Abstract
Adult male baboons (Papio cynocephalus) give loud two-syllable 'wahoo' calls during dawn choruses, interactions between groups, when chasing females, and in aggressive interactions with other males. These 'contest' wahoos are acoustically different from 'alarm' wahoos given to predators. In a study of free-ranging baboons in the Okavango Delta, Botswana, we found no significant correlations between the acoustic features of wahoos and adult male size; however, acoustic features were correlated with male dominance rank, age, and calling bout length. Here we show that other measures of calling behavior also appear to function as honest indicators of stamina and competitive ability. High-ranking males were more likely than middle- or low-ranking males to participate in wahoo bouts. They called at significantly higher rates, and their bouts were longer and contained more calls. All males were significantly more likely to participate in wahoo bouts with another male if their opponent's rank was similar to their own. Bouts involving males of similar ranks were longer, contained more wahoos, and involved calling at higher rates, than other bouts. In contests between males of similar ranks, the subordinate and dominant were equally likely to end the bout, whereas in contests involving males of disparate ranks, subordinates were significantly more likely to end the bout. Bouts involving males of similar rank were significantly more likely than others to escalate and result in physical fighting.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intrasexual competition can result in injuries that are costly to both winner and loser (e.g., LeBoeuf 1974). Models developed using evolutionary game theory suggest that, regardless of their competitive ability, individuals should display before fighting and attempt to resolve conflicts at the lowest possible cost (Maynard Smith 1982; reviewed in Bradbury and Vehrencamp 1998). When signaler and receiver have different interests, however, there will always be an incentive for signalers to lie, exaggerate, or mislead their opponents in order to achieve a beneficial outcome (Hasson 1997; Vehrencamp 2000). Despite the benefits of such 'deceptive' signaling, most displays are reliable indicators of an individual's competitive ability. Reliable signals are maintained whenever there is some type of cost or constraint on signalers that makes cheating a less optimal strategy than revealing the truth (e.g., Maynard Smith and Harper 1995). The costs that constrain signaler behavior take a variety of forms and may, for example, be physiological (signal acoustics are constrained by body size or condition), ecological (signals are limited by vulnerability to predation), or social (signals are limited by vulnerability to receiver attack; see Vehrencamp 2000 for a recent review).
Game theoretical models also predict that contests involving individuals of widely disparate competitive abilities will end relatively quickly, with the weaker individual retreating, whereas contests involving individuals of similar competitive abilities will escalate and be more intense, longer, and more likely to result in physical fighting (e.g., Enquist and Leimar 1983). In the latter case, models assume that contestants are either unwilling to retreat or unable to detect a difference between their own and their opponent's competitive ability (Enquist and Leimar 1990; Bradbury and Vehrencamp 1998).
In amphibians, birds, and some mammals, males produce loud repetitive calls that apparently function as displays of size, condition, or fighting ability (e.g., amphibians: Davies and Halliday 1978; Bee and Gerhardt 2001; red deer: Clutton-Brock and Albon 1979; Reby and McComb 2003; elephants: Poole 1989, 1999; reviewed in Andersson 1994). In toads, frogs, and birds, for example, the fundamental frequency (F0) of calls is significantly correlated with body size (Davies and Halliday 1978; Ryan 1985; Bee et al. 1999; Ryan and Brenowitz 1985). Similarly, among adult red deer, the resonant, or formant, frequencies of male roars are significantly correlated with weight and age (Reby and McComb 2003). In these species, calls are reliable indicators of size and fighting ability because the acoustic features of calls are constrained by body size and large size typically confers an advantage in fights (Andersson 1994). Playback studies of frogs and toads support the view that listeners decide whether to escalate or retreat depending upon the spectral properties of an opponent's calls (e.g., Arak 1983; Given 1987; Wagner 1989). Further supporting this hypothesis, in both toads (Davies and Halliday 1978) and red deer (Clutton-Brock and Albon 1979; Clutton-Brock et al. 1979), contests are longer in duration and more likely to lead to fighting when males are similar in size and produce calls with similar acoustic properties.
Loud calls are also produced by males in many non-human primates. Loud calling during inter-group encounters may attract, repel, or induce counter-calling by the members of a rival group (chimpanzees: Clark 1993; Mitani and Nishida 1993; Mitani and Brandt 1994; Wilson et al. 2001; howler monkeys: Sekulic 1982; Chiarello 1995; gibbons: Mitani 1988; Cowlishaw 1992, 1996; langurs: Steenbeek et al. 1999; mangabeys: Waser 1977; blue monkeys: Butynski et al. 1992; members of the Cercopithecus genus: Gautier and Gautier-Hion 1977). In some species living in multi-male groups, males give loud calls in the context of intra-group aggression. Although such calls appear to function as displays of competitive ability, to date they have received little attention.
Savannah baboons (Papio cynocephalus) live in large groups that may include a dozen or more adult males who have immigrated from neighboring groups and who are unrelated to each other. Male baboon dominance hierarchies are typically linear (Hausfater 1975; Packer 1979b; Smith 1986; Bulger 1993), though rank reversals are common. Competition for dominance appears to be driven by access to receptive females. Both DNA evidence (Altmann et al. 1996) and field observations (Packer 1979b; Smith 1986; Altmann et al. 1988; Bulger 1993; Drews 1996; Weingrill et al. 2000) indicate a high positive correlation between high rank and mating success. Similar data are available from several other primates (Silk 1987; Cowlishaw and Dunbar 1991). Preliminary evidence, however, suggests no clear relationship between body size and dominance rank among adult male baboons (see below), probably because dominance ranks are more transient and impermanent than body size.
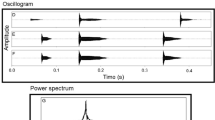
Male baboons in the Okavango Delta of Botswana produce loud, two-syllable 'wahoo' calls (Fig. 1) in a variety of contexts: (1) as alarm calls to leopards or lions ('alarm' wahoos); (2) when a male is separated from the group and appears to be lost ('contact' wahoos); (3) in pre-dawn 'choruses'; (4) during inter-group encounters; and (5) during aggressive interactions within the group. Wahoos given during the last three contexts are collectively termed 'contest' wahoos. Contest wahoos are often accompanied by aggressive displays that include chasing other males, herding females, and leaping and running through trees. Despite sounding alike and overlapping in some acoustic features, by other measures alarm and contest wahoos show significant acoustic differences (Fischer et al. 2002). Contact wahoos are acoustically similar to contest wahoos, but given at a much lower rate. In playback experiments, alarm and contest wahoos elicit different responses from listeners (Kitchen et al. 2003).
Although baboon contest wahoos have been widely observed and seem to function as vocal displays of a male's stamina and competitive ability (Hall and DeVore 1965; Saayman 1971; Buskirk et al. 1974; Hamilton et al. 1975; Cheney and Seyfarth 1977; Packer 1979b; Byrne 1981; Ransom 1981; Byrne et al. 1987), this hypothesis has never been tested. Contest wahoos and the behavior that accompanies them appear to be energetically costly. Wahoos are very loud calls, consistently greater than 90 dB at 5 m, and can be heard from at least a kilometer away. They are often accompanied by aggressive chases and arboreal displays that can last for over an hour. Toward the end of a bout of calling and chasing, males appear to become exhausted and their wahoos change in several ways; fundamental frequency declines and 'hoo' duration become shorter (J. Fischer, D. Kitchen, R. Seyfarth, D. Cheney, unpublished data). Wahoos also alter as a male ages: F0 declines, 'hoo' duration becomes shorter, and formant dispersion decreases (J. Fischer, D. Kitchen, R. Seyfarth, D. Cheney, unpublished data). Although there is no consistent relation between the acoustic features of contest wahoos and adult male size, both fundamental frequency and 'hoo' duration are significantly correlated with dominance rank, with high-ranking males having higher F0s and longer 'hoo' syllables than low-ranking males (J. Fischer, D. Kitchen, R. Seyfarth, D. Cheney, unpublished data).
Contest wahoos may therefore function as 'index' or 'quality handicap' signals (Vehrencamp 2000) that are physiologically constrained by stamina, health, or condition, and that convey information about intrinsic sender attributes. If this were true, we would predict that males would also vary their frequency of participation in displays, calling rate, and calling duration to advertise their stamina and willingness to escalate. Alternatively, wahoos could be 'conventional' signals (Vehrencamp 2000), not limited by energetic costs, but constrained instead by some other factor, such as retaliation from recipients.
Whatever their costs, contest wahoos and the displays of which they form a part are clearly less risky than fighting. Male baboons frequently injure each other in fights, and such injuries may result not only in the loss of dominance rank but also in permanent disabilities. In the study discussed below, for example, injuries sustained by males included the loss of an eye, the loss of the lower lip, deep gashes on the face, neck, ears, thighs, and arms, and sprained wrists. One male disappeared a few weeks after a canine bite to the shoulder became severely infected (see also Drews 1996).
Here we use data from free-ranging baboons to test whether a male's dominance rank, the dominance rank of his opponent, or both of these factors influence the intensity of calling and the frequency with which males participate in wahoo bouts. On the assumption that wahoos function as index or quality handicap signals, we examine whether high-ranking males are the most frequent participants in wahoo bouts, wahoo at the highest rates, and participate most often when their opponents are also high-ranking. On the assumption that rivals use wahoos to assess their opponent's competitive ability, we examine whether bouts involving males of similar ranks (and, presumably, similar competitive abilities) last longer than those involving males of disparate ranks, and are more likely to escalate into physical fighting.
Methods
Study site and subjects
Research was conducted in the Moremi Game Reserve, located in the Okavango Delta, Botswana. Grasslands in the delta flood annually, leaving elevated 'islands' edged with forest exposed. Islands can be less than one to hundreds of hectares in size (Hamilton et al. 1976; Ross 1987; Ellery et al. 1993). During floods, baboons continue to ford the submerged plains and move between islands throughout approximately 5-km2 range (Bulger and Hamilton 1987).
Like most Old World monkeys, female baboons remain in their natal groups throughout their lives, while males usually emigrate to neighboring groups at around 9 years of age. Males form linear dominance hierarchies that are stable over short periods of time (see below). Natal males often rise in rank shortly before emigrating, and some natal males occasionally remain in their natal groups to breed (Bulger and Hamilton 1988; Hamilton and Bulger 1990; personal observation). Subjects for this study included all immigrant and natal males 8.5 years of age or older from two free-ranging groups.
The main study group, C, has been observed since 1977. All individuals are identifiable and the matrilineal relatedness of all natal animals is known. Subjects are fully habituated to observers on foot. During this study, C group contained 78–88 animals, including 17 different adult males (3 additional males who never gave wahoo vocalizations were excluded from analysis). The mean number of adult males on any given day was 12. The number fluctuated due to immigrations, emigrations, maturation and death. Fourteen males had been resident in the group for at least a year before the study began. The six males that immigrated into the group during the course of the study were observed for at least a year.
A second group, Q, occupies a home range adjacent to that of C. This group has been observed intermittently since 1992 and all individuals are identifiable. During the study, Q group contained 20–31 individuals including 2–10 adult males. Because seven of these males had been born in C, most were fully habituated. Other males were approachable to within a few meters but females were only approachable to within 6–10 m.
Data collection
During the 23-month study (June 1999 to May 2001), we typically located a group between 0500 and 0830 hours and then followed it for 5–7 h. On most days, observers (usually three and never fewer than two) distributed themselves throughout the group in an attempt to maximize coverage of adult males. They maintained contact via two-way radios. Each observer carried a Psion LZ64 computer for recording data on social behavior, and a digital audio tape recorder (Sony PCM-M1) and directional microphone (Sennheiser ME66) for tape-recording vocalizations. The Psion computer included software written by J.B. Silk for collection of data on social behavior timed to the nearest second. The tape recorder was programmed to note the time of each recording of either a vocalization or observer commentary.
One method of data collection involved 10-min focal animal samples (Altmann 1974) conducted on all male subjects. During focal sampling, an observer recorded continuous data on all interactions involving the subject and other group members, including approaches, supplants, threats, chases, physical fights, and vocalizations. The two observers who conducted focal sampling selected subjects from a randomized list and, when possible, did not sample any individual twice until all males had been sampled once. All male subjects in C group were sampled for approximately 3 h per month, for a total of 3,560 focal animal samples during the study. All three observers also collected ad libitum data (Altmann 1974) on all supplants, threats, chases, and fights involving adult males.
Females in Q group were not fully habituated, so we did not conduct focal animal sampling on the males in this group. However, throughout the study Q was small, did not disperse widely, and normally occupied open, short grass habitat. Thus both (or all three) observers were able to see most members of the group concurrently, increasing the probability that ad libitum sampling captured a large proportion of all social interactions involving adults.
Data on the time and occurrence of each wahoo were recorded on either the Psion computer or the tape recorder with spoken commentary identifying the male(s) involved. We defined a bout as a continuous period of wahooing by one male that contained no silent periods longer than five minutes. From these data we calculated the probability that each male would participate (by giving at least one call) in a wahoo contest involving one or more males, the total number of wahoos produced, the total duration and rate of wahooing by each participant, and the identity of the male who vocalized last in any two-male contest.
Because we were not always close to a male when he began to call, we were in some cases unable to record a portion of his wahoo bout. Occasionally we also temporarily lost a male as he ran through tall grass or dense bush, or we were unable reliably to identify a male's calling due to the simultaneous calling of several males. When this happened, we used only the total number of positively identified wahoos to estimate a male's calling rate.
At least two wahoo bouts were recorded from each of 17 males in C group, for a total of 401 bouts. The number of calls per bout ranged from 1 to 359 (mean±SE=23.6±1.9).
The context of male calling behavior
Contest wahoos were produced in three distinct contexts: prior to or at first light, before the group had left its sleeping site ('dawn chorus'); at any time when the study group encountered another group or an extra-group male ('inter-group encounter'); and during aggressive interactions with other resident males ('intra-group contest'). Because we found no consistent differences across individuals in patterns of wahoo production (Kruskal-Wallis one-way analysis of variance, number of wahoos per bout: H 2=0.57, n=332, P=0.75; bout duration: H 2=0.29, n=202, P=0.86; rate of wahoos per minute: H 2=4.55, n=228, P=0.11), the analysis pools data from these three contexts.
Dominance rank
Dominance ranks were established using data collected by both focal animal and ad libitum methods. Dyadic dominance relations were defined according to the direction of approach-retreat interactions and submissive behaviors. Submissive behaviors included the 'fear grimace' (lips pulled back exposing clenched teeth), 'fear bark' (a cough-like vocalization) and 'lean-away' (animal glances at and then turns head and extends body away from approaching dominant).
In most cases, dominance rank orders were linear and unidirectional, at least over the short term. However, in C group a few triangular rank relations (F>G, G>H, and H>F) were stable over several months. Because in these triads each male outranked one individual and was outranked by another, for purposes of analysis all three males were assigned the same middle rank. Rank reversals were common in C group, occurring on average 1.5 times per month.
In every bout a dominance rank was assigned to each participant based on his place in the hierarchy at the time. The highest-ranking male was assigned rank 1, the second-ranking male rank 2, and so on. In tests of the relation between dominance rank and wahooing behavior, results were unaffected when these ranks were replaced with a variable representing the percent of males dominated, which controlled for changes in the total number of males residing in the group. For each bout, we calculated the number of rank positions separating males in the dominance hierarchy (e.g., adjacently-ranked males were separated by 1 rank position, the first- and third-ranked males were separated by 2 rank positions, etc).
In C group, the alpha, or first, rank was held by two different individuals during the 23-month study. Overall, we recorded wahoos from 17 different adult males occupying 12 different rank positions. An average of 3.2 males occupied each of the 12 rank positions (range: 2–6), and each male occupied an average of 3.4 different ranks (range: 2–7).
In Q group, the alpha position was held by two different individuals over 23 months. An average of 1.5 males occupied each of seven rank positions, and each male occupied an average of two different ranks. Five different adult males occupying seven different rank positions were observed wahooing.
Age and size
The ages of natal adult males were known. Although we could use residence length to estimate the ages of non-natal adult males who had lived in the group for several years, it was more difficult to estimate the ages of recent immigrants because male baboons often transfer more than once during their lives. We therefore estimated the ages of the 13 immigrant males through tooth wear. Using photographs obtained while adult males with fully erupted canines were yawning, we scored teeth on a 1–5 scale, with 5 = white teeth with sharp, unchipped points; 4 = white teeth or slight yellowing on one or two teeth, some chipping or wear on one tooth; 3 = some discoloration on several teeth, breaks, chipping, or tooth wear very evident; 2 = extensive discoloration, one or both canines missing or broken; 1 = extensive discoloration, one or both canines missing or worn to the level of premolars and substantial damage to other teeth.
Shoulder height was used as one estimate of relative body size. To obtain this measure, one observer followed an adult male until he stood upright next to a fairly straight and rigid piece of vegetation on level ground; for example, a small tree or shrub. The observer then crouched approximately 2 m away and waited for the male's shoulder to brush an overhanging object (a branch, for example) without otherwise disturbing the vegetation. Then, after the male had left the area, the observer measured the distance from the overhanging vegetation to the ground. To make our measurements as independent as possible, we conducted two bouts of sampling that were separated by 18 months. We obtained height measurements from 13 of the 17 males. Most males were sampled at least 6 times on at least two different days; one male was sampled 3 times on one day. To determine the reliability of our measurements, we compared the variation within subjects to the variation between subjects using an inter-class correlation ([icc=(F-1)/(F-1+n)], where n is the number of samples per individual and F is obtained using a one-way ANOVA with subjects as treatments; Winer et al. 1991). Measures of male shoulder height were repeatable and reliable; the intra-class correlation was high regardless of whether we used all 13 males and three randomly chosen samples per male (F=17.65, icc=0.847, n =3 replicates on 13 males, P<0.01) or 12 males and six randomly chosen samples per male (F=27.32, icc=0.814, n =6 replicates on 12 males, P<0.01).
As a second estimate of male body size, we obtained data on the weights of nine adult males using a large grocery scale that was buried so that the weighing platform rested at ground level on a path frequently used by the baboons. When a male stood on the platform, his weight was recorded by one observer who could not see the platform. Another observer noted the identity of the male being weighed. Five of nine males were weighed twice; four were weighed once.
Statistical analyses
As in any study of individuals who live together for long periods of time, who change dominance ranks periodically, and who interact repeatedly in various dyadic, triadic, or larger combinations, the data presented here are not perfectly independent. Because one of our goals was to examine the relation between dominance rank and calling behavior, in many of our analyses we used rank as a predictor variable and pooled data from all individuals who occupied each rank. To control for non-independence and possible pseudo-replication (Hurlbert 1984), we conducted four additional analyses. First, we calculated the mean rank occupied by each male during the study, weighted for the time spent in each rank, and compared this rank with several measures of calling. Note that, although this method ensures that all individuals contribute equally to the analysis, it also obfuscates data by treating a middle-ranking male who occupied roughly the same ranks throughout the study as equivalent to a male who occupied both high and low ranks. Second, to test hypotheses about the relation between male rank and behavior we present data on one individual (TH) who occupied seven different rank positions during the study. TH was the only individual whose rank changed sufficiently often to allow this analysis. Third, to test hypotheses about the relation between a male's behavior and his opponent's rank we present data on one individual (PO) whose rank remained fairly constant but whose opponents occupied many different rank positions during the study (7 different rank position differences, ranging from adjacently-ranked opponents to those separated by 11 places in the hierarchy). PO was the only individual whose rank remained sufficiently stable to allow this analysis. Finally, wherever sample size permits we include data from Q group. We used two-tailed tests with α set at 0.05.
Results
Age, size, and dominance rank
Although the exact ages of most adult males were not known, data obtained from tooth wear suggested a negative but non-significant correlation between age and rank (Table 1; Spearman rank correlation, r s=-0.234, n =17, P>0.10), with older males typically occupying lower-ranks. While the highest dominance ranks were occupied by young adult males, not all young adults were high-ranking. As a result, the correlation between age and rank was not strong.
When a male's rank by shoulder height was compared to his average dominance rank, no significant relation was found (r s=0.017, n =13, P>0.10). We also found no relation between rank and male weight (r s=-0.167, n =9, P>0.10). Neither male age nor male size, therefore, accurately predicted dominance rank.
Mean male rank and wahoo production
As a first test of the relation between male dominance rank and calling behavior, we compared each male's mean dominance rank during the study with his average number of wahoos per bout, bout duration, and wahoo rate. Only males who participated in at least four wahoo bouts were included in this analysis, reducing the sample to 15 males. There was a significant correlation between male rank and all three measures (Fig. 2). Higher-ranking males gave more wahoos per bout (r s=-0.545, n =15, P<0.05), called for longer bouts (r s=-0.450, n =15, P<0.05), and gave wahoos at higher rates than lower-ranking males (r s=-0.461, n =15, P<0.05).
The relationship between rank and participation in wahoo bouts
Although most bouts in C group involved one or two males, bouts involving up to six males were also observed. Across all wahoo bouts involving different numbers of males, the likelihood of a male's participation decreased with decreasing dominance rank (Fig. 3; r s=-0.923, n =12 ranks, P<0.001). To compare the effects of a male's rank and the number of individuals involved on the likelihood of his participation in wahoo bouts, we used Friedman's nonparametric method for randomized blocks, with male ranks as treatments and groups of one, two, three, and four or more males as blocks (Sokal and Rohlf 1995, pp. 440ff). The effect of male rank was significant (χ 2=36.93, df=11, P<0.001), but there was no significant effect of the number of males involved (χ 2=0.78, df=4, P=0.94). High-ranking males, therefore, were more likely to participate in wahoo bouts regardless of the number of males involved.
Data in Fig. 3 have been pooled from observations involving several different males in each dominance rank (see Methods). To test whether the relation between rank and participation in wahoo bouts remained the same when we controlled for individual differences, we examined the participation of one male (TH) who occupied seven different ranks during the study period. TH was involved in 78 wahoo bouts, or 30.6%, of all bouts observed in C group. The probability of TH's participation in wahoo bouts was significantly correlated with his rank (Fig. 4; r s=-0.964, n =7 rank positions occupied, P<0.005). Results were similar when we separately examined TH's participation in bouts involving one, two, three, and four or more males (Friedman's method for randomized blocks, n =7 rank positions for all analyses, n=26 single-male bouts, 28 two-male bouts, 14 three-male bouts, and 10 bouts involving four or more males). The effect of TH's rank on his participation was significant (χ 2=14.52, df=6, P=0.024), but the number of males involved had no effect on behavior (χ 2=1.34, df=4, P=0.85). TH was therefore more likely to participate in wahoo bouts when he was high-ranking than when he was middle- or low-ranking, regardless of the number of males involved.
Although most bouts in Q group involved one or two males, bouts of up to five males were also observed. As with C group, the likelihood of a male's participation decreased with decreasing dominance rank (r s=-0.631, n =7 rank positions occupied by six males in 41 bouts, P<0.10). When data were tested for the separate effects of male rank and the number of males involved in a bout, the effect of male rank was positive but non-significant (χ 2=9.80, df=6, P=0.13). There was no significant effect of the number of males involved (χ 2=0.64, df=2, P=0.73).
Effect of opponent's rank in multiple-male bouts
Participation in wahoo bouts
In C group, we identified the ranks of both contestants in 60 of 61 dyadic bouts. These bouts involved 15 different males who occupied ranks 1–12. We first tested whether overall participation decreased as the difference in rank between contestants increased. Results revealed a sharp decline in participation as rank differences increased.
To test whether closely-ranked males were more likely than disparately-ranked males to participate in wahoo bouts against each other, we compared the frequency with which males of different ranks competed against each other with the frequency that would have been expected had males been equally likely to join in a dyadic bout with males of any ranks (Fig. 5). To calculate expected probabilities, we assumed that all individuals were equally likely to interact with all possible opponents and then calculated how many interactions would have involved adjacently-ranked individuals, how many would have involved individuals separated by two rank positions, and so on. We pooled data from all bouts involving individuals separated by five or more rank positions because of the small number of interactions involving males of greatly disparate ranks. Overall, the observed distributions were significantly different from expected (Fig. 5; χ 2 2=21.07, n=60, P<0.001). Post-hoc paired comparisons revealed that bouts involving adjacently-ranked males and males separated by two rank positions occurred significantly more often than expected (χ 1 2=11.64, n =34, P<0.001). Bouts involving males separated by three or four rank positions occurred no more frequently than expected (χ 1 2=0.14, n =14, P >0.10), and bouts involving males separated by five or more rank positions occurred significantly less often than expected (χ 1 2=7.11, n =12, P<0.01).
The frequency of dyadic wahoo bouts involving males separated by different numbers of positions in the dominance rank hierarchy. X-axis shows the rank difference between opponents, with 1 = adjacently-ranked males. Y-axis shows the observed proportion (shaded histogram) of 61 bouts involving males separated by different numbers of rank positions, and the proportion that would have been expected (dark histogram) if males had interacted at random (see text)
To test whether this result also applied to bouts involving three or more males, we examined the identity of 136 participants in 40 multi-male interactions and asked whether each male was more likely than expected by chance to participate if the bout involved at least one closely-matched opponent. Closely-matched opponents were defined as adjacently-ranked males or males separated by two rank positions. To calculate expected probabilities, we treated three-, four-, and five-male bouts as if they were composed of many dyads simultaneously, and calculated expected probabilities as described above, on the null assumption that all males were equally likely to participate. Hence the probability that any male would interact with a closely matched opponent in a bout involving three or more males was the sum of the probabilities that the male would have an adjacently-ranked individual or an individual separated by two rank positions among his three or more opponents (see Fig. 5). Results indicated that 113 of 136 participants (or 83.1%) in bouts of three or more males interacted with at least one such closely-ranked opponent significantly more than expected by chance (expected = 43.28, χ 1 2=112.34, P<0.001).
As in earlier analyses, these results have been pooled from observations involving several different males at each dominance rank. To test whether the relation between opponent's rank and participation in wahoo bouts remained the same when we controlled for individual differences, we examined the participation of one male (TH) who was involved in the most wahoo bouts and another (PO) who was involved in bouts with males of the most different ranks. For both males, involvement in dyadic interactions decreased as the rank disparity with their opponents increased. Using the methods described above, we determined the possible opponents at each rank difference when PO occupied ranks 1 and 2 and when TH occupied ranks 1–7. Because of the small number of interactions involved, we pooled the closely-matched categories (1 or 2 rank differences) and the disparately-matched categories (3 or more rank differences) for analysis. In dyadic bouts, both individuals were more likely than expected to participate with closely-ranked opponents (TH: observed = 20, expected = 9.09, χ 2=38.36, n =28, P<0.001; PO: observed = 16, expected = 6.14, χ 2=46.69, n =27, P<0.001). In bouts involving three or more males, both individuals were more likely to participate in interactions involving at least one closely-ranked opponent (TH: observed = 22, expected = 7.79, χ 2=19.39, n=24, P<0.001; PO: observed = 20, expected = 5.68, χ 2=18.10, n =25, P<0.001).
Taken together, these results suggest that, regardless of their absolute rate of participation in wahoo bouts, males selectively sought out similarly-ranked opponents. It remains possible, however, that high rates of interaction between males of similar rank occurred simply as a consequence of frequent participation by high-ranking males. Given that high-ranking males were more likely than others to participate in wahoo bouts, it naturally follows that a disproportionate number of dyadic interactions involved closely-ranked, high-ranking individuals.
To test between these two hypotheses, we used data from Fig. 3 to calculate the probability that a male occupying a specific rank would participate in a dyadic wahoo bout. For example, out of 61 dyadic bouts the probability that the alpha male would be involved was 0.227 and the probability that the third-ranking male would be involved was 0.168. Based on their overall probability of participation, therefore, the probability that any dyadic interaction would involve the first- and third-ranking males was 0.227×0.168=0.038. For each dyad we then compared the observed frequency with the frequency that would have been expected based on the two males' overall rates of interaction. Males in nine of 12 rank positions interacted more often than expected with individuals of adjacent rank (Wilcoxon signed ranks test, T=6, n =12, P<0.005). Males in 10 of 12 rank positions interacted more often than expected with individuals who were either adjacently ranked or two steps away in the hierarchy (T=3, n =12, P<0.005). We therefore conclude that males did not participate in wahoo bouts with opponents whose ranks were similar to their own simply as a consequence of high-ranking individuals interacting at high rates with one another. Regardless of their absolute rates of participation, individuals of all ranks interacted at higher rates than expected with similarly-ranked opponents.
Wahoo production
We next examined whether the rank difference between opponents predicted calling behavior. In this analysis we first classified each dyadic bout according to whether opponents were closely-ranked (separated by one or two rank positions), intermediately-ranked (separated by three or four rank positions), or disparately-ranked (separated by five or more rank positions). The number of wahoos produced per male decreased significantly as the disparity between opponents increased (mean±SE: closely-ranked = 26.0±5.3, intermediately-ranked = 15.5±4.0, disparately-ranked = 3.8±0.7; Kruskal-Wallis H 2=10.64, n =92, P=0.005). Bout length also decreased as the rank disparity between opponents increased (mean ± SE: closely-ranked = 116.7±14.2 s, intermediately-ranked = 109.9±22.4 s, disparately-ranked=71.3 ± 30.1 s), but the difference was not significant (H 2=2.04, n =57, P=0.36) and there was no apparent effect of rank difference on wahoo rate (H 2=3.10, n=63, P=0.21).
Second, for bouts involving three or more males we assigned each male a score based on whether or not at least one other opponent was closely-ranked (separated by one or two rank positions) or disparately-ranked (separated by three or more rank positions). No significant differences were found (number of wahoos: Mann-Whitney U 1=957.0, n =100, P=0.28; bout length: U 1=448.0, n =77, P=0.47; rate: U 1=593.5, n =86, P=0.65).
As a final test, we analyzed data from male PO, who was involved in bouts with males that covered the widest range of rank position differences in C group. When interacting in dyadic interactions with closely-ranked opponents, PO called for significantly longer durations, gave significantly more wahoos, and called at a faster rate than when interacting with more distantly-ranked opponents (Table 2). In interactions involving three or more males, PO had a significantly faster wahoo rate when he faced at least one closely-ranked opponent than when he faced opponents of more disparate ranks. PO's bout length and number of wahoos were also greater when at least one opponent was closely-ranked, but these differences were not statistically significant (Table 2).
Who ends a wahoo bout?
Because males occasionally raced out of sight while chasing each other as a wahoo bout ended, we were only able to identify the last male to wahoo in 38 of 61 dyadic bouts. If male X in a dyadic encounter was the last to wahoo and male Y did not reply, we assumed that Y had ended the interaction. When males were closely-ranked or separated by three or four rank positions, the subordinate and dominant were equally likely to end the interaction (closely-ranked: χ 1 2=0.18, n =22, P>0.10; other: χ 1 2=0.14, n =7, P>0.10). When males were separated by five or more rank positions, the subordinate was significantly more likely to end the interaction (χ 1 2=5.44, n =9, P<0.05).
Consequences of wahoo bouts: the distribution of physical fights
The preceding results indicate that wahoo bouts involving males of similar dominance rank were more frequent, lasted longer, and involved higher rates of calling than wahoo bouts involving males of disparate ranks. If opponents use these bouts to assess relative fighting ability, contests should be more likely to escalate to physical fights when males are closely matched and contestants are either unwilling to retreat or unable to detect a difference between their own and their opponent's competitive ability. Consistent with this view, thirty-five (68.6%) of 51 observed wahoo bouts that escalated into physical fighting involved males separated by one or two rank positions, significantly more than expected by chance (χ 2 2=31.85, P<0.001).
Discussion
A variety of evidence supports the hypothesis that, in both their acoustic features and their manner of delivery, male baboon wahoos are physiologically costly to produce and therefore serve as honest indicators of an individual's competitive ability. High-ranking males, who are presumably in the best condition and have the best competitive ability, were more likely than middle- or low-ranking males to participate in wahoo contests. They gave significantly more wahoos, called for significantly longer bouts, and called at significantly higher rates than did low-ranking males. Two acoustic features of wahoos, F0 and 'hoo' duration were also correlated with dominance rank, age, and stamina, further suggesting that wahoos are energetically costly to produce. Male baboon wahoos therefore appear to function as quality handicap signals (Vehrencamp 2000) whose reliability is maintained because of the stamina and strength required for their production.
An alternative hypothesis argues that wahoos are 'conventional' signals, not limited by energetic costs but constrained instead by, for example, high rates of receiver retaliation (e.g., Enquist et al. 1985). This hypothesis predicts that high-ranking males call at higher rates than low-ranking males because they are not punished for doing so, whereas a lower-ranking male would be. We found no support for this view. The probability of an escalated fight depended not on the rank of any one male but on the magnitude of the rank difference between opponents.
In many species, the vocalizations used by males in aggressive displays vary systematically with body size, and body size accurately predicts a male's fighting success (e.g., Bee et al. 1999; see Archer 1988 for review). As a result, listeners can, and apparently do, assess the competitive ability of their opponent from the acoustic features of his calls alone, without any additional supporting cues (e.g., Robertson 1986; but see Bee 2002). In a previous study of baboons, Packer (1979a) found a significant positive correlation between size and dominance rank. However, we found no relationship between size and dominance rank in our study. Given frequent changes in male dominance rank, the lack of a strong relation between age, size, competitive ability, and wahoo acoustics is not surprising.
If a male baboon's dominance rank (and hence his competitive ability) changes often, and if rank changes are unrelated to body size, then a male cannot advertise his competitive ability simply by giving calls that reflect his size. He can, however, advertise his competitive ability by giving calls whose acoustic features or mode of delivery reflect his rank.
The dominance rank of a male's opponent had a significant effect on his participation in wahoo bouts. Regardless of their status, all males participated in bouts at higher rates when their opponent's rank was similar to their own. Like red deer that roar at higher rates when they hear an opponent roaring at higher rates (Clutton-Brock and Albon 1979), male baboons increased their participation in wahoo bouts and called at higher rates as the dominance ranks of their opponents became more like their own.
Bouts involving males of similar dominance ranks differed quantitatively and qualitatively from those involving males of disparate ranks. Bouts between males of similar ranks occurred more often, included more wahoos, were longer, and involved higher rates of calling than other bouts. In bouts involving males of similar rank, the subordinate and dominant were equally likely to end the bout, whereas in bouts involving males of disparate ranks the subordinate was significantly more likely to end the bout. Perhaps most important, physical fighting was more likely to occur between males of similar rank, and less likely to occur between males of disparate rank, than would have been expected by chance. Results support the prediction that displays involving individuals of very different competitive abilities will end relatively quickly with the weaker individual retreating, whereas displays involving individuals of similar competitive abilities, when contest outcome is least clear, will be more intense and more likely to escalate to physical challenges (e.g., Enquist and Leimar 1983; Gerhardt et al. 2000; Vehrencamp 2000).
As noted earlier, this study focused on individuals who interacted repeatedly with one another over relatively long periods of time. By their very nature, therefore, many of our observations could not achieve perfect statistical independence. Nonetheless, two points seem important. First, results remained consistent regardless of the number of individuals involved in a bout. Thus, high-ranking males were the most active participants in single-male displays, and this result was replicated in displays involving two, three, and four individuals (Fig. 3). Second, the results that emerged most consistently from analyses of pooled data also held when we tested the behavior of one male (TH) who occupied many different ranks and another male (PO) who interacted with many different opponents. These observations suggest that our overall results are not an artifact of combining data from many different individuals.
Much of the theory developed to explain competitive displays in animals has been developed from, or tested on, species in which individuals encounter each other rarely. Theory assumes that displays are adaptive because they allow both participants to assess each other's fighting abilities without incurring a significant cost, and because they permit individuals to assess the motivations of a competitor who is largely unfamiliar. In contrast, baboon wahoo bouts typically involve individuals who live in the same social group and who have a long history of prior interactions. Results presented here demonstrate that, under these very different conditions, predictions derived from game theory are nonetheless supported.
References
Altmann J (1974) Observational study of behaviour: sampling methods. Behaviour 49:227–265
Altmann J, Hausfater G, Altmann S (1988) Determinants of reproductive success in savannah baboons. In Clutton-Brock T (ed) Reproductive success. University of Chicago Press, Chicago, pp 403–418
Altmann J, Alberts SC, Haines SA, Dubach J, Muruthi P, Coote T, Geffen E, Cheesman DJ, Mututua RS, Saiyalel SN, Wayne RK, Lacy RC, Bruford MW (1996) Social structure predicts genetic structure in a wild primate group. Proc Natl Acad Sci USA 93:2597–5801
Andersson M (1994) Sexual selection. Princeton University Press, Princeton, N.J.
Arak A (1983) Sexual selection by male-male competition in natterjack toad choruses. Nature 306:261–262
Archer J (1988) The behavioural biology of aggression. Cambridge University Press, Cambridge
Bee MA (2002) Territorial male bullfrogs (Rana catesbeiana) do not assess fighting ability based on size-related variation in acoustic signals. Behav Ecol 13:109–124
Bee MA, Gerhardt, HC (2001) Neighbour-stranger discrimination by territorial male bullfrogs (Rana catesbeiana): I. Acoustic basis. Anim Behav 62:1129–1140
Bee MA, Perrill SA, Owen PC (1999) Size assessment in simulated territorial encounters between male green frogs (Rana clamitans). Behav Ecol Sociobiol 45:177–184.
Bradbury JW, Vehrencamp SL (1998) Principles of animal communication. Sinauer, Sunderland, Mass.
Bulger J (1993) Dominance rank and access to estrous females in male savanna baboons. Behaviour 124:89–122
Bulger J, Hamilton WJ (1987) Rank and density correlates of inclusive fitness measures in a natural chacma baboon (Papio ursinus) troop. Int J Primatol 8:635––650
Bulger J, Hamilton WJ (1988) Inbreeding and reproductive success in a natural chacma baboon, Papio cynocephalus ursinus, population. Anim Behav 36:574–578
Buskirk WH, Buskirk RE, Hamilton WJ (1974) Troop-mobilizing behavior of adult male chacma baboons. Folia primatol 22:9–18
Butynski TM, Chapman CA, Chapman LJ, Weary, DM (1992) Use of male blue monkey 'pyow' calls for long-term individual identification. Am J Primatol 28:183–189
Byrne RW (1981) Distance vocalisations of Guinea baboons (Papio papio) in Senegal: an analysis of function. Behaviour 78:283–313
Byrne RW, Whiten A, Henzi SP (1987) One-male groups and intergroup interactions of mountain baboons. Int J Primatol 8:615–633
Cheney DL, Seyfarth RM (1977 Behavior of adult and immature male baboons during inter-group encounters. Nature 269:404–406
Chiarello AG (1995) Role of loud calls in brown howlers, Alouatta fusca. Am J Primatol 36:213–222
Clark AP (1993) Rank differences in the production of vocalizations by wild chimpanzees as a function of social context. Am J Primatol 31:159–179
Clutton-Brock TH, Albon SD (1979) The roaring of red deer and the evolution of honest advertisement. Behaviour 69:145–170
Clutton-Brock TH, Albon SD, Gibson RM, Guinness FE (1979) The logical stag: adaptive aspects of fighting in red deer (Cervus elaphus L.). Anim Behav 27:211–225
Cowlishaw G (1992) Song function in gibbons. Behaviour 121:131–153
Cowlishaw G (1996) Sexual selection and information content in gibbon song bouts. Ethology 102:272–284
Cowlishaw G, Dunbar RI (1991) Dominance rank and mating success in male primates. Anim Behav 41:1045–1056
Davies NB, Halliday TR (1978) Deep croaks and fighting assessment in toads (Bufo bufo). Nature 274:683–684
Drews C (1996) Contexts and patterns of injuries in free-ranging male baboons (Papio cynocephalus). Behaviour 133:443–474.
Ellerry WN, Ellery K, McCarthy TS (1993) Plant distribution in island of the Okavango Delta, Botswana: determinants and feedback interactions. Afr J Ecol 31:118–134
Enquist M, Leimar O (1983) Evolution of fighting behaviour: decision rules and assessment of relative strength. J Theor Biol 102:387–410
Enquist M, Leimar O (1990) The evolution of fatal fighting. Anim Behav 39:1–9
Enquist M, Plane E, Röed J (1985) Aggressive communication in fulmars (Fulmarus glacialis) competing for food. Anim Behav 33:1007–1020
Fischer J, Hammerschmidt K, Cheney DL, Seyfarth RM (2002) Acoustic features of male baboon loud calls: influences of context, age, and individuality. J Acoust Soc Am 111:1465–1474
Gautier JP, Gautier-Hion A (1977) Communication in old world monkeys. In: Sebeok T (ed) How animals communicate. Indiana University Press, Bloomington, Ind., pp 890–964
Gerhardt HC, Roberts JD, Bee MA, Schwartz JJ (2000) Call matching in the quacking frog (Crinia georgiana). Behav Ecol Sociobiol 48:243–251
Given MF (1987) Vocalizations and acoustic interactions of the carpenter frog, Rana virgatipes. Herpetologica 43:467–481
Hall KRL, DeVore I (1965) Baboon social behavior. In: DeVore I (ed) Primate behavior. Holt, Rinehart, and Winston, New York, pp 53–110
Hamilton WJ, Bulger JB (1990) Natal male baboon rank rises and successful challenges to resident alpha males. Behav Ecol Sociobiol 26: 357–363
Hamilton WJ, Buskirk, RE Buskirk, WH (1975) Chacma baboon tactics during intertroop encounters. J Mammal 56:857–870
Hamilton WJ, Buskirk RE, Buskirk WH (1976) Defense of space and resources by chacma (Papio ursinus) baboon troops in an African desert and swamp. Ecology 57:1264–1272
Hasson O (1997) Towards a general theory of biological signaling. J Theor Biol 185:139–156.
Hausfater G (1975) Dominance and reproduction in baboons (Papio cynocephalus). Contributions to primatology, vol 7. Karger, Basel
Hurlbert SH (1984) Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:172–173
Kitchen DM, Cheney DL, Seyfarth RM (2003) Female baboons' responses to male loud calls. Ethology (in press)
LeBoeuf BJ (1974) Male-male competition and reproductive success in elephant seals. Am Zool 14:163–176
Maynard Smith J (1982) Evolution and the theory of games. Cambridge University Press, Cambridge
Maynard Smith J, Harper DGC (1995) Animal signals: models and terminology. J Theor Biol 177:305–311
Mitani JC (1988) Male gibbon (Hylobates agilis) singing behavior: natural history, song variations and function. Ethology 79:177–194
Mitani JC, Brandt KL (1994) Social factors influence the acoustic variability in the long-distance calls of male chimpanzees. Ethology 96:233–252
Mitani JC, Nishida T (1993) Contexts and social correlates of long-distance calling by male chimpanzees. Anim Behav 45:735–746
Packer C (1979a) Intertroop transfer and inbreeding avoidance in Papio anubis. Anim Behav 27:1–36
Packer C (1979b) Male dominance and reproductive activity in Papio anubis. Anim Behav 27:37–45
Poole JH (1989) Announcing intent: the aggressive state of musth in African elephants. Anim Behav 37:140–152
Poole JH (1999) Signals and assessment in African elephants: evidence from playback experiments. Anim Behav 58:185–193
Ransom TW (1981) Beach troop of the Gombe. Associated University Presses, East Brunswick, N.J.
Reby D, McComb K (2003) Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav (In press)
Robertson JGM (1986) Male territoriality, fighting and assessment of fighting ability in the Australian frog Uperoleia rugosa. Anim Behav 34:763–772
Ross K (1987) Okavango: jewel of the Kalahari. Macmillan, New York
Ryan MJ (1985) Energetic efficiency of vocalization by the frog Physalaemus pustulosus. J Exp Biol 116:47–52
Ryan MJ, Brenowitz EA (1985) The role of body size, phylogeny, and ambient noise in the evolution of bird song. Am Nat 126:87–100
Saayman GS (1971) Behavior of the adult males in a troop of free-ranging chacma baboons. Folia Primatol 15:36–57.
Sekulic R (1982) The function of howling in red howler monkeys (Alouatta seniculus). Behaviour 81:38–54
Silk JB (1987) Social behavior in evolutionary perspective. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT (eds) Primate societies. University of Chicago Press, Chicago, pp 318–329
Smith KS (1986) Dominance and mating strategies of chacma baboons, Papio ursinus, in the Okavango Delta, Botswana. PhD. dissertation, University of California, Davis
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. Freeman, New York
Steenbeek R, Assink P, Wich SA (1999) Tenure related changes in wild Thomas's langurs II: loud calls. Behaviour 136:627–650
Vehrencamp S (2000) Handicap, index, and conventional elements of bird song. In: Espmark Y, Amundsen T, Rosenqvist G (eds) Animal signals: signalling and signal design in animal communication. Tapir, Trondheim, Norway, pp 277–300
Wagner RE (1989) Fighting, assessment, and frequency alteration in Blanchard's cricket frog. Behav Ecol Sociobiol 25:429–436
Waser PH (1977) Individual recognition, intragroup cohesion, and intergroup spacing: evidence from sound playback to forest monkeys. Behaviour 60:28–74
Weingrill T, Lycett JE, Henzi SP (2000) Consortship and mating success in chacma baboons (Papio cynocephalus ursinus). Ethology 106:1033–1044
Wilson ML, Hauser MD, Wrangham RW (2001) Does participation in intergroup conflict depend on numerical assessment, range location, or rank for wild chimpanzees? Anim Behav 61:1203–1216
Winer BJ, Brown DR, Michaels KM (1991) Statistical principles in experimental design, 3rd edn. McGraw-Hill, New York
Acknowledgements
We are grateful to the Office of the President of the Republic of Botswana and the Botswana Department of Wildlife and National Parks for permission to conduct this research. The tireless support of J. Nicholson was essential to the project. M. Mokupi, M. Mpitsang, M. Kehaletse, and C. Seyfarth provided invaluable assistance in the field. We also thank G. Dudley, L. Bester-Dudley, J. Rawle, C. McAllister, Game Trackers, Mack Air, and Ensign Agencies for their friendship and logistical support. This paper was also improved by comments from C. Nunn and three anonymous reviewers. Research was supported by NSF grant IBN 9514001, NIH grant MH62249 and the University of Pennsylvania. This research complied with the laws of the Republic of Botswana and was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pennsylvania.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by C. Nunn
Rights and permissions
About this article
Cite this article
Kitchen, D.M., Seyfarth, R.M., Fischer, J. et al. Loud calls as indicators of dominance in male baboons (Papio cynocephalus ursinus). Behav Ecol Sociobiol 53, 374–384 (2003). https://doi.org/10.1007/s00265-003-0588-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-003-0588-1