Abstract
Purpose
Post-operative surgical site infection (SSI) is one of the most significant complications after instrumented spinal surgery. However, implant retention feasibility for early-onset multidrug-resistant SSI is still controversial. We aimed to verify our therapeutic strategy, surgical debridement with implant retention and long-term antimicrobial therapy for post-operative early-onset multidrug-resistant SSI.
Methods
We retrospectively analyzed the clinical course of 11 cases [eight men and three women, with a mean age of 70.4 (54–82) years] with early-onset multidrug-resistant SSI out of 409 consecutive cases of spinal instrumentation surgery performed between 2007 and 2013 at our institution.
Results
The median duration of follow-up was 868 (178–1,922) days. All SSIs were controlled, without recurrence during follow-up. The microbial pathogens were methicillin-resistant Staphylococcus aureus (seven cases), multidrug-resistant Corynebacterium (two cases), methicillin-resistant Staphylococcus epidermidis (one case), and methicillin-resistant coagulase-negative Staphylococcus aureus (one case). The mean duration from SSI diagnosis to surgery was 2.9 (1–6) days. Ten patients underwent surgical debridement with implant retention. No patients required multiple operations. All patients were given antimicrobial treatments. Mean duration of intravenous antimicrobials (vancomycin, vancomycin+ piperacillin/tazobactam, or gentamicin) was 66.5 (12–352) days and 336 (89–1,673) days for oral antimicrobials (rifampicin + sulfamethoxazole/trimethoprim, sulfamethoxazole/trimethoprim, or minomycin). The mean duration of clinical signs and symptom recovery was 31.0 (7–73) days, and the mean time for normalization of C-reactive protein was 54.5 (7–105) days.
Conclusions
Early-onset multidrug-resistant SSI was successfully treated by surgical debridement with implant retention and long-term antimicrobial therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Post-operative surgical site infection (SSI) is one of the most significant complications after spinal instrumentation surgery, and it is closely related with morbidity and mortality. The incidence of SSI after spinal implant surgery reported in the literature varies from 0.7 to 4.2 % [1–5]. According to several recent studies, most patients that develop SSI require an intensive debridement treatment and antibiotic therapy for SSI eradication [1–4, 6–13]. In many cases, implant removal is particularly important for the management of SSIs in patients who undergo spinal instrumentation surgery.
The treatment strategy depends largely on the timing of SSI, which is classified as either early or late onset. Many previous studies reported that an intensive debridement and implant removal were essential for the management of late-onset SSI because the biofilm on the surface of implants resists antimicrobial therapy and the host’s immune defense mechanisms, such as antibodies and phagocytes [1–3, 6, 7]. In such cases, the implant removal did not lead to post-operative malalignment because the bone union was almost complete. In contrast, patients with early-onset SSI do not generally obtain bone union; therefore, removal of the spinal implant may lead to post-operative malalignment and pseudarthrosis. Consequently, revision surgeries to insert new instrumentation or prolonged immobility to achieve the solid fusion without implant are required after the improvement of SSI [1, 9–11, 13].
To address these issues, we have treated SSI patients with surgical debridement and irrigation with retention of the spinal implant and long-term antimicrobial therapy since 2007. Recently, several authors have reported high success rates of debridement, implant retention, and systematic antimicrobial therapy for patients with early SSI. Nowadays, multidrug-resistant bacteria are more frequently identified as the pathogenic bacteria in SSIs. Furthermore, an intensive debridement, along with implant removal, is conventionally required in such cases [2–4, 6, 7, 10, 14]. However, there have been few reports focusing on multidrug-resistant SSI. Therefore, there is no consensus on preferred surgical and medical treatment strategies, in particular, on surgical procedures. Thus, whether retention or removal of spinal implants is the best-suited strategy is still controversial. In the current study, we aimed to verify our therapeutic strategy for SSI. In addition, to our knowledge, this is the first study to focus on multidrug-resistant SSI.
Materials and methods
Patient population
A total of 409 consecutive patients, who underwent spinal instrumentation surgery between 2007 and 2013 were retrospectively analyzed. Eighteen patients (4.4 %) presented SSI. Of those, we enrolled 11 patients with SSI caused by multidrug-resistant bacteria within 90 days after the index surgery. There were eight men and three women, and their mean ± standard deviation (SD) age at the time of surgery was 70.4 ± 8.7 (range, 54–82) years.
All index surgeries were performed by three senior orthopedic spine surgeons; all surgical procedures were performed in the same block of operating rooms. Standard preparation of the surgical site was performed using povidone iodine. The surgical site was surrounded by sterile surgical drapes. Cefazolin was used for antibiotic prophylaxis. Prophylaxis began at least 30 minutes before skin incision, and it was re-administered every six hours or 1,500 ml of blood loss during the procedure. Additionally, cefazolin was administered every eight hours up to 48 hours post-operatively.
SSI
The diagnosis of SSI was based on clinical symptoms (temperature elevation, back pain, and wound dehiscence associated with drainage of purulent fluid), findings on diagnostic imaging studies (radiographs and magnetic resonance imaging [MRI]), laboratory examinations (mean white blood cell [WBC] count, C-reactive protein [CRP] levels and erythrocyte sedimentation rate [ESR]), and positive cultures (obtained from blood, wound discharge or intra-operative tissue specimens).
Surgery and antimicrobial therapy
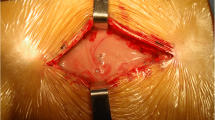
Debridement surgery was recommended for all suspected cases of SSI. During surgery, the spinal implant and the intervertebral disc cage used for interbody fusion were routinely retained, and any necrotic tissue and all grafting bone in the facet joint were removed. The wound was cleaned by a lavage with at least 6 l of normal saline solution without antimicrobials. Then, the wound was primarily closed over suction drains, which were removed when the drainage decreased to less than 30 ml/day.
Antimicrobials were not administered until after blood, wound discharge or intra-operative cultures were obtained. Based on microbial identification and determination of sensitivity, intravenous antimicrobials, consisting of broad-spectrum coverage in combination with or without oral antimicrobials were administered until the CRP level decreased to 1.0 mg/dl or less. Then, patients only received oral antimicrobials for a period of three months or more.
Clinical and radiological evaluation
The resolution of the SSI was established by improvement of clinical signs and symptoms such as pain relief, wound healing, and decline of fever, in addition to normalization of laboratory parameters such as WBC, CRP and ESR lasting more than two weeks. The timing of debridement surgery, the individual durations of intravenous and oral antimicrobial treatments, as well as the duration of the entire treatment were analyzed. The content of antimicrobial treatment, and SSI recurrence were evaluated. MRI was also used to evaluate the presence of abscess in the iliopsoas or the intervertebral region proximal to the surgical site, as required.
Radiological evaluation, including evidence of pseudarthrosis, loss of primary fixation, and implant failure, was performed by the anteroposterior and lateral radiographs at final follow-up. Radiographic fusion was defined as the observation of continuous bridging bone on computed tomography (CT), and lack of instability on dynamic lateral radiographs.
Results
Patient comorbidities were as follows: diabetes mellitus (three cases), hypertension (three cases), rheumatoid arthritis (two cases), alcohol-related liver disease (two cases), and obesity (one case). Additionally, three patients (27 %) had undergone at least one previous surgery at the same spinal level. The preoperative diagnoses of patients who subsequently developed infections consisted of eight patients with lumbar spinal stenosis, two with thoracolumbar or lumbar burst fracture, and one with vertical and atlanto-axial subluxation. All cases of infections associated with spinal instrumentation involved a posterior approach to the spine, whereas no cases of infection resulted from anterior approach. All patients underwent posterior instrumented fusion using the pedicle screw and rod system. Furthermore, 10 of 11 patients (91 %) underwent transforaminal lumbar interbody fusion. The mean number of fused segments was 2.8 ± 2.1 (range, 1–8) segments (Table 1).
The most common microbial pathogens were methicillin-resistant Staphylococcus aureus (MRSA) (seven cases [64 %]), multidrug-resistant Corynebacterium (two cases [18 %]), methicillin-resistant Staphylococcus epidermidis (one case [9 %]) and methicillin-resistant coagulase-negative Staphylococcus aureus (one case [9 %]). Infection was monomicrobial in ten patients and polymicrobial in one patient. The mean duration from index surgery to the day of SSI diagnosis was 21.0 ± 7.2 (range, 11–37) days.
Ten patients (91 %) underwent surgical debridement with implant retention, and the mean duration from the day of SSI diagnosis to surgery was 2.9 ± 1.5 (range, 1–6) days. Furthermore, none of the patients required multiple surgery. In one case, surgical debridement was not performed because of dramatic improvement of clinical signs and symptoms and normalization of CRP levels in response to antimicrobial therapy.
All patients were given intravenous antimicrobials. Furthermore, all but one patient, who was diagnosed with superficial SSI based on intra-operative findings, were also given oral antimicrobials. Intravenous antimicrobial therapy with vancomycin (five cases), vancomycin + piperacillin/tazobactam (four cases), or gentamicin (two cases) had a mean duration of 66.5 ± 98.2 (range, 12–352) days (Fig. 1). In two of nine cases, vancomycin was substituted by linezolid (Case 1) or teicoplanin (Case 6) because treatment with vancomycin was affecting the renal function in these two cases. Oral antimicrobial therapy with rifampicin + sulfamethoxazole/trimethoprim (six cases), minomycin (three cases), or sulfamethoxazole/trimethoprim (one case) had a mean duration of 336 ± 477 (range, 89–1,673) days.
An illustrative case report: Case 5. A 71-year-old woman who presented with intermittent claudication. a–c Pre-operative radiographs and magnetic resonance imaging (MRI) show L4 spondylolisthesis and L3/4, L4/5 lumbar spinal stenosis (LSS). d She underwent L3/4 postero-lateral fusion and L4/5 transforaminal lumbar interbody fusion (TLIF). e–g Thirty-seven days after surgery, she presented fever and increased back pain. On MRI, abscess formation was evidenced in the epidural space. Three days after the diagnosis, surgical debridement and irrigation with implant retention were performed. The bacterial pathogen isolated from the intra-operative culture was methicillin-resistant Staphylococcus aureus. Vancomycin was administered for 37 days until the C-reactive protein (CRP) level decreased to 1.0 mg/dl or less. Then, the patient began oral antimicrobial therapy with minomycin only. The treatment was continued for 374 days. As a result, the fever and back pain resolved 13 days after the debridement surgery. Furthermore, CRP levels returned to normal 35 days after surgery. h–j One year after surgery, she presented L2/3 lumbar disc herniation at the adjacent segment and required additional fusion surgery (L2/3 TLIF). k, l At the final follow-up, solid fusion was obtained
The mean duration of recovery of clinical symptoms and signs from SSI diagnosis was 31.0 ± 23.3 (range, 7–73) days. Furthermore, CRP levels of ten patients normalized, except for one patient, who had rheumatoid arthritis. The mean duration for CRP normalization was 54.5 ± 29.8 (range, 7–105) days. The mean hospital stay from the time of SSI diagnosis to hospital discharge was 100.3 ± 79.1 (range, 15–212) days. Of 11 patients that presented SSI, nine patients (82 %) were discharged from the hospital to their homes and usual activities. However, two patients could not be discharged to their homes because of disuse syndrome and were transferred to another hospital. Subsequently, these two patients were able to return to their homes. The median duration of follow-up in this study was 868 ± 686 (range, 178–1,922) days. All cases of SSI were resolved and none of the patients presented recurrence during the follow-up period.
Pseudarthrosis was documented in one patient (9 %) who presented a radiolucent area around the distal pedicle screws and the intervertebral cage in both radiograph and CT at final follow-up; however, there were no clinical symptoms or signs suggesting the need for additional treatment (Table 2).
Discussion
The results of the present case series indicate that 11 consecutive patients with multidrug-resistant SSI were successfully treated with surgical debridement and antimicrobial therapy. Further, there were no recurrences of SSI during the follow-up period. Consequently, all patients experienced improvements of their primary complaint; low back pain and leg pain were the main surgical indications.
Eradication of late-onset SSI when implants are retained is fraught with potential difficulties because implants can be colonized by bacteria-harboring biofilms, which provide relative immunity against the host’s defense mechanisms and antimicrobial pharmacotherapy [2, 3, 6, 7]. Therefore, surgical debridement and implant removal have been considered as the “gold standard” treatment for SSIs [1, 6–11, 13]. In contrast, regarding early-onset SSI, general consensus has not been achieved and a treatment gold standard has yet to be established. Implant removal was considered the best option in terms of SSI control [1, 7]. However, in most of the cases with early-onset SSI, solid fusion was not achieved yet. Therefore, removal of implants increases the risk of fusion failure, which could lead to revision surgery. From the multivariate analysis of a cohort of 81 patients with SSI after spinal instrumentation surgery, Kowalski et al. [7] concluded that oral antimicrobial suppression therapy and implant removal were significant prognostic factors for early-onset and late-onset SSI, respectively. In recent years, several reports detailing the management of early-onset SSI have advocated surgical debridement with implant retention and systematic antimicrobial therapy [2–4, 6, 7, 10, 11, 13, 14]. Qualie [13] mentioned that the implant retention is essential to achieve the satisfactory results of early-onset SSI treatment, the main thrust is early diagnosis and treatment. Radial debridement is especially required for deep infections because these penetrate the deep fascia muscle layer and around spinal implants. Mok et al. [2] reported a study of 16 patients with SSI after spinal surgery that were treated by debridement and antimicrobial therapy, which allowed implant preservation. Treatment included six weeks of intravenous antimicrobials and long-term oral antimicrobials. None of the patient with early-onset SSI required implant removal, whereas all patients with late SSI required implant removal. In their study, there were six patients with multidrug-resistant SSI; however, there was no specific reference to their outcome. Closed suction irrigation system and vacuum-assisted wound closure are also effective for treating wound complications after spinal instrumented surgery and they are thought to contribute to implant retention [11, 13].
To the best of our knowledge, no previous report has referred to the feasibility of implant retention in cases of early-onset multidrug-resistant SSI after spinal instrumentation surgery. In the present study, 11 consecutive patients with multidrug-resistant SSI were treated by surgical debridement with implant retention and antimicrobial therapy. None of the patients presented recurrence, nor did they require revision surgeries to achieve spinal fusion. Based on these findings, the authors emphasize the feasibility of implant retention for early-onset multidrug-resistant SSI after spinal instrumentation surgery. However, for successful SSI control, long-term intensive antimicrobial therapy holds a key role in our treatment protocol.
Reports in the literature regarding antimicrobial therapy for SSI related to spinal surgery revealed a varying range of treatments and recommendations; however, the standard treatment strategy has not yet been established. Rihn et al. [9] recommended a minimum six weeks of intravenous antimicrobials followed by oral antimicrobials. In the report by Hong et al. [15], although CRP levels did not return to normal limits after four to six weeks of intravenous antimicrobials, levels did decrease progressively and returned to normal after a mean antimicrobial treatment duration of six weeks. Kowalski et al. [7] advocated the administration of intravenous antimicrobials for at least two weeks and the use of oral antimicrobials for at least six months until spine fusion occurs in patients with early SSI. In cases of multidrug-resistant SSI, Meredith et al. [12] recommended that intravenous antimicrobial therapy should be continued for eight weeks. In our study, intravenous antimicrobials combined with or without oral antimicrobials were administered until the CRP level decreased to less than approximately 1.0 mg/dl. Then, patients were treated with oral antimicrobials only for at least three months or more. All cases of SSI that were managed by our protocol were controlled and did not recur. Therefore, we suggest that oral antimicrobials should be continued for at least three months after CRP levels decrease to 1.0 mg/dl or less to prevent an infection flare-up for as long as possible. This is particularly relevant in cases of immunocompromised patients.
An issue for the treatment of MRSA strains is the relatively limited options for oral antimicrobial therapy because many strains are multidrug-resistant. When choosing oral antimicrobials for MRSA, the chosen drug should be bactericidal against microorganisms that grow in matrix-enclosed biofilms adherent to surface of implants, have good oral bioavailability, and penetrate bone and joint tissues. Rifampicin, clindamycin, sulfamethoxazole/trimethoprim, quinolones, fusidic acid and linezolid are the only drugs that have excellent bioavailability for MRSA when given orally to treat bone infections [16]. Among them, rifampicin plays an important role [17]. It has all the qualities needed to treat biofilm organisms, is active against MRSA, and has excellent bioavailability and tolerability [16, 17]. However, since the single use of rifampicin causes rapid development of resistance, it should be administered with another anti-staphylococcal agent with similar pharmacokinetic properties [16–19].
Previously, several reports also referred to the effectiveness of sulfamethoxazole/trimethoprim on SSI after orthopaedic surgery [20, 21]. The combination of rifampicin and sulfamethoxazole/trimethoprim blocks the folate biosynthetic pathway, resulting in significantly improved therapeutic activity against MRSA in both in vivo and in vitro studies [22]. In a case reported by Nemoto et al. [23], MRSA prosthetic arthritis and osteomyelitis were successfully treated with a combination of vancomycin and rifampicin for 17 weeks, followed by sulfamethoxazole/trimethoprim. Fujino et al. [24] also reported a case with MRSA tricuspid valve infective endocarditis treated by combination therapy with vancomycin, rifampicin, and sulfamethoxazole/trimethoprim. Based on the evidence described above, rifampicin and sulfamethoxazole/trimethoprim were included as the standard drugs in our protocol, in addition to other oral antimicrobials.
The main limitations to this study are its retrospective design and small sample size. However, even for the patients with multidrug-resistant SSI managed according to our protocol (surgical debridement with implant retention and long-term antimicrobial therapy), none presented recurrence of SSI or needed multiple surgeries to control infection. Therefore, we propose that our protocol can be beneficial for the treatment of early-onset multidrug-resistant SSI after spinal instrumentation surgery.
References
Hedequist D, Haugen A, Hresko T, Emans J (2009) Failure of attempted implant retention in spinal deformity delayed surgical site infections. Spine 34:60–64. doi:10.1097/BRS.0b013e31818ed75e
Mok JM, Guillaume TJ, Talu U, Berven SH, Deviren V, Kroeber M, Bradford DS, Hu SS (2009) Clinical outcome of deep wound infection after instrumented posterior spinal fusion: a matched cohort analysis. Spine 34:578–583. doi:10.1097/BRS.0b013e31819a827c
Weinstein MA, McCabe JP, Cammisa FP Jr (2000) Postoperative spinal wound infection: a review of 2,391 consecutive index procedures. J Spinal Disord 13:422–426
Pull ter Gunne AF, van Laarhoven CJ, Cohen DB (2010) Incidence of surgical site infection following adult spinal deformity surgery: an analysis of patient risk. Eur Spine J 19:982–988. doi:10.1007/s00586-009-1269-1
Glassman SD, Dimar JR, Puno RM, Johnson JR (1996) Salvage of instrumental lumbar fusions complicated by surgical wound infection. Spine 21:2163–2169
Ahmed R, Greenlee JD, Traynelis VC (2012) Preservation of spinal instrumentation after development of postoperative bacterial infections in patients undergoing spinal arthrodesis. J Spinal Disord Tech 25:299–302. doi:10.1097/BSD.0b013e31821fbf72
Kowalski TJ, Berbari EF, Huddleston PM, Steckelberg JM, Mandrekar JN, Osmon DR (2007) The management and outcome of spinal implant infections: contemporary retrospective cohort study. Clin Infect Dis 44:913–920. doi:10.1086/512194
Viola RW, King HA, Adler SM, Wilson CB (1997) Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine 22:2444–2450, discussion 2450–2441
Rihn JA, Lee JY, Ward WT (2008) Infection after the surgical treatment of adolescent idiopathic scoliosis: evaluation of the diagnosis, treatment, and impact on clinical outcomes. Spine 33:289–294. doi:10.1097/BRS.0b013e318162016e
Bachy M, Bouyer B, Vialle R (2012) Infections after spinal correction and fusion for spinal deformities in childhood and adolescence. Int Orthop 36:465–469. doi:10.1007/s00264-011-1439-8
Gerometta A, Rodriguez Olaverri JC, Bitan F (2012) Infections in spinal instrumentation. Int Orthop 36:457–464. doi:10.1007/s00264-011-1426-0
Meredith DS, Kepler CK, Huang RC, Brause BD, Boachie-Adjei O (2012) Postoperative infections of the lumbar spine: presentation and management. Int Orthop 36:439–444. doi:10.1007/s00264-011-1427-z
Quaile A (2012) Infections associated with spinal implants. Int Orthop 36:451–456. doi:10.1007/s00264-011-1408-2
Nunez-Pereira S, Pellise F, Rodriguez-Pardo D, Pigrau C, Bago J, Villanueva C, Caceres E (2013) Implant survival after deep infection of an instrumented spinal fusion. Bone Jt J 95-B:1121–1126. doi:10.1302/0301-620X.95B8.30784
Hong HS, Chang MC, Liu CL, Chen TH (2008) Is aggressive surgery necessary for acute postoperative deep spinal wound infection? Spine 33:2473–2478. doi:10.1097/BRS.0b013e3181894ff0
Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ (2009) Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis 48:922–930. doi:10.1086/597291
Falagas ME, Bliziotis IA, Fragoulis KN (2007) Oral rifampin for eradication of Staphylococcus aureus carriage from healthy and sick populations: a systematic review of the evidence from comparative trials. Am J Infect Control 35:106–114. doi:10.1016/j.ajic.2006.09.005
Samuel JR, Gould FK (2010) Prosthetic joint infections: single versus combination therapy. J Antimicrob Chemother 65:18–23. doi:10.1093/jac/dkp398
Walsh TJ, Standiford HC, Reboli AC, John JF, Mulligan ME, Ribner BS, Montgomerie JZ, Goetz MB, Mayhall CG, Rimland D et al (1993) Randomized double-blinded trial of rifampin with either novobiocin or trimethoprim-sulfamethoxazole against methicillin-resistant Staphylococcus aureus colonization: prevention of antimicrobial resistance and effect of host factors on outcome. Antimicrob Agents Chemother 37:1334–1342
Adra M, Lawrence KR (2004) Trimethoprim/sulfamethoxazole for treatment of severe Staphylococcus aureus infections. Ann Pharmacother 38:338–341. doi:10.1345/aph.1D156
Stein A, Bataille JF, Drancourt M, Curvale G, Argenson JN, Groulier P, Raoult D (1998) Ambulatory treatment of multidrug-resistant Staphylococcus-infected orthopedic implants with high-dose oral co-trimoxazole (trimethoprim-sulfamethoxazole). Antimicrob Agents Chemother 42:3086–3091
Elwell LP, Wilson HR, Knick VB, Keith BR (1986) In vitro and in vivo efficacy of the combination trimethoprim-sulfamethoxazole against clinical isolates of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 29:1092–1094
Nemoto T, Yamasaki Y, Torikai K, Ishii O, Fujitani S, Matsuda T (2012) A case of MRSA infection in multiple artificial joints successfully treated with conservative medical treatment. Kansenshogaku Zasshi 86:411–414
Fujino T, Amari Y, Mohri M, Noma M, Yamamoto H (2009) MRSA tricuspid valve infective endocarditis with multiple embolic lung abscesses treated by combination therapy of vancomycin, rifampicin, and sulfamethoxazole/trimethoprim. J Cardiol 53:146–149. doi:10.1016/j.jjcc.2008.06.007
Acknowledgments
We thank Dr Keyra Martinez, of Edanz Group Ltd., for her editorial assistance during the manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Rights and permissions
About this article
Cite this article
Miyazaki, S., Kakutani, K., Maeno, K. et al. Surgical debridement with retention of spinal instrumentation and long-term antimicrobial therapy for multidrug-resistant surgical site infections after spinal surgery: a case series. International Orthopaedics (SICOT) 40, 1171–1177 (2016). https://doi.org/10.1007/s00264-015-3073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-3073-3