Abstract
Purpose
It is unclear whether late THA periprosthetic femoral fractures are related to a mechanical mechanism that decreases strength of the femur (for example, loosening) or to a biological problem as osteolysis. It is also unknown if ceramic on ceramic bearing couples decrease the risk of late periprosthetic fractures as a result of the absence of wear and osteolysis.
Material and methods
We therefore asked whether the cumulative long-term fractures were different according to the couple of friction ceramic on ceramic or ceramic on polyethylene in 327 patients (654 hips) with bilateral THA (one ceramic-ceramic, and the contralateral ceramic-polyethylene) who had THA with cemented stems performed between from 1978 to 2000 for osteonecrosis.
Results
There were two intra-operative fractures (0.3%). The median follow-up was 22 years (range, 15–40 years), and at the most recent follow-up, the cumulative number of late (after 7 years of follow-up) post-operative fractures was 32 (5% of 654 hips). Fractures were unilateral, which means for the 327 patients, a 10% rate of fractures. Periprosthetic fractures increased in number with follow-up: seven fractures (1% of 654 hips) occurred within ten years of THA implantation, 20 (3%) within 20 years, 26 (4%) within 30 years, and 32 (5%) within 40 years. The risk of fracture was influenced (p < 0.001) by the bearing surfaces at the time of prosthetic implantation, low (0.3%) for ceramic on ceramic (1/32 fractures; 1/327 hips), high (10%) for ceramic on PE (31/32 fractures; 31/327 hips).
Conclusion
In summary, when the contralateral hip of the same patient is the control, after 40 years of follow-up, post-operative fractures occur 30 times more often on the side with PE cup than on the side with ceramic/ceramic bearing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periprosthetic fractures are considered as a rare complication of total hip arthroplasty; however, it is an increasing cause of revision associated with higher morbidity and mortality compared with other causes of aseptic revision surgery. It damages the functional ability of patients and the treatment can be technically demanding for surgeons with a high risk frequency of complications. Periprosthetic fracture after THA can occur early or late, although definitions of what constitutes “early” and “late” vary. The occurrence of early fractures appears highest with uncemented stems [1, 2]. Intra-operative fractures occurred 14 times more often with uncemented stems [1]. However, late fractures after hip replacement occur also with cemented stems and it is not clear whether the bearing surfaces with hard on hard friction will change the risk of late fractures due to the absence of wear and osteolysis. In our 20-year study [3] of patients with alumina on alumina (AL/AL) bearing in one hip and AL-on-PE (AL/PE) on the contralateral side, we were surprised to have no late periprosthetic fractures in the hips with ceramic on ceramic contrary in contrast to our experience with arthroplasties with AL/PE bearings. We were also surprised that these fractures could arrive without important osteolysis when remodeling with osteopenia was observed.
Femoral bone loss in the femur around the stem may be considered as a risk factor for periprosthetic fracture. There are different causes for femoral bone loss after total hip arthroplasty as osteolysis or remodeling with cortical thinning and osteopenia [4, 5]. We found no information in the literature comparing radiologic long-term changes in the cortical bone after THA with the same type of cemented stem in patients with different bearing surfaces.
We therefore studied a cohort of 327 patients who had been treated for the same disease (osteonecrosis), with a single implant design, with the same operative technique, and who had bilateral arthroplasties one ceramic-ceramic (CoC) and the contralateral ceramic-polyethylene (PE) performed from 1978 to 2000. The first purpose of the study was to determine whether the cumulative long-term periprosthetic fracture were different according to the couple of friction. The secondary purpose was to investigate patient’s factors (age, gender, and cause of osteonecrosis) and arthroplasty’s (osteolysis, osteopenia) factors that could affect the risk of fracture.
Patients and methods
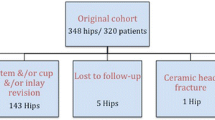
We retrospectively reviewed 327 patients (654 hips; 185 men, 142 women) with bilateral primary THA (one ceramic on ceramic, AL/AL, and the contralateral side ceramic-on-PE, AL/PE) who had THA performed from 1978 to 2000. Osteonecrosis in young patients was usually an indication for a ceramic on ceramic hip. Because there was sometimes concern about fixation of the ceramic cup with cement or with metal back, some patients had one side with ceramic on ceramic and the contralateral with a conventional PE cup. The mean age at surgery was 42 years (range, 27–62 years). The median follow-up was 22 years (range, 15–40 years). The indication for surgery was stage IV osteonecrosis with collapse of the articular surface. Of the initial cohort of 327 patients, 17 patients were lost to follow-up after 25 years of follow-up, and 39 died at an average follow-up of 25 years (range, 13–36 years).
Surgery was performed with a posterolateral approach under general anesthesia. All patients received the same implants except for the cups. The prostheses were manufactured by Ceraver (Ceraver Osteal, Roissy, France). The stem was the same on each side; it was made of anodized titanium alloy (TiAl6V4) and was smooth and always cemented with cement (Palacos G; Heraeus Medical GmbH, Hanau, Germany) containing antibiotics (gentamicin).
Periprosthetic femoral fractures were analyzed based on demographic details (age, gender), timing after the fracture, mechanism of injury, anatomical location. Medical data and radiographs of all patients with periprosthetic fractures were reviewed for this study. Institutional Review Board approval was obtained prior to initiation of the study. Post-operative periprosthetic femoral fractures were classified according to the Vancouver system, which incorporates the site of the fracture, stability of implant, and quality of surrounding bone.
Concerning osteolysis, when a periprosthetic fracture was observed, CT scan was performed on both hips in 28 of the 32 patients with fractures (the patient in a supine position) and in 25 matched patients for follow-up. Hips were scanned from 5-cm proximal to the acetabular component to a point 5-cm distal to the end of the femoral implant as described previously [3]. The maximum thickness of the cuts ranged from 1 to 3 mm. Osteolysis was defined as a newly (on radiographs) developed endosteal bone loss with a diameter greater than 3 mm with an either scalloping or bead-shaped lucency at the cement-bone interface. To calculate the volume of the osteolysis, lesions were identified and traced on each axial cut with use of a semiautomated edge-detection module (Adobe Photoshop; Adobe Systems Software Ireland Ltd., Dublin, Ireland). The areas of the lytic lesions were then calculated from each tracing by determining the number of pixels per square millimeter. The volume between adjacent cuts was calculated by averaging the areas between adjacent cuts multiplied by the thickness of the cuts. Summation of the volumes on each of these cuts was used to determine the total volume of bone loss resulting from lysis as previously described.
Cortical thinning was assessed on the two femurs by comparing the initial and the last follow-up radiographs. Thicknesses of cortices were measured in millimeters (mm) medially and laterally at the same levels in areas without osteolysis. Proximal and distal levels (Figs. 1 and 2) were defined as horizontal lines at the distal end of the minor trochanter and at the tip of the prosthesis perpendicular to the axis of the femur; between these two lines the distance was divided in four equidistance segments to draw the other lines. The intramedullary canal width was also measured on the same levels. The mean annual changes (in millimeters per year) in cortical thickness and canal width were calculated at each level.
Qualitative data (gender, cause of osteonecrosis) were expressed as counts and percentages; quantitative data were expressed by mean ± SD or range. Qualitative data between the two groups were compared with use of the chi-square test or Fisher’s exact test. Kaplan-Meier survivorship analysis, with 95% confidence intervals, was used to estimate the cumulative probability of not having a periprosthetic fracture in the whole series.
Results
There were two intra-operative fractures (0.3%). The median follow-up was 22 years (range, 15–40 years), and at the most recent follow-up, the cumulative number of late (after 7 years of follow-up) post-operative fractures was 32 (5% of 654 hips). The number increased in number with follow-up: 7 fractures (1% of 654 hips) occurred within ten years of THA implantation, 20 (3%) within 20 years, 26 (4%) within 30 years, and 32 (5%) within 40 years. Fractures were unilateral, which means for the 327 patients, a 10% rate of fractures.
More hips with PE liners at the time of index arthroplasty had late periprosthetic fractures than did hips with ceramic liners (10% [31 of 327] compared with 0.3% [one of 327]; odds ratio [OR], 34.14; and 95% confidence interval [CI], 4.6318–251.6640; p = 0.0005). For the 327 hips with CoC as bearing surface and cemented stem, one fracture (0.3%) was observed at 21 years of follow-up; the mechanism of fracture was car accident with high energy traumatism. For the 327 hips with PE liners as bearing surface, the 31 fractures were due to a fall from standing height. Among these 327 primary THA with a PE cup and a cemented stem, the cumulative probabilities of a periprosthetic fracture were 2% (n = 7) between years seven to ten, 6% (n = 20) between years 11 and 20, 8% (n = 25) between years 21 and 30, and 10% (n = 31) between years 31 and 40 (Fig. 3).
When stratified based upon gender, there was no increased risk of post-operative femoral fracture in females: 15 fractures were observed among the 142 women and 17 fractures among the 185 men (p = 0.67). The 163 patients > 42 years of age at operation had no increased risk (p = 0.13) of post-operative femoral fracture (n = 20) when compared with patients ≤ 42 years of age (n = 12).
Cause of osteonecrosis affected the number of periprosthetic fractures. Alcohol (p = 0.04) as the underlying cause of osteonecrosis was associated with a greater risk of fractures as compared with other causes (corticosteroids and sickle cell disease). This may be related to a higher number of falls from standing height observed in these patients. However, fractures occurred only in hips with PE bearings.
Based upon the Vancouver classification, there were 25 type-B1 fractures (with solidly fixed stems), 4 type-C fractures that occurred distal to the tip of the stem without loosening, and 2 type-B3 fractures on loose stems with osteolysis at the site of fracture. Most of fractures (29 among 32 cases) occurred at a site where there was not osteolysis; they occurred at a site with cortical thinning with medullary canal expansion.
No osteolysis was observed on radiographs in the 53 CoC hips (28 patients with a fracture, and 25 matched patients), whereas all of the PE hips demonstrated some osteolysis on radiographs (Figs. 1 and 2). For hips with PE, osteolytic lesions on acetabulum and femur were observed in 100% of the hips with a volume of osteolysis ranging from 20 to 70 cm3 (mean, 39 cm3) when measured on CT scan. For 27 hips, the fracture did not occurred at a site of osteolysis on the femur. However, increased amounts of total (acetabulum and femur) osteolysis (mean 51 cm3; range 41 to 70 cm3) were observed in the PE hips of patients with fractures as compared with amount of osteolysis (mean 31 cm3; range 20 to 38 cm3) in the PE hips of patients without fractures (p = 0.02).
Cortical thinning and intramedullary canal expansion was observed on all THA hips when the first and last follow-up radiographs were compared at all levels. With a mean of 22 years (range, 15–40 years) of follow-up, the mean annual cortical thinning was calculated as 0.0 ± 0.06 mm/year, and the mean intramedullary canal expansion as 0.17 ± 0.11 mm/year. However, we found a significantly (p = 0.021) greater cortical thinning in hips with PE bearings (0.12 ± 0.07 mm/year) as compared with (0.02 ± 0.01 mm/year) on the CoC hips (Figs. 1 and 2). The PE hip had also more intramedullary femoral canal expansion (0.25 ± 0.17 mm/year) compared with the ceramic on ceramic bearings side (0.07 ± 0.06 mm/year; Mann-Whitney U test, p = 0 .015). Cortical thinning and intramedullary canal expansion were correlated (p = 0.024; p = 0.031) to the amount of total osteolysis measured on CT scan.
Discussion
The cumulative risk of late post-operative periprosthetic femoral fracture for patients who were followed during more than 30 years was around 10%, making post-operative periprosthetic fracture one of the most frequent long-term complications of THA in the hips with conventional PE bearings. This is concordance with the increased frequency observed in registries like in Sweden [6], where periprosthetic femoral fractures is the second most common reason for revision.
Although the etiology of periprosthetic fractures is probably multifactorial [7, 8], longer-term changes in bone such as osteolysis may influence the risk of prosthetic fractures. Biological differences in wear products generated by different bearing surfaces may influence differences in the appearance of fractures after THA. However, no one has looked at fractures and bone changes related to different bearing surfaces to see whether the bearing used at the index arthroplasty was associated with a difference in likelihood of periprosthetic fractures in long-term follow-up. We have evaluated the risk of fractures after long-term follow-up in these patients according to their bearing surfaces and investigated CT-based measures of skeletal osteolysis in patients with fractures presenting different bearing surfaces (CoC or PE). We found that ceramic surfaces used in THAs at the index arthroplasty were associated with fewer fractures as compared with PE bearing surfaces. This difference may be related to the reduced amount of osteolysis in patients whose THA included a CoC bearing compared with those with ceramic-on-PE bearings.
Remodeling with osteopenia and cortical thinning was different on each side suggesting that in the same patient with the same stem on each side a different pattern of bone loss distribution may be observed when bearing surfaces are different. On the side with PE, femoral remodeling with bone loss and cortical thinning around was observed; the femur and the mean cortical thinning was seven times greater than on the contralateral side. The contralateral femur with ceramic on ceramic bearing showed less cortical thinning in all studied levels, with a remodeling similar to that observed on femur in natural aging without arthroplasty. This could be explained by a different mechanical transmission of load on the stem by a hard on hard bearing as compared with the elasticity of the PE or explained by a different biologic response to an undetected osteolysis on CT and radiographs.
We note several limitations to our study. First, ours was a retrospective study of a nonrandomized patient population. Thus, our study is a study of association, not causation, and not all important variables have been controlled for. Likewise, activity level was not controlled for or even measured. These factors, and others, could well have influenced the findings. Some patients were lost to follow up during this study; others died. It is possible that some patients had unknown fractures treated in another hospital and not related. Fourth, only patients who had a primary arthroplasty with the same implant during the study period were included, which may have affected the ability to detect differences associated with other implants. However, one of the advantages of our series is that surgery was performed in the same patients with a consistent surgical technique and the same arthroplasty, which reduced some variability. A single implant design (with two different bearing couples) was chosen for this study to minimize variables that might confound analysis.
In summary, as the indications for THA continue to increase in young population with a greater life expectancy, the prevalence of periprosthetic fractures [9, 10] might continue to increase if the risk is linked with follow-up and osteolysis in patients with conventional PE cups. These fractures are challenging to treat [11, 12] and are a source of considerable morbidity and mortality [13, 14] and often. Future studies should quantify the role played by subclinical osteolysis and whether newer bearing couples have an impact on subsequent fracture.
References
Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ (2016) Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J 98–B:461–467
Berry DJ (2003) Periprosthetic fractures associated with osteolysis: a problem on the rise. J Arthroplast 18:107–111
Hernigou P, Zilber S, Filippini P, Poignard A (2009) Ceramic-ceramic bearing decreases osteolysis: a 20-year study versus ceramic polyethylene on the contralateral hip. Clin Orthop Relat Res 467:2274–2280
Sidler-Maier CC, Waddell JP (2015) Incidence and predisposing factors of periprosthetic proximal femoral fractures: a literature review. Int Orthop Sep 39(9):1673–1682
Arabmotlagh M, Sabljic R, Rittmeister M (2006) Changes of the biochemical markers of bone turnover and periprosthetic bone remodeling after cemented hip arthroplasty. J Arthroplast 21:129
Lindahl H, Malchau H, Herberts P, Garellick G (2005) Periprosthetic femoral fractures classification and demographics of 1049 periprosthetic femoral fractures from the Swedish National Hip Arthroplasty Register. J Arthroplast 20:857–865
Cook RE, Jenkins PJ, Walmsley PJ, Patton JT, Robinson CM (2008) Risk factors for periprosthetic fractures of the hip: a survivorship analysis. Clin Orthop Relat Res 466:1652–1656
Ricioli W Jr, Queiroz MC, Guimarães RP, Honda EK, Polesello G, Fucs PM (2015) Prevalence and risk factors for intra-operative periprosthetic fractures in one thousand eight hundred and seventy two patients undergoing total hip arthroplasty: a cross-sectional study. Int Orthop. Oct 39(10):1939–1943
Lindahl H (2007) Epidemiology of periprosthetic femur fracture around a total hip arthroplasty. Injury 38:651–654
Frenzel S, Vécsei V, Negrin L (2015) Periprosthetic femoral fractures—incidence, classification problems and the proposal of a modified classification scheme. Int Orthop. Oct 39(10):1909–1920
Abdel MP, Cottino U, Mabry TM (2015) Management of periprosthetic femoral fractures following total hip arthroplasty: a review. Int Orthop. Oct 39(10):2005–2010
Amenabar T, Rahman WA, Avhad VV, Vera R, Gross AE, Kuzyk PR (2015) Vancouver type B2 and B3 periprosthetic fractures treated with revision total hip arthroplasty. Int Orthop. Oct 39(10):1927–1932
Lindahl H, Oden A, Garellick G, Malchau H (2007) The excess mortality due to periprosthetic femur fracture. A study from the Swedish national hip arthroplasty register. Bone 40:1294–1298
Jakubowitz E, Seeger JB (2015) Periprosthetic fractures: concepts of biomechanical in vitro investigations. Int Orthop Oct 39(10):1971–1979
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hernigou, P., Auregan, J.C., Bastard, C. et al. Higher prevalence of periprosthetic fractures with ceramic on polyethylene hip bearing compared with ceramic on ceramic on the contralateral side: a forty year experience with hip osteonecrosis. International Orthopaedics (SICOT) 42, 1457–1461 (2018). https://doi.org/10.1007/s00264-018-3863-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-018-3863-5