Abstract
Purpose
Three-dimensional (3D) imaging using computed tomography (CT) has made it possible to accurately evaluate ossification of the posterior longitudinal ligament (OPLL). Recently, we developed a novel technique to measure ossification volume using the 3D analysis. The purpose of this study was to investigate the natural course of OPLL and the risk factors for volume progression.
Methods
Forty-one patients (22 males and 19 females) diagnosed with cervical OPLL who had been non-surgically treated were included in this study. We evaluated clinical examination, radiological findings, and the volume of ossified lesions during at least 1-year intervals. Furthermore, we performed risk factor analysis for OPLL volume progression.
Results
The mean ossification volume was 2047.4 ± 1437.3 mm3 in the first examination and 2201.0 ± 1524.1 mm3 in the final examination, indicating a significant increase during the follow-up period (p < 0.001). The mean annual rate of lesion increase was 4.1 ± 2.7%. Univariate regression analysis demonstrated significant relationships between the annual rate of lesion increase and age (β = −0.48; p = 0.001), body weight (BW) (β = 0.36; p = 0.02), and body mass index (BMI) (β = 0.35; p = 0.03). Furthermore, age was the only significant predictor of OPLL progression (R2 = 0.23; p = 0.001) in multivariate liner regression analysis.
Conclusions
Younger age, higher BW, and higher BMI are predictors of OPLL progression. Younger age is the most significant predictor in non-surgically treated patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ossification of the posterior longitudinal ligament (OPLL) is a progressive disease resulting in a narrowed spinal canal. In addition, OPLL has been recognized as one of the main causes of cervical myelopathy [1,2,3]. Several studies demonstrated that approximately 70% of patients showed radiographic evidence of OPLL progression after laminoplasty [4,5,6,7]. Knowing the natural course of OPLL and especially the risk factors of OPLL progression is important for careful observation or indication for surgical treatment. Based on two-dimensional (2D) images of OPLL using plain radiography and plain computed tomography (CT), previous reports described that OPLL progression occurred more frequently in surgically treated cases than in non-surgically treated cases [7, 8]. Because conventional 2D image analysis could not evaluate lesion volume accurately, CT-based 3-dimensional (3D) imaging analysis has been developed and has made evaluation of OPLL accurate [9,10,11]. We have developed a novel technique to measure ossification volume based on the creation of a 3D model using CT images [10, 11].
There have been no reports regarding risk factors for OPLL volume progression in non-surgically treated patients based on 3D image analysis. We conducted an explorative pilot study using novel 3D image analysis to investigate the OPLL progression, and this study aimed to investigate the natural course of OPLL and the risk factors for volume progression.
Materials and methods
This study was approved by the ethics committee of our university hospital. Informed consent was obtained from all patients before enrollment. Forty-one OPLL patients (22 males, 19 females; mean age, 61.8 years; range, 35–80 years) who had been followed conservatively at the university hospital or affiliated hospitals between 2006 and 2015 were included for analysis. Thirty-seven patients had no or slight myelopathy, and conservative treatment included close observation and/or pain management medication. The mean follow-up period from the first examination to the final examination was 25.6 ± 17.1 months (range, 12–84 months). According to the criteria proposed by the Investigation Committee on Ossification of Spinal Ligaments of the Japanese Ministry of Public Health and Welfare based on multi-planar reconstruction CT (MPR-CT), OPLL types were classified as continuous in three patients, segmental in five, and mixed in 33 [12]. After 3D analysis of OPLL, segmental OPLL was reclassified as mixed OPLL for one patient during the follow-up period. The location of ossification was classified as localized upper cervical spine (C1–C4) in four patients, and extended middle to lower cervical spine in 37. We evaluated clinical examination, radiological examination, and 3D measurements of the ossified lesion, and performed risk factor analysis for OPLL progression.
Clinical examinations
Body height, body weight, and body mass index (BMI) were recorded for all patients. We evaluated each patient for coexistence of diabetes mellitus (DM) and family history of OPLL. Neurologic severity was evaluated using the Japanese Orthopedic Association (JOA) score [13], which involves a 17-point instrument. Points are assigned based on the ratings of motor function (upper extremity and lower extremity), sensory function (upper extremity, lower extremity, and trunk), and urinary bladder function.
Radiological examinations
The numbers of ossified vertebrae were recorded using MPR-CT. Ossification thickness and space available for the spinal cord (SAC) were measured using plain CT via the axial view. Spinal canal occupation rates were expressed as the percentage ratio of the maximum thickness of ossification to the midsagittal diameter of the canal using the CT axial view [14]. The C2–C7 lordotic angle was measured between the lower endplates of C2 and C7 on lateral radiographs. Additionally, we evaluated the C2–C7 range of motion (ROM) and segmental ROM at the maximum cord compression level based on flexion and extension radiographs.
Measurement of the ossified lesion
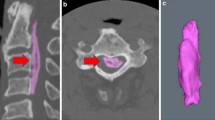
All ossifications of the vertebrae were identified and detached from the posterior aspect of the vertebral body semi-automatically by two observers based on CT images using MIMICS® software (Materialise Japan Co., Ltd., Yokohama, Japan) and a 3D model was created automatically (Fig. 1) [10]. OPLL measurements were obtained twice with an interval of at least one year (initial examination and final examination). The ossified lesion volume was calculated twice during each examination to determine the mean volume and evaluate the intra-observer error. We evaluated the volume change of OPLL using the annual rate of lesion increase. The annual rate of lesion increase between the initial and final measurements was calculated using the following formula: (%): (V2 - V1) ÷ V1 × 100 × 12 ÷ (Int1–2) (volume of the ossified lesion in the initial measurement: V1 [mm3], volume of the ossified lesion in the final measurement: V2 [mm3], interval from the initial to the final measurement: Int1–2 [month]).
a and b Ossification was detached from the affected vertebral body semi-automatically using computed tomography (CT) axial and sagittal planes (so-called segmentation). b Axial planes of CT at C2 (straight line) and C3 (dotted line). c The region of ossification was isolated and a three-dimensional model was created
Statistical analyses
Data were expressed as mean and standard deviation. The change from baseline within each group was evaluated using paired t tests or Wilcoxon signed-ranks tests for clinical and radiological outcomes. Differences between groups were evaluated using Student’s t tests, Mann–Whitney U tests, and one-way analysis of variance (ANOVA) for continuous variables; χ2 tests were used for categorical variables. Stepwise regression analysis was performed to identify variables independently associated with the annual rate of lesion increase as a dependent variable. First, significance of the variable was evaluated using univariate analysis. Next, variables with p < 0.1 were subjected to multivariate analysis; p < 0.05 was considered significant. Beta indicated the standardized partial regression coefficient. Statistical analyses were performed using SPSS software (version 19; SPSS Inc., Chicago, IL, USA).
Results
Body height, body weight, and BMI were 160.7 ± 8.7 cm (range, 145–180 cm), 63.6 ± 11.6 kg (range, 43–87 kg), and 24.5 ± 3.3 kg/m2 (range, 17.6–32.9 kg/m2), respectively. Twelve of the 41 patients (29.3%) had DM and eight (19.5%) had a family history of OPLL. The JOA score was 15.6 in the initial examination and 15.5 in the final examination.
The mean number of ossified vertebrae was 3.7 (range, 1–6 vertebrae) and the mean spinal canal occupation rate was 43.2% (range, 19.0–71.6%). The mean ossification thickness was 5.1 mm (range, 2.4–8.6 mm) and the mean SAC was 6.7 mm (range, 3.4–10.2 mm). The mean C2–C7 lordotic angle, C2–C7 ROM, and segmental ROM were 11.2 °, 32.5 °, and 3.8 ° in the initial examination and 11.5 °, 30.0 °, and 3.7 ° in the final examination, respectively. There were significant differences in the C2–C7 ROM between the initial and final examinations (p < 0.05) (Table 1).
The mean ossification volume was 2047.4 mm3 in the first examination and 2201.0 mm3 in the final examination, indicating a significant mean increase in ossification volume (153.6 mm3; p < 0.001) over the course of 25 months (Table 1). The mean intra-observer intraclass correlation coefficients (ICCs) were 0.995 (range, 0.993–0.996) for observer 1 and 0.997 (range, 0.994–0.999) for observer 2. The mean inter-observer ICC was 0.997 (range, 0.966–0.999). The mean rate of increase in volume between the initial and final examinations was 8.8 ± 8.5% (range, 0.1–36.5%). The mean annual rate of lesion increase was 4.1 ± 2.7% (range, 0.1–10.8%).
The initial JOA score had no correlations with spinal canal occupation rate, thickness of ossification, SAC, initial ossification volume, and location of ossification. While the annual rate of lesion increase had significant correlation with menopausal status (p < 0.05), there were no significant differences in the annual rate of lesion increase between sex, OPLL types, location of ossification, coexistence of DM, and family history of OPLL (Table 2).
To investigate the risk factors for OPLL progression, univariate linear regression analyses demonstrated significant correlations between the annual rate of increase and age (β = −0.48; p = 0.001) (Fig. 2), body weight (β = 0.36; p = 0.02), and BMI (β = 0.35; p = 0.03). Since both BW and BMI, initial and final C2–C7 lordotic angle were highly correlated (r = 0.80; p < 0.001 and r = 0.77; p < 0.001, respectively), BMI and final C2–C7 lordotic angle were excluded from the independent variables, and multivariate linear regression analysis demonstrated that age was the only significant predictor of OPLL progression (R2 = 0.23; p = 0.001) (Table 3).
The mean annual rate of lesion increase was 10.2 ± 1.0% by age 30–49 years (n = 3), 3.9 ± 2.2% by age 50–59 years (n = 14), 3.0 ± 1.9% by age 60–69 years (n = 17), and 4.3 ± 2.6% by age ≥ 70 years (n = 7).In the comparison among the four age groups, the mean ossification volume in the initial examination was 1356.5 ± 612.8 mm3 by age 30–49 years, 2145.0 ± 1252.8 mm3 by age 50–59 years, 1943.0 ± 1738.7 mm3 by age 60–69 years, and 2401.6 ± 1321.9 mm3 by age ≥ 70 years. There was no significant difference of the ossification volume in the initial examination among the four age groups, whereas there were significant differences in the annual rate of lesion increase between the 30–49 years age group and other age groups (p < 0.05).
Illustrative case
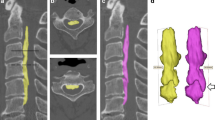
A 62-year-old man with continuous OPLL (Fig. 3a) had slight numbness in both hands and a JOA score of 16. In the 3D imaging analysis, the ossified lesion volume was 3654.1 mm3 in the initial examination (Fig. 3c). Three years later, OPLL had increased 12.0 mm in the craniocaudal direction, and the ossified lesion volume increased to 4092.7 mm3 (Fig. 3d). The annual rate of lesion increase was 4.0%.
a Plain radiography of a 62-year-old man with continuous ossification of the posterior longitudinal ligament (OPLL) at C2–C4. b Plain radiography at 3 years after initial examination showed approximately 10 mm of OPLL progression in the longitudinal axis. The arrows indicate the edge of OPLL. c The length and volume of the ossified lesion were 54.4 mm and 3654.1 mm3 according to three-dimensional (3D) imaging analysis in the initial examination. d Three years later, OPLL had grown 12.0 mm in the craniocaudal direction, and the volume of the ossified lesion increased to 4092.7 mm3. The annual rate of lesion increase was 4.0%. 3D imaging analysis revealed growth of not only the cranial part (arrow) but also the caudal part (arrowhead)
Discussion
According to previous reports, risk factors for OPLL progression after surgical treatment are younger age at surgery, continuous or mixed type of OPLL, higher JOA score at last follow-up, and wide anterior-posterior width of the SAC [2, 4,5,6, 15, 16]. In particular, several studies indicated that younger age was the most significant risk factor for OPLL progression [4,5,6, 16]. However, Taketomi and Kajiura et al. investigated OPLL progression in non-surgically treated patients and noted no correlation between age and OPLL progression [17, 18]. Because most previous studies have reported OPLL progression according to 2D imaging analysis using plain radiographs or conventional CT, accurate measurement was difficult and it was more difficult to clarify the risk factors for OPLL progression. Therefore, 3D imaging analysis using CT must be suited for measuring OPLL volume; we have developed a novel measurement technique using 3D CT [10, 11].
In the present study, the mean annual rate of lesion increase was significantly higher for those in the 30–49 years age group, and multivariate linear regression analysis showed that younger age was the only risk factor for OPLL progression. The results of the present study indicate that younger age is the best predictor of OPLL progression and reflect the natural course of OPLL. Therefore, we consider the natural course of non-surgically treated OPLL as follows: 1) the volume of OPLL grows rapidly in those during the ages of 30–49 years, 2) OPLL progression decelerates after age of 50 years and the aging process tends to slow the OPLL progression throughout the lifetime. There are some hypotheses about why younger patients increase risk for OPLL progression: high bioactivity, large biomechanical stress such as wider ROM and increased load of the cervical spine [3].
Previous reports showed a significant relationship between obesity, glucose intolerance, and presence of OPLL [2, 19, 20]. Obesity and upregulation of insulin production have been shown to be correlated with the extent of OPLL [19]. The present study demonstrated that obesity (body weight and BMI) are significant predictors of OPLL progression in univariate linear regression analyses. To the best of our knowledge, no reports have described the relationship between obesity and OPLL progression. It is well known that obesity causes insulin resistance and glucose intolerance, and insulin is thought to be an osteogenic factor of bone anabolism [21]. Akune et al. [19] reported that the upregulation of insulin production due to the impairment of insulin action may stimulate osteoprogenitor cells to induce ossification. These metabolism-related factors could affect the presence and volume progression of OPLL. Moreover, the annual rate of lesion increase was significantly higher in pre-menopausal status, but there were only three pre-menopausal women in this subject. Sex hormone may affect the OPLL progression, and further study is necessary to clarify the findings.
Several reports described that OPLL progression occurred more commonly in patients with mixed or continuous type OPLL than in those with segmental type OPLL [2, 4,5,6]. These types were classified according to the conventional criteria based on plain radiographs. Because Chang et al. [22] and Fujimori et al. [23] reported that many cases of continuous ossification classified by radiography were reclassified as the mixed type based on CT evaluation, we believe that additional 3D imaging analyses according to OPLL classification criteria based on MPR-CT are necessary. In the present study, the continuous type indicated bridging all levels of ossification and tended to demonstrate lower annual rate of OPLL increase. Using 3D imaging analysis, we previously reported that additional posterior instrumented fusion following laminoplasty, which could be considered as a stabilizing effect, significantly suppressed OPLL progression compared to laminoplasty alone [11]. Therefore, we hypothesize that OPLL progression of the continuous type classified by MPR-CT can be restricted by the stabilizing effect of bridging ossified lesions.
OPLL has been most commonly treated with posterior decompression surgery such as laminoplasty for patients with the K-line (+) type of OPLL, which has been reported to be a safe procedure with satisfactory long-term outcomes [5, 24]. However, we have recommended laminoplasty concomitant with posterior instrumented fusion for patients with the K-line (−) type of OPLL [25], which suppressed OPLL progression [11]. The results of the present study suggest that posterior decompression concomitant with fusion might be considered for young, especially those 50 years or younger, and/or obese patients with a relatively large volume of OPLL. Furthermore, even with conservative treatment, special attention should be given to ossified volume progression or myelopathic signs in patients with these risk factors of OPLL progression.
Limitations of the present study
During our 3D analysis, the process used for identification of ossification was semi-automatic; therefore, measurement errors may have occurred. However, we believe that our 3D analysis was satisfactorily accurate and valid because of the high intraobserver and interobserver ICCs. Other limitations were the small number of patients and short follow-up period because cervical OPLL is a relatively rare disease, especially in patients younger than 50 years. Therefore, we plan to perform a long-term longitudinal analysis with a larger number of patients as a future assignment. However, this is the first pilot study investigating the natural course of cervical OPLL focused on lesion volume progression using a novel 3D imaging analysis in non-surgically treated patients, and we believe that the results of this study provide informative data for physicians.
Conclusions
The present study revealed that younger age, higher body weight, and higher BMI are significant predictors of OPLL progression in non-surgically treated patients; younger age is the most important predictor. Moreover, the annual rate of OPLL progression tends to gradually decrease for patients older than 50 years. These findings reflect the natural course of OPLL, which is important in the clinical management of patients with the aforementioned risk factors.
References
Iwasaki M, Okuda S, Miyauchi A, Sakaura H, Mukai Y, Yonenobu K, Yoshikawa H (2007) Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: part 1: clinical results and limitations of laminoplasty. Spine (Phila Pa 1976) 32:647–653. https://doi.org/10.1097/01.brs.0000257560.91147.86
Fragen KM, Cox JB, Hoh DJ (2012) Does ossification of the posterior longitudinal ligament progress after laminoplasty? Radiographic and clinical evidence of ossification of the posterior longitudinal ligament lesion growth and the risk factors for late neurologic deterioration. J Neurosurg Spine 17:512–524. https://doi.org/10.3171/2012.9.SPINE12548
Matsunaga S, Nakamura K, Seichi A et al (2008) Radiographic predictors for the development of myelopathy in patients with ossification of the posterior longitudinal ligament: a multicenter cohort study. Spine (Phila Pa 1976) 33:2648–2650. https://doi.org/10.1097/BRS.0b013e31817f988c
Kawaguchi Y, Kanamori M, Ishihara H, Nakamura H, Sugimori K, Tsuji H, Kimura T (2001) Progression of ossification of the posterior longitudinal ligament following en bloc cervical laminoplasty. J Bone Joint Surg Am 83:1798–1802
Iwasaki M, Kawaguchi Y, Kimura T, Yonenobu K (2002) Long-term results of expansive laminoplasty for ossification of the posterior longitudinal ligament of the cervical spine: more than 10 years follow up. J Neurosurg 96:180–189
Hori T, Kawaguchi Y, Kimura T (2007) How does the ossification area of the posterior longitudinal ligament thicken following cervical laminoplasty? Spine (Phila Pa 1976) 32:E551–E556. https://doi.org/10.1097/BRS.0b013e31814614f3
Matsunaga S, Sakou T, Taketomi E, Komiya S (2004) Clinical course of patients with ossification of the posterior longitudinal ligament: a minimum 10-year cohort study. J Neurosurg 100:245–248
Takatsu T, Ishida Y, Suzuki K, Inoue H (1998) Radiological study of cervical ossification of the posterior longitudinal ligament. J Spinal Disord 12:271–273
Fujimori T, Iwasaki M, Nagamoto Y et al (2012) Three-dimensional measurement of growth of ossification of the posterior longitudinal ligament. J Neurosurg Spine 16:289–895. https://doi.org/10.3171/2011.11.SPINE11502
Izumi T, Hirano T, Watanabe K, Sano A, Ito T, Endo N (2013) Three-dimensional evaluation of volume change in ossification of the posterior longitudinal ligament of the cervical spine using computed tomography. Eur Spine J 22:2569–2574. https://doi.org/10.1007/s00586-013-2989-9
Katsumi K, Izumi T, Ito T, Hirano T, Watanabe K, Ohashi M (2016) Posterior instrumented fusion suppresses the progression of ossification of the posterior longitudinal ligament: a comparison of laminoplasty with and without instrumented fusion by three dimensional analysis. Eur Spine J 25:1634–1640. https://doi.org/10.1007/s00586-015-4328-9
Investigation Committee on OPLL of the Japanese Ministry of Public Health and Welfare (1981) The ossification of the posterior longitudinal ligament of the spine (OPLL). Nihon Seikeigeka Gakkai Zasshi 55:425–440
Japanese Orthopaedic Association (1994) Scoring system for cervical myelopathy. J Jpn Orthop Assoc 68:134–147 (in Japanese)
Jayakumar PN, Kolluri VR, Vasudev MK, Srikanth SG (1996) Ossification of the posterior longitudinal ligament of the cervical spine in Asian Indians: a multiracial comparison. Clin Neurol Neurosurg 98:142–148. https://doi.org/10.1016/0303-8467(96)00004-2
Tokuhashi Y, Ajiro Y, Umezawa N (2009) A patient with two re-surgeries for delayed myelopathy due to progression of ossification of the posterior longitudinal ligaments after cervical laminoplasty. Spine (Phila Pa 1976) 34:E101–E105. https://doi.org/10.1097/BRS.0b013e31818a3135
Chiba K, Yamamoto I, Hirabayashi H, Iwasaki M, Goto H, Yonenobu K, Toyama Y (2005) Multicenter study investigating the postoperative progression of ossification of the posterior longitudinal ligament in the cervical spine: a new computer-assisted measurement. J Neurosurg Spine 3:17–23
Taketomi E (1997) Progression of ossification of the posterior longitudinal ligament in the cervical spine. J Spine Res 8:359–366 (in Japanese)
Kajiura K, Ikata T, Katoh S, Sairyo K, Chikawa T, Hamada Y (1998) The progression of ossification of the posterior longitudinal ligament: a long-term follow-up study of more than 10 years (author’s translation). Investigation committee 1998 report on the ossification of the spinal ligaments of the Japanese Ministry of Public Health and welfare. Springer, Tokyo, pp 146–148 (in Japanese)
Akune T, Ogata N, Seichi A, Ohnishi I, Nakamura K, Kawaguchi H (2001) Insulin secretory response is positively associated with the extent of ossification of the posterior longitudinal ligament of the spine. J Bone Joint Surg Am 83-A:1537–1544
Shingyouchi Y, Nagahama A, Niida M (1996) Ligamentous ossification of the cervical spine in the late middle-aged Japanese men. Its relation to body mass index and glucose metabolism. Spine (Phila Pa 1976) 21:2474–2478
Thomas DM, Hards DK, Roġers SD, Ng KW, Best JD (1997) Insulin and bone, clinical and scientific view. Endocrinol Metab North Am 4:5–17
Chang H, Kong CG, Won HY, Kim JH, Park JB (2010) Inter- and intra-observer variability of a cervical OPLL classification using reconstructed CT images. Clin Orthop Surg 2:8–12
Fujimori T, Iwasaki M, Nagamoto Y et al (2012) Three-dimensional measurement of intervertebral range of motion in ossification of the posterior longitudinal ligament: are there mobile segments in the continuous type? J Neurosurg Spine 17:74–81
Matsumoto M, Chiba K, Toyama Y (2012) Surgical treatment of ossification of the posterior longitudinal ligament and its outcomes: posterior surgery by laminoplasty. Spine (Phila Pa 1976) 37:E303–E308. https://doi.org/10.1097/BRS.0b013e318239cca0
Fujiyoshi T, Yamazaki M, Kawabe J et al (2008) A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine (Phila Pa 1976) 33:E990–E993. https://doi.org/10.1097/BRS.0b013e318188b300
Funding
This work was supported by a grant-in-aid from the Investigation Committee on the Ossification of the Spinal Ligaments of the Japanese Ministry of Health, Labor, and Welfare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
ESM 1
(XLSX 23 kb)
Rights and permissions
About this article
Cite this article
Katsumi, K., Watanabe, K., Izumi, T. et al. Natural history of the ossification of cervical posterior longitudinal ligament: a three dimensional analysis. International Orthopaedics (SICOT) 42, 835–842 (2018). https://doi.org/10.1007/s00264-017-3667-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3667-z