Abstract
Background
Fluorodeoxyglucose positron emission tomography (FDG-PET) is a novel method of assessing suspected periprosthetic hip infection. However, a heterogeneity of sensitivity and specificity using different diagnostic criteria across clinical studies has been published. The objective of this study is to evaluate the various diagnostic criteria using FDG-PET in diagnosing periprosthetic hip infection.
Methods
FDG-PET scans of patients suffering from painful hip prostheses between 2008 and 2015 were retrospectively reviewed. The PET images were considered positive for infection using five criteria: any increased uptake at the (1) bone-prosthesis-interface, (2) periprosthetic soft tissue (PST), or (3) both, (4) increased uptake in the bone-prosthesis-interface compared to the PST, and (5) increased uptake along the femoral bone-prosthesis-interface. The final diagnosis of infection was based on the pre-operative and intra-operative findings with clinical follow-up > 12 months.
Results
A total of 33 hip prostheses were evaluated in this study, of which 16 were determined to be infected and 17 uninfected. Any periprosthetic FDG uptake was found in all symptomatic prostheses (sensitivity 100%; specificity 0%). When increased uptake in the bone-prosthesis-interface (sensitivity 100%; specificity 65%) or PST (sensitivity 94%; specificity 59%) was considered infected, specificity increased. A higher intensity of uptake at the bone-prosthesis-interface than PST demonstrated only moderate specificity (sensitivity 44%; specificity 71%). The most specific criterion for infection was an increased FDG uptake along the femoral bone-prosthesis-interface (sensitivity 81%; specificity 94%).
Conclusions
Our results demonstrated that the accuracy of FDG-PET is highly dependent of the diagnostic criteria used for periprosthetic hip infection. Only an acceptable diagnostic accuracy (sensitivity 81%; specificity 94%) was found when increased FDG uptake along the femoral bone-prosthesis-interface was considered positive for infection.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Total hip arthroplasty is one of the most clinically successful and cost-effective surgical treatments of patients suffering from end-stage hip disease, such as severe osteoarthritis, post-traumatic arthritis, developmental dysplasia, inflammatory arthritis, and osteonecrosis of the femoral head [1]. However, 10% of the treated patients eventually develop a significant painful hip prosthesis, the majority resulting from aseptic loosening [2]. Although less frequent, one of the most devastating complications is infection which can result in substantial morbidity and decline in functional outcome. Crucial for initiating the appropriate medical and surgical treatment is the timely identification and precise localization of a periprosthetic infection. A delay in diagnosing and treatment of a periprosthetic hip infection can have critical impact on loosening or maintaining the prosthesis and joint function.
Diagnosing periprosthetic hip infection, including a differentiation of mechanical loosening, remains challenging. The diagnostic algorithm is characterized by a multi-modality work-up including microbiologic, laboratory (elevated erythrocyte sedimentation rate [ESR], C reactive protein [CRP]), synovial marker, and histologic tests [3, 4]. In addition to these diagnostic tests, various imaging techniques including bone, leukocyte, bone marrow, antigranulocyte scintigraphy, and positron emission tomography (PET) can be used in the assessment of suspected periprosthetic infection, especially in the challenging diagnosis of a chronic or low-grade infection [5,6,7]. Currently, combined leukocyte and bone marrow scintigraphy is considered the preferred imaging modality in the evaluation of a suspected periprosthetic infection by most authors [8]. Although most studies indicate that this imaging technique is accurate, it is hampered by time-consuming procedures, technically difficulties, limited availability, and sub-optimal spatial resolution. In recent literature, FDG-PET imaging has been proposed for detecting orthopaedic infections. This relative novel method of assessing periprosthetic infection has important advantages compared to combined leukocyte and bone marrow scintigraphy, such as increased resolution and time efficiency which results in a less inconvenient procedure for patients. Although most reports indicate that FGD-PET is highly accurate in the evaluation of infection associated with total hip arthroplasty, inconsistent results of diagnostic accuracy has been reported [7]. In literature, the sensitivity and specificity ranged between 33 and 95% and 62 and 96%, respectively. This heterogeneity may be explained by the use of different criteria in image interpretation for periprosthetic hip infection [7]. The present study was undertaken to evaluate the predefined diagnostic criteria in literature and determine the optimal criteria for diagnosing periprosthetic hip infection.
Methods
Patient population
A cohort study was performed. The ICPC codes (International Classification of Primary Care) and operation codes were used to collect the patients with a painful hip prosthesis between 2008 and 2015 in two general teaching hospitals in the Netherlands. After this selection, all patients were screened whether FDG-PET imaging was used in the evaluation of symptomatic hip prostheses by the departments of orthopaedic surgery. The data were collected and these patients were allocated to the symptomatic group when the following inclusion criteria were met: (1) the diagnosis of the painful prosthesis was unclear before performing the FDG-PET imaging in the evaluation of infection; (2) the patients who underwent surgery were included when surgical exploration was performed within one year after performing the FDG-PET imaging; (3) a clinical follow-up of at least 12 months. Patients were excluded from this study if the imaging and clinical data were incomplete, such that retrospective analysis could not be performed. A priori, no differentiation was made regarding the stage of infection (acute or chronic), the sort of implant or the imaging time after surgery. When FDG-PET was not primarily used for the evaluation of symptomatic hip prosthesis or when patients had a bilateral, asymptomatic hip prosthesis, the data were also collected. These patients were allocated to the asymptomatic, control group. The study was performed according to the ethical and judicial guidelines of the Central Committee on Research Involving Human Subject (the Netherlands) and approved by the Ethical Committee, NWZ.
Reference standard
The diagnosis of periprosthetic hip infection was based on one or more the following criteria: (1) a systemic infection with pain in the hip and purulent fluid within the joint and/or (2) a positive result on at least three tests (ESR, CRP, joint aspiration, and intra-operative culture) or (3) a sinus tract communicating with the joint [4]. A final diagnosis was given to each patient based on the interpretation of the clinical presentation and/or the pre-operative and intra-operative findings. For patients in which periprosthetic infection was excluded, the clinical follow-up was at least 12 months.
Imaging procedures
FDG-PET studies were performed with a Philips Gemini TF PET/CT scanner or a Siemens Biograph PET-CT 16 Truepoint. Patients fasted four hours prior to administering FDG. The images were obtained 60 minutes after the FDG injection of 156–351 MBq. Corrected and uncorrected transaxial images were acquired. Image reconstruction was performed with a multiplicative iterative reconstruction algorithm for improvement of image quality and reduction of computation time. Primarily, the PET images were used for the interpretation of uptake patterns. The CT-portion, without a metal artifact reduction algorithm, was not used for the interpretation of periprosthetic soft tissue uptake.
Imaging interpretation
Two readers (one senior nuclear medicine physician and one nuclear medicine trainee) blinded to any clinical information, evaluated individual FDG studies. Each study was read once, and disagreement between the two observes was resolved with consensus. Any area of increased uptake was identified and the location of uptake was registered. The combination of non-attenuation and attenuation-correction images were used. The interpretation of images was generated using predefined criteria published in the literature. The femoral and acetabulum component of the hip prosthesis was divided into three zones (A, B, C and I, II, III, respectively) and the presence of FDG uptake in the bone-prosthesis-interface and soft tissue was documented (Fig. 1). The images were visual evaluated for infection using the following diagnostic criteria: (1) any increased uptake adjacent of the prosthesis [9, 10]; (2) uptake in the bone-prosthesis-interface (zone A–C, I–III) [10, 11]; (3) uptake in periprosthetic soft tissue [8]; (4) an increased uptake in the bone-prosthesis-interface compared to the periprosthetic soft tissue [12, 13]; and the (5) FDG uptake extending along the femoral bone-prosthesis-interface (zone B, Fig. 1), and involving more than one contiguous zone and not limited to only the femoral neck, head zone, or periprosthetic soft tissue [14,15,16,17,18] (Table 1).
Overview of the various regions that could demonstrate increased FDG uptake on FDG-PET imaging. An increased uptake in region A includes the femoral head and neck that could persist for years after implantation in both symptomatic and asymptomatic hip prostheses. An increased uptake or activity along the femoral bone-prosthesis-interface in region B is highly suggestive for infection. An increased uptake at the distal tip of the hip prosthesis in region C is not suggestive for infection (non-specific uptake)
Analysis
The diagnosis of the FDG-PET studies, the consensus score, was compared to the reference standard in order to determine the sensitivity and specificity of the symptomatic group, with a 95% confidence interval (CI). The results were differentiated and analyzed regarding the stage of infection (acute or chronic), sort of implant, and time after surgery. The use of antibiotic treatment during FDG imaging was not analyzed, because it is assumed not to affect the sensitivity in delineating sites of infections, since uptake is independent of leukocyte migration. The Cohen’s kappa (κ) coefficient was calculated in order to evaluate inter-observer reliability. Kappa’s coefficient is a measure that adjusts for the agreement that is expected by chance and ranges between—1.0 and 1.0. Absolute agreement (100%) is represented with κ = 1.0 and κ = 0.0 is considered as random agreement. The interpretation of kappa coefficients was performed using the criteria of Landis and Koch and implies κ = > 0.8, κ = 0.6–0.8, κ = 0.4–0.6, κ = 0.2–0.4, and κ = < 0.2 as almost perfect, substantial, moderate, fair, and slight interobserver agreement, respectively [20]. Calculation was performed using computer-calculated kappa statistics (Microsoft Office Excel 2007, Redmon, Washington USA) and the web-based statistical tool http://www.hsls.pitt.edu/medcalc/Kappa_MC.htm.
Results
Inclusion
A total of 43 hip prostheses were included for evaluation (age ranges 54–92 years, mean 76.4 year). Of these patients, 33 prostheses were allocated to the symptomatic group. This group comprised 21 primary (15 uncemented, 6 cemented) and 12 revised hip prostheses (7 uncemented, 5 cemented). The main reason for implantation of the primary prostheses was osteoarthritis, while revision surgery was performed after mechanical loosening. The time between implantation and imaging ranged between one and 415 months (mean 47.0). Details of the patient characteristics, infection, and implants are detailed in Table 2. Of the symptomatic hip prostheses, 16 were determined to be infected. No sinus tract or open wound was found in this group. These patients demonstrated elevated CRP and/or ESR and no signs of loosening or infection on plain radiographs. Of the remaining 17 prostheses, infection was excluded based on either surgical and aspiration findings or clinical follow-up. A total of ten asymptomatic hip prostheses were used as control group. The reason for total hip arthroplasty in this group was osteoarthritis. All prostheses were primary implants (two were cemented, eight uncemented). The time between implantation and imaging was three to 108 months.
Evaluation of control group
Of the ten asymptomatic hip prostheses in the control group, eight prostheses demonstrated some uptake of FDG adjacent to the prosthesis (Tables 3 and 4). Uptake in the head zone was found in eight cases. One hip prosthesis, implanted less than one year before imaging, demonstrated uptake in periprosthetic soft tissue adjacent to the femoral component. No increased uptake was found in the femoral bone-prosthesis-interface. The time after implantation ranged between three and 108 months (Table 2).
Uptake areas in the symptomatic group
The FDG-PET studies demonstrated any increased uptake (zone A, B, or C) at the bone-prosthesis-interface in all 16 infected cases (Table 4), located at the middle portion of the femoral component in 13 cases (zone B). Any uptake in the periprosthetic soft tissue was observed in 15 prostheses. Of the 17 uninfected hip prostheses, any uptake at the bone-prosthesis-interface was observed in six cases. Five of these six cases demonstrated (not extended) uptake involving zone A and/or zone C (Fig. 1), not considered to represent an increased uptake to zone B. In one case, there was increased uptake at zone B (Fig. 1). Uptake in the periprosthetic soft tissue around the femoral component was found in seven cases, all within one year after implantation. Without exception, uptake in the neck and head zone was observed in all symptomatic cases.
Comparison of diagnostic criteria
In the comparison of the different diagnostic criteria (Table 3), the most accurate interpretation was FDG uptake extending along the femoral bone-prosthesis-interface (zone B), not limited to only the femoral neck, head zone, or periprosthetic soft tissue (Fig. 2), with a sensitivity of 81% and specificity of 94%. Any FDG accumulation adjacent to the prosthesis resulted in a sensitivity of 100% and a specificity of 0%. Only uptake at the bone-prosthesis-interface demonstrated comparable sensitivity of 100% and higher specificity of 65%. A sensitivity of 94% and specificity of 59% was found when only uptake in the periprosthetic soft tissue was used as criterion for infection. When the diagnostic criterion was used that uptake of the bone-prosthesis-interface was relatively more than uptake in the periprosthetic soft tissue, sensitivity decreased to 44% and specificity increased to 71%.
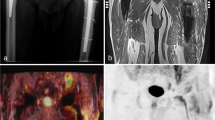
Example of infection. This 84-year-old patient had left hip replacement 10 months previously. He suffered from increasing pain at the prosthesis. The laboratory blood tests showed elevated CRP and ESR. Clinical findings and prior diagnostic tests, including negative arthrography, did not demonstrate signs of loosening or infection. A variety of uptake patterns are demonstrated in the FDG-PET images. All the different criteria, as showed in Table 3, are positive in this example: (1) there is some increased uptake adjacent to the prosthesis; (2) there is increased uptake at the bone-prosthesis-interface of the femoral and acetabulum component; (3) any increased uptake in the periprosthetic soft tissue (dotted arrow); (4) uptake at the femoral bone-prosthesis-interface is more than the surrounding soft tissue; and (5) an increased FDG uptake along the bone-prosthesis-interface of the femoral shaft, not limited to the femoral head, neck of periprosthetic soft tissue. The arrow points at the region that reflects the most specific uptake pattern for infection. a PET coronal image; b PET transverse non-attenuation-correction image; c PET transverse attenuation-correction image. After FDG-PET imaging, cultures samples taken from the proximal and distal femur, grew Staphylococcus epidermidis
Inter-observer agreement
The inter-observer agreement between the two readers demonstrated substantial to almost perfect agreement. In the interpretation of uptake in bone-prosthesis-interface zone B, disagreement was found in four of the 33 symptomatic hip prostheses, which resulted in an almost perfect inter-observer agreement (κ = 0.85). This disagreement was only found in four infected cases. In the acetabulum component, periprosthetic soft tissue and head zone uptake, inter-observer agreement was substantial to almost perfect (κ = 0.81, κ = 0.77, and κ = 0.79 respectively).
Discussion
In the evaluation of suspected periprosthetic hip infection, various diagnostic tests including blood laboratory tests, aspiration results and synovial fluid, and microbiologic and histopathological analysis can be used. However, inconsistent diagnostic accuracies with these various tests across studies have been published and accurate diagnosis of periprosthetic hip infection remains challenging, especially in chronic- or low-grade infections. Because of that, imaging tests remain important, and could contribute in the diagnosis. FDG-PET is a novel method of assessing suspected periprosthetic hip infection; however, a heterogeneity of sensitivity and specificity using different diagnostic criteria across clinical studies have been published [7]. The objective of this study was to evaluate the predefined diagnostic criteria when FDG-PET was used in the assessment of periprosthetic hip infection. Our results demonstrated that accumulation of FDG along the bone-prosthesis-interface, not limited to only the femoral neck, head zone, or periprosthetic soft tissue was the most accurate diagnostic criterion with a sensitivity of 81% and specificity of 94%.
FDG-PET has been successfully used the evaluation patients with both malignant and infectious disorders [21]. Subsequently, this imaging modality was introduced as potential useful tracer in detecting periprosthetic infections. Although differentiation between inflammatory uptake resulting from mechanical loosening and periprosthetic infection was presumed difficult [19, 22], the first clinical investigations demonstrated specific uptake pattern in infected hip prostheses. The visual interpretation of site and pattern of FDG uptake appeared more important and reliable than intensity of uptake, standardized uptake values (SUV), in the region of interest. However, the sequential published investigations demonstrated heterogeneity in diagnostic accuracies across clinical studies, with sensitivity and specificity ranging between 33 and 95% and 62 and 96%, respectively (Table 1) [7]. A possible explanation for the heterogeneity may lie in the use of different diagnostic criteria for periprosthetic infection.
Various diagnostic criteria for infection were used in previous investigations (Table 1). When any periprosthetic uptake was considered as infection, Love et al. reported sensitivity of 100% and specificity of 0.1%, similarly to our results (Table 3) [19]. However, any uptake was also found in our control group, which illustrates the lack of efficacy of this criterion [12, 22]. Specificity increased when more specific uptake patterns were used as criterion for infection. Reinartz et al. evaluated exclusively the accuracy of uptake in the periprosthetic soft tissue, with a reported sensitivity of 94% and specificity of 95% [10]. Our results demonstrated similar sensitivity but lower specificity of 59%. When using the criterion of an increased uptake in the bone-prosthesis-interface compared to the periprosthetic soft tissue, Garcia et al. found a sensitivity of 64% and a specificity of 62% [12] In our study, sensitivity was lower with 44% and specificity was more or less equivalent with 71%. The most specific criteria for diagnosing periprosthetic hip infection in previous studies was found to be an increased uptake along the bone-prosthesis-interface, not limited to only the femoral neck, head zone, or periprosthetic soft tissue (Fig. 1). Our results demonstrated a sensitivity of 81% and specificity of 94%, which is comparable with the pooled sensitivity of 86% and pooled specificity of 93% found in a recently performed meta-analysis [7].
When periprosthetic uptake of FDG is absent, a periprosthetic hip infection can be excluded (Table 3). However, in case uptake is observed, it is important to differentiate in uptake patterns, as mentioned above. The asymptomatic group showed non-specific uptake around the head and neck in 80% (Fig. 3), as described by previous reports [15, 23].Similar to conventional tracers, the post-operative remodeling conditions after implantation results in non-infectious uptake patterns around the neck and/or head of the asymptomatic prosthesis for years after surgery. This non-specific postsurgical inflammation pattern does not affect the ability of FDG-PET to diagnose or exclude infection associated with total hip arthroplasty. It is assumed that the uptake at the femoral component provides a larger area that facilitates the possibility to differentiate between non-specific and infection uptake patterns. Uptake at the femoral component is not necessarily a sign of progression of infection from the acetabulum, area A or C. Therefore, in this setting, recognition of the commonly increased FDG uptake pattern (area A) is important and should not be interpreted as a finding suggestive of infection. In only one case, the interpretation of uptake in the head and neck zone was different between the two readers, which resulted in a substantial inter-observer agreement (κ = 0.79).
Example of non-specific uptake. This 54-year-old patient had bilateral hip replacement. Total hip arthroplasty (uncemented) on the right side was performed 2 years before FDG-PET imaging. This prosthesis was not painful and asymptomatic. The FDG-PET image demonstrated non-specific uptake which is the result of postoperative remodeling. The (uncemented) hip replacement on the left side was performed 3 years before performing FDG-PET imaging. One year after implantation, the patient suffered from ongoing pain in the left hip region. The prior diagnostic tests, including radiographs, laboratory blood tests, aspiration results, and bone scintigraphy were inconclusive. FDG-PET imaging was performed to rule out a low-grade periprosthetic infection. The FDG-PET scan demonstrated a non-specific periprosthetic uptake pattern, not suggestive for infection
In the evaluation of suspected periprosthetic infection, the diagnosis relies on a combination of clinical judgment, pre-operative hematologic testing, information obtained from aspiration, and microbiologic as well as histopathologic testing of tissue or fluid obtained at the time of surgery. Although important developments have been described [3], there is not yet a single gold standard diagnostic test for identification of a periprosthetic infection. It is not recommended to use nuclear imaging as isolated diagnostic; however, imaging can contribute in the challenging diagnosis of a periprosthetic infection [6, 7, 24]. In clinical practice, when there is a low suspicion of an infected hip implant, a highly sensitive imaging modality can rule out an infection. For this purpose, bone scintigraphy could be the preferred imaging modality because it is widely available with low costs. Unfortunately, this technique lacks the specificity needed to differentiate between various conditions causing periprosthetic uptake and is of limited use in the first post-operative years because of increased bone turnover after surgery. When prior diagnostic tests are inconclusive, especially in case of a low-grade or chronic infection (Fig. 2), including inconclusive periprosthetic uptake on a bone scan (Fig. 4), a more specific imaging modality can contribute in diagnosing infection. For this purpose, most authors reported that combined leukocyte and bone marrow scintigraphy is the preferred imaging modality [8]. However, a recent meta-analysis compared the diagnostic accuracies of all currently used imaging modalities in the assessment of periprosthetic hip infection and concluded that FDG-PET could potentially be used as preferred imaging modality [7]. Compared to combined bone marrow and leukocyte scintigraphy, FDG-PET has important advantages as time efficiency, increased resolution, and the use of low-dose computed tomography. However, in the setting of assessing periprosthetic infection, FDG-PET is often depicted as an expensive and complicated imaging modality, and therefore gained limited popularity in clinical daily practice. In the perspective of combined costs of multiple diagnostic tests including the use of conventional nuclear medicine methods, clinical investigating is needed to evaluate the cost-effectiveness and exact role of FDG-PET in the diagnostic algorithm of a periprosthetic infection. Our results demonstrated that, when the appropriate diagnostic criteria were used, this imaging modality could provide additional value in more complex clinical patients using relatively simple and visual interpretation.
Example of infection. Because of osteoarthritis, this 90-year-old patient underwent hip replacement in 2000 on the left side (cemented) and in 2005 on the right side (cemented). Two years after implantation of the right hip prosthesis, the patient complained about pain in the right hip region. Radiographs did not show any signs of loosening or infection. Laboratory blood results demonstrated elevated ESR. A triple-phase bone scintigraphy a was performed to rule out infection. However, periprosthetic uptake was found in all three phases, suggestive for infection. To confirm a chronic periprosthetic infection, FDG-PET imaging b was performed. The FDG-PET images demonstrated periprosthetic uptake in the lateral soft tissue region and an extended uptake at the femoral bone-prosthesis-interface, specific for infection on the right side. The arrow defines the extended uptake from zone A to zone B. The intraoperative findings and the culture samples separately taken from the distal femur, were positive for Staphylococcus epidermidis. The asymptomatic left prosthesis demonstrated uptake in the head and neck region, not specific for infection
Our investigation is a blinded study that involves a sufficient cohort of patients; however, there are several limitations. (1) Based on routine practice with multiple diagnostic tests including laboratory tests, radiographs, and aspiration results, the diagnosis of infection was initially not clear in the symptomatic group before performing FDG-PET imaging. Subsequently, these symptomatic patients represent a group in which the diagnosis was more complex compared to patients with more obvious signs of infection. Therefore, our results should be interpreted with caution when applying to all patients with painful hip prostheses. (2) This study defined test performance of FDG-PET as an isolated diagnostic test. Because we performed a retrospectively study, the influence of FDG-PET on the diagnostic decisions in the algorithm could not properly be analyzed. It is important to note that this technique can be used in concert with other elements of other diagnostic tests to arrive at a more precise diagnosis than is possible with imaging testing alone. Further, prospective studies should investigate the exact role of FDG-PET in the multi-modality work-up of a periprosthetic infection. (3) Although we used stringent criteria for periprosthetic hip infection and we were convinced of the diagnosis, the universally lack of a golden standard could potentially distort some results due to a reference-test bias [4]. For obvious reasons, surgery with microbiologic evaluation was not performed in all patients with a painful hip prosthesis, and clinical follow-up sometimes had to be used as the method for the final diagnosis. Potentially, this could have resulted in more false-negative results. (5) Although we observed no influences of the stage of infection and time after surgery, more patient data and members are needed in order to analyze these variables properly. These variables are often insufficient described in published investigations. In our study, the majority of the included patients were not considered acute infections. The differentiation between acute and chronic infection is important because nuclear imaging is presumed more useful when there are less obvious signs of infection, which is more likely in chronic infections. The most used classification systems defined periprosthetic joint infection either as acute or chronic, or as early (occurring within 3 months postoperatively), delayed (3–24 months), and late (> 24 months) [25]. Of the 33 included patients, only two patients developed symptoms within four weeks and three patients within three months after implantation (early infection). The remaining patients were considered chronic infection and developed symptoms within three to 24 months (delayed) or after 24 months (late infection). Therefore, our results should be interpreted with caution when applying to patients with acute infected hip prostheses. (4) Furthermore, in our study, we defined the interobserver variability of the interpretation using FDG imaging, and a substantial to almost perfect inter-observer agreement was found. However, we only investigated two observers with a limited number of patients and disagreement between the two observers was found in four of the 16 infected cases. The diagnostic results in clinical practice could be potentially be hampered by a larger inter-observer variability. A larger number of patients and observers are needed to properly define the reproducibility of FDG imaging in the assessment of periprosthetic infection including the influences of experience of the observers and diversity of periprosthetic uptake patterns.
In conclusion, FDG-PET can be used as a sensitive and specific imaging modality in diagnosing periprosthetic hip infection. Our results demonstrated that the accuracy of FDG-PET is highly dependent of the diagnostic criteria used for periprosthetic infection. Only an acceptable diagnostic accuracy was found using increased FDG uptake along the femoral bone-prosthesis-interface, not limited to only the femoral neck, head zone, or periprosthetic soft tissue as a criterion for infection.
References
Learmonth ID, Young C, Rorabeck C (2007) The operation of the century: total hip replacement. Lancet 370:1508–1519. https://doi.org/10.1016/s0140-6736(07)60457-7
Soderman P, Malchau H, Herberts P, Zugner R, Regner H, Garellick G (2001) Outcome after total hip arthroplasty: Part II. Disease-specific follow-up and the Swedish National Total Hip Arthroplasty Register. Acta Orthop Scand 72:113–119. https://doi.org/10.1080/000164701317323345
Wyatt MC, Beswick AD, Kunutsor SK, Wilson MJ, Whitehouse MR, Blom AW (2016) The alpha-defensin immunoassay and leukocyte esterase colorimetric strip test for the diagnosis of periprosthetic infection: a systematic review and meta-analysis. J Bone Joint Surg Am 98:992–1000. https://doi.org/10.2106/jbjs.15.01142
Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, Garvin KL, Mont MA, Wongworawat MD, Zalavras CG (2011) New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res 469:2992–2994. https://doi.org/10.1007/s11999-011-2102-9
Verberne SJ, Temmerman OPP (2017) 12 - Imaging of prosthetic joint infections A2- arts, J.J. Chris. In: Geurts J (ed) Management of periprosthetic joint infections (PJIs). Woodhead Publishing, Cambridge, pp 259–285
Verberne SJ, Sonnega RJ, Temmerman OP, Raijmakers PG (2017) What is the accuracy of nuclear imaging in the assessment of periprosthetic knee infection? A meta-analysis. Clin Orthop Relat Res 475:1395–1410. https://doi.org/10.1007/s11999-016-5218-0
Verberne SJ, Raijmakers PG, Temmerman OPP (2016) The accuracy of imaging techniques in the assessment of periprosthetic hip infection: a systematic review and meta-analysis. J Bone Joint Surg 98:1638–1645. https://doi.org/10.2106/jbjs.15.00898
Reinartz P (2009) FDG-PET in patients with painful hip and knee arthroplasty: technical breakthrough or just more of the same. Q J Nucl Med Mol Imaging 53:41–50
Vanquickenborne B, Maes A, Nuyts J, Van AF, Stuyck J, Mulier M, Verbruggen A, Mortelmans L (2003) The value of (18)FDG-PET for the detection of infected hip prosthesis. Eur J Nucl Med Mol Imaging 30:705–715. https://doi.org/10.1007/s00259-002-1109-6
Reinartz P, Mumme T, Hermanns B, Cremerius U, Wirtz DC, Schaefer WM, Niethard F, Buell U (2005) Radionuclide imaging of the painful hip arthroplasty: positron-emission tomography versus triple-phase bone scanning. J Bone Joint Surg Br 87:465–470. https://doi.org/10.1302/0301-620x.87b4.14954
Mayer-Wagner S, Mayer W, Maegerlein S, Linke R, Jansson V, Muller PE (2010) Use of 18F-FDG-PET in the diagnosis of endoprosthetic loosening of knee and hip implants. Arch Orthop Trauma Surg 130:1231–1238. https://doi.org/10.1007/s00402-009-1000-z
Garcia-Barrecheguren E, Rodriguez FM, Toledo SG, Valenti Nin JR, Richter Echevarria JA (2007) 18FDG-PET: a new diagnostic approach in hip prosthesis infection. In: Revista Espanola de Medicina Nuclear La 18FDG-PET: Una nueva aproximacion diagnostica en la infeccion protesica de cadera. pp. 208–220
Mumme T, Reinartz P, Alfer J, Muller-Rath R, Buell U, Wirtz DC (2005) Diagnostic values of positron emission tomography versus triple-phase bone scan in hip arthroplasty loosening. Arch Orthop Trauma Surg 125:322–329. https://doi.org/10.1007/s00402-005-0810-x
Chacko TK, Zhuang H, Stevenson K, Moussavian B, Alavi A (2002) The importance of the location of fluorodeoxyglucose uptake in periprosthetic infection in painful hip prostheses. Nucl Med Commun 23:851–855
Pill SG, Parvizi J, Tang PH, Garino JP, Nelson C, Zhuang H, Alavi A (2006) Comparison of fluorodeoxyglucose positron emission tomography and (111)indium-white blood cell imaging in the diagnosis of periprosthetic infection of the hip. J Arthroplasty 21:91–97. https://doi.org/10.1016/j.arth.2006.05.021
Zhuang H, Duarte PS, Pourdehnad M, Maes A, Van AF, Shnier D, Garino JP, Fitzgerald RH, Alavi A (2001) The promising role of 18F-FDG PET in detecting infected lower limb prosthesis implants. J Nucl Med 42:44–48
Chryssikos T, Parvizi J, Ghanem E, Newberg A, Zhuang H, Alavi A (2008) FDG-PET imaging can diagnose periprosthetic infection of the hip. Clin Orthop Relat Res 466:1338–1342
Basu S, Kwee TC, Saboury B, Garino JP, Nelson CL, Zhuang H, Parsons M, Chen W, Kumar R, Salavati A, Werner TJ, Alavi A (2014) FDG PET for diagnosing infection in hip and knee prostheses: prospective study in 221 prostheses and subgroup comparison with combined (111)In-labeled leukocyte/(99m)Tc-sulfur colloid bone marrow imaging in 88 prostheses. Clin Nucl Med 39:609–615. https://doi.org/10.1097/RLU.0000000000000464
Stumpe KD, Notzli HP, Zanetti M, Kamel EM, Hany TF, Gorres GW, von Schulthess GK, Hodler J (2004) FDG PET for differentiation of infection and aseptic loosening in total hip replacements: comparison with conventional radiography and three-phase bone scintigraphy. Radiology 231:333–341. https://doi.org/10.1148/radiol.2312021596
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Glaudemans AW, de Vries EF, Galli F, Dierckx RA, Slart RH, Signore A (2013) The use of (18)F-FDG-PET/CT for diagnosis and treatment monitoring of inflammatory and infectious diseases. Clin Dev Immunol 2013:623036. DOI https://doi.org/10.1155/2013/623036
Love C, Marwin SE, Tomas MB, Krauss ES, Tronco GG, Bhargava KK, Nichols KJ, Palestro CJ (2004) Diagnosing infection in the failed joint replacement: a comparison of coincidence detection 18F-FDG and 111In-labeled leukocyte/99mTc-sulfur colloid marrow imaging. J Nucl Med 45:1864–1871
Zhuang H, Chacko TK, Hickeson M, Stevenson K, Feng Q, Ponzo F, Garino JP, Alavi A (2002) Persistent non-specific FDG uptake on PET imaging following hip arthroplasty. Eur J Nucl Med Mol Imaging 29:1328–1333. https://doi.org/10.1007/s00259-002-0886-2
Zmistowski B, Della VC, Bauer TW, Malizos KN, Alavi A, Bedair H, Booth RE, Choong P, Deirmengian C, Ehrlich GD, Gambir A, Huang R, Kissin Y, Kobayashi H, Kobayashi N, Krenn V, Drago L, Marston SB, Meermans G, Perez J, Ploegmakers JJ, Rosenberg A, Simpendorfer C, Thomas P, Tohtz S, Villafuerte JA, Wahl P, Wagenaar FC, Witzo E (2014) Diagnosis of periprosthetic joint infection. J Arthroplasty 29:77–83. https://doi.org/10.1016/j.arth.2013.09.040
Zimmerli W (2006) Infection and musculoskeletal conditions: prosthetic-joint-associated infections. Best Pract Res Clin Rheumatol 20:1045–1063. https://doi.org/10.1016/j.berh.2006.08.003
Acknowledgments
We greatly appreciate the contribution of J.W. Kuiper and W.A.M. Broos for collecting the raw data used for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The study was performed according to the ethical and judicial guidelines of the Central Committee on Research Involving Human Subject and approved by the Ethical Committee, NWZ.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
The research was performed at Centre for Orthopaedic Research Alkmaar (CORAL), NWZ, Alkmaar, the Netherlands.
Rights and permissions
About this article
Cite this article
Verberne, S.J., Temmerman, O.P.P., Vuong, B.H. et al. Fluorodeoxyglucose positron emission tomography imaging for diagnosing periprosthetic hip infection: the importance of diagnostic criteria. International Orthopaedics (SICOT) 42, 2025–2034 (2018). https://doi.org/10.1007/s00264-018-3931-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-3931-x