to females may be critical in the early stages of competition with their faster growing brothers. Furthermore, in conditions where food is limiting, early male-biased brood-reduction may be favoured by selection because daughters are less costly to rear and the production of low quality sons may be of little reproductive value. In this scenario, mothers should produce females earlier in the clutch or provision them with larger eggs.
To evaluate these alternative hypotheses we determined first, if one sex was elevated higher in the laying (and hatching) order or hatched from larger eggs, and second, if either of these potential advantages persisted into the nestling period.
Methods
Study sites
The study was conducted in the semi-arid grass- and shrub-lands of south-western New South Wales, Australia. Data were collected between September and December in the 3 years from 1998 to 2000. The locations and sizes of the study sites were 33°21′S, 144°58′E (60 ha) in 1998, 33°72′,S 145°30′E (120 ha) in 1999, and 34°22′S, 145°02′E (120 ha) in 2000.
Data collection
Following discovery, nests were checked daily until either the clutch/brood was depredated or fledged. If the nest was found before or during laying, eggs were numbered with a permanent marker in the order in which they were laid, otherwise they were numbered randomly. The length and width of all eggs was measured to the nearest 0.1 mm using vernier callipers, and the mass of freshly laid eggs was measured with a pesola balance to the nearest 0.05 g. Most data on the laying order of eggs were collected in 2000.
Complete or partial hatch order was determined in the field for 72 clutches. These data were obtained by frequent visits to nests (about every 3 h) during daylight hours over the period of hatching. Recently hatched chicks were individually marked on the tarsus with a permanent pen, weighed (to the nearest 0.1 g) and had their wing, bill and tarsus length measured (to the nearest 0.1 mm). The complete laying and hatching order of chicks was known for seven of these broods.
Another 31 clutches (17 with complete known laying order) were collected from the field and hatched in an incubator. These clutches were collected approximately 1 day before hatching was anticipated (day 11 of incubation) and replaced by dummy eggs of approximately equal size and colour. The eggs from these clutches were then placed in separate compartments within a polystyrene incubator (BellSouth), so that the hatching order and morphological characteristics could be accurately determined for each chick in the brood. The time of hatching was recorded for each egg, along with the hatchling mass (to the nearest 0.001 g using an analytical balance) and other morphometric measurements (as above). Chicks were then marked and returned to their nest within hours of hatching, or early the following morning if they hatched during the night.
Five and ten days after the first chick(s) in a brood hatched, the mass (to the nearest 0.1 g) and tarsus, bill, and wing length of each chick was measured. Chicks had usually left the nest by 12 days of age.
Sex determination
From most hatchlings, a blood sample was taken, suspended in 500 µl of lysis buffer (0.1 M Tris-HCl, pH 8.0; 0.1 M EDTA, pH 7.4; 10 mM NaCl; 0.5% SDS) and stored at 5°C. Unhatched eggs were collected and the embryos frozen. DNA was extracted from blood and tissue samples using a standard NaCl/ethanol extraction method (Lahiri et al. 1992). Molecular sexing was performed by PCR amplification of introns in two homologous genes (CHD-W and CHD-Z) using the P2/P8 primer couple (Griffiths et al. 1998). Products were resolved on 2% agarose gels stained with ethidium bromide, that revealed either one (male) or two (female) distinct bands. This technique was verified as reliable by the correct sexing of six male and six female adults.
Data analyses
The laying order and sex of each egg in the clutch was determined for a total of 24 clutches (71 eggs). For another 16 clutches, the laying order and sex was known for only some eggs in the clutch (24 eggs) because of partial predation, egg infertility or discovery of the nest after initiation of egg-laying. The hatching order and sex of each chick in the brood was determined for 61 broods (156 chicks), while the hatch order and sex for only some chicks in the brood was known for a further 42 broods (51 chicks). We always used the largest available data set appropriate to each analysis. Nevertheless, our results were always similar regardless of whether analyses were performed on the largest data set or the sub-set of data where the sex and laying/hatching order was known for all eggs/chicks in the clutch/brood.
The eggs comprising our laying data set were from clutches ranging in size from 2 to 5 (3 with 2 eggs; 34 with 3 eggs; 2 with 4 eggs; 1 with 5 eggs), while chicks in the hatching data set were from broods ranging from 1 to 4 (4 with 1 chick; 22 with 2 chicks; 62 with 3 chicks; 15 with 4 chicks). Because the implications of being laid third in a clutch of three may be quite different to being third in a clutch of four, we ranked each egg into one of three categories for analysis; (1) first laid egg, (2) middle laid egg(s), and (3) last laid egg. The same three categories were used for chicks to define their position in the hatching order. Actual laying/hatching order was usually the same as the rank order because most eggs (86/95) and chicks (133/207) were from clutches/broods of three. In the few clutches and broods of two, the second egg/chick was assigned to the 'middle' category because second eggs usually hatched shortly after first eggs irrespective of whether the clutch had only two eggs. The results of our analyses were always similar regardless of whether actual or rank order was used, or if second eggs in two egg clutches were categorised as middle or last eggs.
Egg volume (V) was calculated according to the formula V=LW 2π/6, where L=egg length and W=egg width (Hoyt 1979). This estimate of volume was highly correlated with mass of freshly laid eggs (r 2=0.81, n=154, P<0.001), so only volume was analysed as the estimation of investment in an egg. An estimate of body condition for hatchlings and nestlings was calculated by deriving residuals from the regression of mass on tarsus length (Packard and Boardman 1987). Only the measurements from chicks that hatched in the incubator were used to evaluate hatchling morphology because most chicks that hatched in the field would have received some food before they were measured.
Daily nest monitoring allowed us to identify cases of partial brood mortality or loss. To assess if the probability of mortality was related to egg volume and hatch order we compared these variables for chicks that were lost versus those that remained within broods that suffered partial loss.
Most analyses were performed using multilevel modelling in MlwiN 1.10 (Rasbash et al. 2000). This approach allows within-clutch (or within-brood) patterns to be examined after correcting for variation attributable to differences between clutches and broods. For these models, the clutch (or brood) was specified as the level two unit of variation and egg (or chick) as the level one unit of variation. When the response variable was egg/chick sex or chick survival, we used a binomial response model with logit-link function, while normal response models were used to examine variation in egg volume and chick morphological characteristic, as these variables were distributed normally.
Each model was derived using backward elimination of possible explanatory variables and their interaction terms. For the binomial response models, the Wald test was applied to determine the significance of explanatory variables as each term was dropped from the final model. For normal response models, the significance of explanatory variables was determined by calculating the change in model deviance (which approximates the χ 2 distribution) as each term was eliminated from the final model. Final models included a constant together with any statistically significant (P<0.05) explanatory variables. Non-significant interaction terms were not included in the model summary tables unless they were of specific interest.
Of those clutches where the laying order and sex of all eggs was determined, we examined whether any particular two-egg gender sequence (i.e. MM, MF, FM, FF) was more common than might be expected by chance. This was tested by comparing the observed frequency of two-egg sex sequences with an expected frequency distribution generated by repeated sampling (n=5,000) of egg sequences from the data set (Manly 1997).
Results
Sex, laying order and hatching order
We found no consistent relationship between the sex of an embryo and the order within a clutch in which eggs were laid (Table 1; Fig. 1). Furthermore, the relationship between embryo sex and laying order did not vary systematically over the course of the breeding season (Table 1). We also found no evidence for a non-random association of egg-sex sequences in the laying order, as the sex of an egg was not related to the sex of the previous or subsequent egg (Table 2).
In clutches of known laying order that hatched in our incubator (n=17 three-egg clutches), the first two eggs hatched an average of 6.8±5.4 h (±SD) apart, while the third egg typically hatched almost a day later (20.5±8.2 h after the first egg). In the larger data-set, however, chick sex was unrelated to hatching order, and this pattern did not vary over the course of the season (Table 1; Fig. 1).
Hatching order corresponded exactly to laying order in 14 of the 17 incubator-hatched clutches where the order of both was known. In two of the remaining cases, the second laid egg hatched before the first laid egg, while in one clutch the third laid egg hatched first. In each of these three cases, a female hatched ahead of a male, raising the possibility that female eggs may have a shorter incubation period than male eggs. This is also suggested by the apparent switch in sex-ratio observed in the laying and hatching order data sets (Fig. 1), which show that more first-laid eggs were male and middle-laid eggs female, but that the majority of middle hatching chicks were male. However, this reversal in sex-ratio was not statistically significant (logistic regression; sex as response variable and rank order and stage (laying or hatching) as factors; interaction between factors, χ 2=3.54, df=2, P=0.17), and it should be noted that clutches contributing to the laying and hatching order data sets were only partially overlapping.
Sex, egg volume and hatchling characteristics
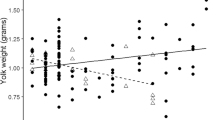
Within clutches, egg volume was related to both sex of the embryo and laying order; females hatched from eggs that were on average 3% larger than their brothers while third laid eggs were about 3% smaller than the first two eggs (Table 3; Fig. 2a). The larger volume of female eggs translated to females also hatching about 3% heavier than their brothers, after controlling for differences in mass associated with laying order (Table 3; Fig. 2b). Female hatchlings also tended to be in better body condition than their brothers, (χ 2=3.1, P=0.08, n=77 chicks from 31 broods), though there was little difference between the sexes in the structural measures of tarsus, bill, or wing length (P>0.1 for each).
a Egg volume in relation to laying order and sex of the embryo. Mean values (±SE) calculated from the model predictions that were derived after correcting for inter-clutch variation in mean egg volume. b Hatching mass in relation to hatching order and sex of the chick. Mean values (±SE) calculated from model predictions that were derived after correcting for inter-brood variation in mean hatchling mass
Across clutches in which all eggs were sexed, there was no correlation between mean egg volume and clutch sex-ratio (r 2=0.09, n=81, P=0.42), indicating that females producing larger eggs did not generally skew brood sex-ratio toward one sex over the other. There were strong correlations between egg volume and hatchling mass (r 2=0.79), tarsus length (r 2=0.30), wing length (r 2=0.36), bill length (r 2=0.35), and body condition (r 2=0.58) (linear regressions; n=77 hatchlings and P<0.001 for each). The nature of these relationships between egg volume and hatchling characteristic was not influenced by the sex of the chick (ANCOVA with 'sex' as factor and 'egg volume' as covariate; n=34 females and 43 males, P>0.1 in each analysis for both sex and the interaction between sex and egg volume).
Effect of egg volume and hatching order on growth and survival
The mass of siblings in five-day-old broods was influenced by both their hatching order and the volume of the egg from which they hatched; chicks hatching earlier and from larger eggs were heavier (Table 4; Fig. 3). Chicks within five-day-old broods were also structurally larger (tarsus length) if they hatched earlier (Table 4). By 10 days of age, chick mass was no longer correlated with egg volume, though hatch order continued to have a strong effect on chick mass and structural size in both sexes (Table 4).
a Nestling mass in 5-day-old broods in relation to chick sex and egg volume. Slopes (±95% confidence interval) calculated from model predictions that were derived after correcting for hatching order and inter-brood variation in mean nestling mass. Egg volume is shown as deviation from the clutch mean. b Nestling mass in 5-day-old broods in relation to chick sex and hatching order. Mean values (±SE) calculated from model predictions that were derived after correcting for egg volume and inter-brood variation in mean nestling mass
Predation was the primary cause of nest loss, and in most cases the predator took the entire brood. Partial brood loss was uncommon; in only 38 of 238 broods (16%) did some (but not all) of the chicks die (n=25 chicks) or disappear (n=28 chicks) at some stage during the nestling period. Some of these deaths and disappearances may have resulted from starvation, though others were probably the result of partial brood predation or disease. Within these broods with partial loss, we tested whether there was any difference in terms of hatching order, egg volume or sex between the chick(s) that died/disappeared earliest compared with those that remained. However, the probability of earlier mortality was apparently unrelated to hatching order rank (χ 2=2.79, P=0.28), chick sex (χ 2=0.48, P=0.49), or egg volume (χ 2=0.71, P=0.40) (n=48 chicks from the 17 broods where all relevant data were available).
Discussion
Female brown songlarks were found to hatch from larger eggs than their brothers, independently of laying order. Disparities in egg size between the sexes have previously been reported in white-crowned sparrows, Zonotrichia leucophrys oriantha (Mead et al. 1987), American kestrels, Falco sparverius (Anderson et al. 1997), house sparrows, Passer domesticus (Cordero et al. 2000), and the spotless starling, Sturnus unicolor (Cordero et al. 2001). Of these species, only nestlings of the American kestrel exhibit appreciable size dimorphism, and in that study the smaller sex (males) were also laid in larger eggs (Anderson et al. 1997). In the remaining three species, males are slightly larger than females at fledging, but the pattern of egg dimorphism was inconsistent; spotless starlings produced larger female eggs (Cordero et al. 2001), while white-crowned and house sparrows laid larger male eggs (Mead et al. 1987; Cordero et al. 2000). Clearly, further data on egg size in relation to sex are needed to assess if there is an inverse association between adult and egg sexual size dimorphism or even if the patterns observed to date are consistent within species.
Sex-related provisioning of eggs suggests that females of at least some bird species, including the brown songlark, can either detect or control the gender of the ova at ovulation and differentially provision the egg according to its sex (Oddie 1998; Cordero et al. 2000). Mechanisms for either control or detection of egg sex remain unknown in birds (Krackow 1999), but the nature of the egg size disparity between the sexes may shed some light on the processes involved. At least in chickens, Gallus domesticus, the yolk of an egg is completely formed before sex is assigned to the developing ovum following the first meiotic division (Johnson 1986). Sex differences in yolk size, therefore, would suggest some form of pre-ovulation control over the process of meiosis. Alternatively, if the mechanism involves the detection of sex following ovulation, only the amount of albumen could be allocated differentially. We have no data on the relative amount of yolk and albumen in male and female eggs, but such information might help reveal both the mechanism and nutritional implications of egg size dimorphism.
The sexual dimorphism in the size of brown songlark eggs resulted in females hatching heavier than their brothers, though they were not skeletally larger in terms of tarsus, bill or wing length. Consequently, the greater mass of females at hatching probably reflected a greater reserve of nutrients (Parsons 1970; Ankney 1980; Williams 1994). Several studies have shown that hatchling mass can influence nestling growth in the short- (Magrath 1992; Williams 1994; Smith et al. 1995) and longer-term (Styrsky et al. 1999; 2000), and even have an effect on the survival prospects of nestlings (Amundsen and Stokland 1990; Reid and Boersma 1990; Williams 1994). In this study, we found that the mass of chicks within 5-day-old broods was still correlated with the volume of the egg from which they hatched, after correcting for the effects of hatch order and sex. This effect of egg volume on nestling mass appeared to be similar for both sexes. However, by the time broods were 10 days of age this association was no longer evident, suggesting that others factors may eventually swamp the initial benefits of hatching from larger eggs (Magrath 1992; Smith et al. 1995; Styrsky et al. 1999).
We also found that hatching order had a strong influence on nestling growth, with chicks that hatched early almost invariably being heavier and larger at both 5 and 10 days of age compared with later hatching siblings of the same sex. Hatching order is known to influence fledging mass and condition in a wide range of altricial birds (e.g. Wiebe and Bortolotti 1995; Kilner 1998; Blanco et al. 2002), and several studies also show that fledging condition can effect the likelihood of recruitment into the breeding population (Magrath 1991; Both et al 1999; Monros 2002). However, unlike egg volume, there was little evidence that position in the hatch order was related to sex of the chick. Similarly, there was no clear association between egg sex and position in the laying order.
Generally, hatching order was closely correlated with laying order, though there was some indication that females may develop faster than their brothers. Firstly, in the three cases where hatching order differed from laying order, a female hatched ahead of a male. Secondly, there was an apparent reversal in the sex ratio of the middle position between laying and hatching, as most middle laid eggs were female, but most middle hatching chicks were male. This pattern could arise if mothers start incubation when the second egg is laid, but it is also consistent with female embryos developing somewhat faster. A difference in the developmental rate of male and female avian embryos has not been reported before, but would perhaps be most likely to occur in species with size dimorphism. Accelerated development in the egg may be another mechanism by which females could gain the advantage of hatching earlier. Unlike adjustment of egg volume or laying order, this mechanism may not be under maternal control. However, the females of some birds are known to adjust the allocation of steroids, such as testosterone, in relation to laying order (Schwabl 1993) and embryo sex (Petrie et al. 2001), so it is plausible that mothers could influence the relative developmental rate of the sexes by the differential allocation of growth hormones. Alternatively, mothers could exploit any difference in developmental rate, either by varying the onset of incubation (later onset, earlier hatching daughters) or by adjusting the laying order of the sexes. While these are intriguing possibilities, further research is clearly required to assess whether there are sex differences in pre-hatching development in the songlark, or indeed in any other avian species.
In birds with altricial young, the smallest chicks in the brood are typically the first to die in the event of brood reduction (Lack 1968; Stoleson and Beissinger 1995). In the years of this study, there were few obvious cases of brood reduction resulting from starvation, suggesting that food was plentiful. Indeed, in 2 of the 3 years, including the 2000 season when most data were collected, winter rainfall was considerably above the long-term average for the region (183% in 1998; 98% in 1999; and 138% in 2000; Bureau of Meteorology). Rainfall over this winter period promotes the growth of grasses and shrubs that result in an abundance of invertebrate prey. At such times, the size advantage of hatching from larger eggs, although evident early in the nestling period, may have little influence on the likelihood of survival and condition in the longer-term (Smith and Bruun 1998; Styrsky et al. 1999; but see Styrsky et al. 2000). Consequently, hatching smaller may only affect males in the short-term, and have no adverse effect on their quality in the longer term. However, in the semi-arid grasslands where songlarks breed, conditions vary dramatically both within and between years. In periods of low food availability, competition between siblings should be more intense and early brood reduction more likely. Under such conditions of food restriction, the initial advantage to daughters of hatching with greater energy reserves may translate to a survival advantage over their brothers early in the nestling period. The resulting shift in brood sex-ratio toward females should be adaptive in years of limited food availability for several reasons. First, smaller daughters are less costly to rear (Magrath et al. unpubl. data), and may be less susceptible to starvation than their more food-demanding brothers. Slow growth (Velando 2002) or greater mortality (Griffiths 1992; Wiebe and Bortolotti 1992; Torres and Drummond 1999) of the larger sex has been reported in several other studies of dimorphic species under situations of low food availability. Second, high quality sons are unlikely to be produced in poor seasons, so it should be more profitable for mothers to produce daughters rather than low quality sons particularly in a species that can be so strongly polygynous (Trivers and Willard 1973; Myers 1978).
The allocation of daughters to larger eggs does not preclude the possibility that mothers use additional tactics to skew sex ratio in poor years. The promotion of daughters in the hatch order or even skewed brood sex ratio may also occur when food is scarce. Indeed, these strategies may appear more efficient mechanisms of adjusting allocation than sex-biased brood reduction (Komdeur et al. 2002). However, brood reduction allows greater responsiveness to the prevailing availability of prey that, in this region, may change appreciably in the three weeks between the start of laying and the early nestling period. Furthermore, the disparity in egg size between the sexes might be even more pronounced in periods of low food availability, promoting the likelihood of sex-biased brood reduction if such conditions persisted. Clearly, it would be informative to repeat this study in a season when food availability was low or, better still, to perform pre-laying manipulations of maternal condition or food supply.
In conclusion, our data indicate that daughters, rather than sons, are afforded a pre-laying advantage in this extremely size dimorphic species. We suggest that the greater resources allocated to female-bearing eggs may partially compensate females for the competitive disadvantage that they encounter during the nestling period. This argument was also advanced to explain larger male eggs in the reverse sexually size dimorphic American kestrel, the only other appreciably size dimorphic species where egg dimorphism has been reported (Anderson et al. 1997). Beyond this explanation, egg size dimorphism in the songlark may also be an adaptation that facilitates sex-biased survival, conditional on food availability. In periods of abundant food availability, the initial disadvantage to males of hatching lighter may have little lasting influence on fledging quality (or survival), and hence in no way compromise the production of high quality sons. However, when food is scarce, early sibling competition may result in male-biased mortality, skewing brood sex-ratios towards the less costly daughters.
References
Albrecht DJ (2000) Sex ratio manipulation within broods of house wrens, Troglodytes aedon. Anim Behav 59:1227–1234
Amundsen T, Stokland JM (1990) Egg size and parental quality influence nestling growth in the Shag. Auk 10:410–413
Anderson DJ, Reeve J, Martinez Gomez JE, Weathers WW, Hutson S, Cunningham HV, Bird DM (1993) Sexual size dimorphism and food requirements of nestling birds. Can J Zool 71:2541–2545
Anderson DJ, Reeve JD, Bird DM (1997) Sexually dimorphic eggs, nestling growth and sibling competition in American kestrels, Falco sparverius. Funct Ecol 11:331–335
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, N.J.
Ankney CD (1980) Egg weight, survival and growth of Lesser Snow Goose goslings. J Wildl Manage 44:174–182
Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA (2002) Sex-biased hatching order and adaptive population divergence in a passerine bird. Science 295:316–318
Bednarz JC, Hayden TJ (1991) Skewed brood sex ratio and sex-biased hatching sequence in Harris's hawks. Am Nat 137:116–132
Blakers M, Davies SJJF, Reilly PN (1984) The atlas of Australian Birds. Royal Australian Ornithologists Union and Melbourne University Press, Melbourne
Blanco G, Davila JA, Septiem JAL, Rodriguez R, Martinez F (2002) Sex-biased initial eggs favour sons in the slightly size-dimorphic Scops owl (Otus scops). Biol J Linn Soc 76:1–7
Bortolotti GR (1986) Influence of sibling competition on nestling sex ratios of sexually dimorphic birds. Am Nat 127:495–507
Both C, Visser ME, Verboven N (1999) Density-dependent recruitment rates in great tits: the importance of being heavier. Proc R Soc Lond B 266:465–469
Charnov EL (1982) The Theory of Sex Allocation. Princeton University Press, Princeton
Clotfelter ED (1996) Mechanisms of facultative sex ratio variation in zebra finches (Taeniopygia guttata). Auk 113:441-449
Cordero PJ, Griffiths SC, Aparicio JM, Parkin DT (2000) Sexual dimorphism in house sparrow eggs. Behav Ecol Sociobiol 48:353–357
Cordero PJ, Vinuela J, Aparicio JM, Veiga JP (2001) Seasonal variation in sex ratio and sexual egg dimorphism favouring daughters in first clutches of the spotless starling. J Evol Biol 14:829–834
Frank SA (1990) Sex allocation theory for birds and mammals. Annu Rev Ecol Syst. 21:13–55
Griffiths R (1992) Sex-biased mortality in the lesser black-backed gull Larus fuscus during the nestling stage. Ibis 134:237–244
Griffiths R, Double MC, Orr K, Dawson RJG (1998) A DNA test to sex most birds. Mol Ecol 7:1071–1075
Howe HF (1976) Egg size, hatching asynchrony, sex, and brood reduction in the Common Grackle. Ecology 57:1195–1207
Hoyt DF (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77
Johnson AL (1986) Reproduction in the female. In: Sturkie PD (ed) Avian physiology. Springer, Berlin Heidelberg New York, pp 403–431.
Kilner R (1998) Primary and secondary sex ratio manipulation by Zebra Finches. Anim Behav 56:155–164
Komdeur J, Magrath MJL, Krackow S (2002) Pre-ovulation control of hatching sex ratio in the Seychelles warbler. Proc R Soc Lond B 269:1067–1072
Krackow S (1999) Avian sex ratio distortions: the myth of maternal control. In: Adams N, Slotov N (eds) Proc Int Ornithol Congr 22:425–433
Krebs EA, Green DJ, Double MC, Griffiths R (2002) Laying date and laying sequence influences the sex ratio of crimson rosella broods. Behav Ecol Sociobiol 51:447–454
Krijgsveld KL, Dijkstra C, Daan S (1998) Energy requirements for growth in relation to sexual size dimorphism in marsh harrier, Circus aeroginosus, nestlings. Physiol Zool 71:693–702
Lack D (1968) Ecological adaptations for breeding in birds. Methuen, London
Lahiri D, Bye S, Nurnberger J, Hodes M, Crisp M (1992) A non-organic and non-enzymatic extraction method gives higher yields of genomic DNA from whole-blood samples than do nine other methods tested. J Biochem Biophys Methods 25:193–205
Legge S, Heinsohn R, Double, MC, Grifiths R, Cockburn A (2001) Complex sex allocation in the laughing kookaburra. Behav Ecol 12:524–533
Magrath RD (1991) Nestling weight and juvenile survival in the blackbird, Turdus merula. J Anim Ecol 60:335–351
Magrath RD (1992) The effect of egg mass on the growth and survival of Blackbirds: a field experiment. J Zool 227:639–653
Manly BJF (1997) Randomization, bootstrap and Monte Carlo methods in biology. Chapman and Hall, London
Mead PS, Morton ML, Fish BE (1987) Sexual dimorphism in egg size and implications regarding facultative manipulation of sex in mountain white-crowned sparrows. Condor 89:798–803
Monros JS, Belda EJ, Barba E (2002) Post-fledging survival of individual great tits: the effect of hatching date and fledging mass. Oikos 99:481–488
Myers JH (1978) Sex ratio adjustment under food stress: maximization of quality or numbers of offspring? Am Nat 112:381–388
Nager RG, Monaghan P, Griffiths R, Houston DC, Dawson R (1999) Experimental demonstration that offspring sex ratio varies with maternal condition. Proc Natl Acad Sci USA 96:570–573
Nilsson JA, Svensson E (1993) Energy constraints and ultimate decisions during egg-laying in the blue tit. Ecology 74:244–251
Oddie KR (1998) Sex discrimination before birth. Trends Ecol Evol 13:130–131
Oddie KR (2000) Size matters: competition between male and female great tit offspring. J Anim Ecol 69:903–912
Packard GC, Boardman TJ (1987) The misuse of ratios to scale physiological data that vary allometrically with body size. In: Feder EF, Bennett AF, Burggren WW, Huey RB (eds) New directions in ecological physiology. Cambridge University Press, Cambridge, pp 216–239
Parsons J (1970) Relationship between egg size and post-hatching chick mortality in the herring gull (Larus argentatus). Nature 228:1221–1222
Petrie M, Schwabl H, Brande-Lavridsen N, Burke T (2001) Sex differences in avian yolk hormone levels. Nature 412:498
Rasbash J, Browne W, Goldstein H, Yang M, Plewis I, Healy M, Woodhouse G, Draper D, Langford I, Lewis T (2000) A user's guide to MlwiN, 2nd edn. Institute of Education, London
Reid WV, Boersma PD (1990) Parental quality and selection on egg-size in the Magellanic penguin. Evolution 44:1780–1787
Schwabl H (1993) Yolk is a source of maternal testosterone for developing birds. Proc Natl Acad Sci USA 90:11446–11450
Sheldon BC (1998) Recent studies of avian sex ratio. Heredity 80:397–402
Smith HG, Bruun M (1998) The effect of egg size and habitat on starling nestling growth and survival. Oecologia 115:59–63
Smith HG, Ohlsson T, Wettermark K-J (1995) Adaptive significance of egg size in the European starling: experimental tests. Ecology 76:1–7
Stamps JA (1990) When should avian parents differentially provision sons and daughters? Am Nat 135:671–685
Stoleson SH, Beissinger SR (1995) Hatching asynchrony and the onset of incubation in birds, revisited. In: Power DM (ed) Current ornithology, vol 12. Plenum Press, New York, pp 191–270
Styrsky JD, Eckerle KP and Thompson CF (1999) Fitness-related consequences of egg mass innestling house wrens. Proc R Soc Lond B 266:1253–1258
Styrsky JD, Dobbs RC, Thompson CF (2000) Food-supplementation does not override the effect of egg mass on fitness-related traits of nestling house wrens. J Anim Ecol 69:690–702
Teather KL, Weatherhead PJ (1988) Sex-specific energy requirements of great-tailed grackle, Quiscalus mexicanus, nestlings. J Anim Ecol 57:659–668
Torres R, Drummond H (1999) Variably male-biased sex ratio in a marine bird with females larger than males. Oecologia 118:16–22
Trivers RL, Willard DE (1973) Natural selection of parental ability to vary the sex ratio of offspring. Science 179:90–92
Velando A (2002) Experimental manipulation of maternal effort produces differential effects in sons and daughters: implications for adaptive sex ratios in the blue-footed booby. Behav Ecol 13:443–449
Wiebe KL, Bortolotti GR (1992) Facultative sex ratio manipulation in American kestrels. Behav Ecol Sociobiol 30:379–386
Wiebe KL, Bortolotti GR (1995) Food-dependent benefits of hatching asynchrony in American kestrels Falco sparverius. Behav Ecol Sociobiol 36:49–57
Williams TD (1994) Intraspecific variation in egg size and egg composition in birds: effects on offspring fitness. Biol Rev 68:35–59
Acknowledgements
We are very grateful to all those land holders and managers throughout the Riverina who allowed us to work and stay on their properties. Particular thanks go to the Jones, McLean, Clarke and Rutledge families on whose properties most of this work was conducted. We are also grateful to Mathew Berg, Wouter van Dongen, Alison Kemp, Emile van Lieshout, Marjan van Meerloo, Cordellia Moore, Arnoud van Petersen, Justin Welbergen and Iain Woxvold for their assistance in the field, and Martijn van de Pol for his help with the re-sampling analysis. We also thank the staff at the NSW National Parks and Wildlife Service (Griffith) and the Rural Lands and Protection board (Hillston and Hay) for advice and assistance. Useful comments on the manuscript were made by Mark Elgar, Theresa Jones, Mathew Symonds and two anonymous reviewers. This research was conducted with the approval of the University of Melbourne Animal Experimentation Ethics Committee, and financially supported by a grant JK from the Australian Research Council (A19802459). LB received financial support from the Marco Polo Fund!
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Dickinson
Rights and permissions
About this article
Cite this article
Magrath, M.J.L., Brouwer, L. & Komdeur, J. Egg size and laying order in relation to offspring sex in the extreme sexually size dimorphic brown songlark, Cinclorhamphus cruralis . Behav Ecol Sociobiol 54, 240–248 (2003). https://doi.org/10.1007/s00265-003-0627-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0627-y