Abstract
Purpose
The purpose of this study is to assess safety and feasibility of intradiscal bone marrow concentrate (BMC) injections to treat discogenic pain as an alternative to surgery.
Methods
A total of 26 patients (11 male, 15 female, aged 18–61 years, 13 single level, 13 two level) that met inclusion criteria of chronic (> 6 months) discogenic low back pain, degenerative disc pathology assessed by magnetic resonance imaging (MRI) with modified Pfirrmann grade of IV–VII at one or two levels, candidate for surgical intervention (failed conservative treatment and radiologic findings) and a visual analogue scale (VAS) pain score of 40 mm or more at initial visit. Initial Oswestry Disability Index (ODI) and VAS pain score average was 56.5 % and 80.1 mm (0–100), respectively. Adverse event reporting, ODI score, VAS pain score, MRI radiographic changes, progression to surgery and cellular analysis of BMC were noted. Retrospective cell analysis by flow cytometry and colony forming unit-fibroblast (CFU-F) assays were performed to characterise each patient’s BMC and compare with clinical outcomes. The BMC was injected into the nucleus pulposus of the symptomatic disc(s) under fluoroscopic guidance. Patients were evaluated clinically prior to treatment and at three, six, 12 and 24 months and radiographically prior to treatment and at 12 months.
Results
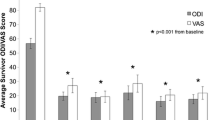
There were no complications from the percutaneous bone marrow aspiration or disc injection. Of 26 patients, 24 (92 %) avoided surgery through 12 months, while 21 (81 %) avoided surgery through two years. Of the 21 surviving patients, the average ODI and VAS scores were reduced to 19.9 and 27.0 at three months and sustained to 18.3 and 22.9 at 24 months, respectively (p ≤ 0.001). Twenty patients had follow-up MRI at 12 months, of whom eight had improved by at least one Pfirrmann grade, while none of the discs worsened. Total and rate of pain reduction were linked to mesenchymal stem cell concentration through 12 months. Only five of the 26 patients elected to undergo surgical intervention (fusion or artificial disc replacement) by the two year milestone.
Conclusions
This study provides evidence of safety and feasibility in the non-surgical treatment of discogenic pain with autologous BMC, with durable pain relief (71 % VAS reduction) and ODI improvements (> 64 %) through two years.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Back pain is the second most common reason for physician visits in the USA and the most common cause of missed work [1]. The cost to the USA for back pain is estimated to be US$100 billion annually [1, 2]. Current treatments for discogenic back pain include activity modification, chiropractic care, exercise, physical therapy, steroid injections and medications [3, 4]. Surgical treatments for chronic, severe, discogenic back pain include spinal fusion or artificial disc replacement [5–7]. Clinical results of a one- or two-level lumbar fusion for back pain are mediocre compared to other orthopaedic procedures [7, 8]. Patients with more than two abnormal discs typically have no surgical options based on a consensus against three-level or more fusion surgeries in the medical community. Many insurance companies will not authorise a lumbar fusion for discogenic back pain because of the expense (US$50,000–100,000) and the published results of patients averaging only a 35 % improvement in pain [8, 9]. However, there remains a void between current nonoperative and surgical treatments [10]. A cell therapy approach may address underlying sources of disc degeneration by mitigating inflammation in the nucleus pulposus or herniation of the annulus, rehydration of the nucleus by remodelling of the tissue or recruiting peripheral cells, nutrients or blood vessels and/or by restoring the disc height to remove pressure from adjacent nerves. Using autologous progenitor cell preparations, including mesenchymal stem cells (MSCs), found in bone marrow concentrate (BMC) may provide a treatment option, which would expand the options beyond nonoperative and operative treatments [11, 12]. This study provides clinical data with 24-month follow-up of the 26 patients suffering from discogenic low back pain who received an intradiscal injection of autologous BMC obtained from aspirate of the iliac wing.
Materials and methods
Study design
This study is a prospective, open-label, non-randomised, two-arm study conducted at a single centre with an Institutional Review Board (IRB) approved clinical protocol. Patients were enrolled as subjects in the study who presented with symptomatic moderate to severe discogenic low back pain as defined according to the following criteria: centralised chronic low back pain that increased with activity and lasted at least six months, nonoperative management for three months without resolution, a change in normal disc morphology as defined by magnetic resonance imaging (MRI) evaluation, have a modified Pfirrmann score of 4–7, a Modic grade II change or less, disc height loss of < 30 % compared to an adjacent non-pathologic disc, pretreatment baseline Oswestry Disability Index (ODI) score of at least 30 on the 100-point scale and pretreatment baseline low back pain of at least 40 mm on the 100-mm visual analogue scale (VAS). An intact annulus was not required to be in the study. Standard exclusion criteria included: an abnormal neurologic exam, symptomatic compressive pathology due to stenosis or herniation or any spondylolisthesis or spondylolysis. Of 26 enrolled patients, seven required discography to confirm affected disc levels at least two weeks prior to treatment.
All patients underwent a pre-injection medical history and physical examination including MRI, ODI and VAS. These ODI and VAS tests were repeated at three, six, 12 and 24 months following the procedure. All patients had a normal neurologic examination of the lower extremities, demonstrated a loss of lumbar range of motion and had pain to deep palpation over the symptomatic disc(s) with associated muscle spasm. Study patient demographics are listed in Table 1. Of the patients, 13 underwent an interdiscal injection of autologous BMC at a single symptomatic lumbar disc and 13 had two adjacent symptomatic disc levels injected. Discography was performed in four patients in the one-level group and three patients in the two-level group to ascertain the symptomatic disc. All other patients were injected based on MRI scanning. MRI scans were repeated at 12 months and assigned a modified Pfirrmann score by a blinded independent reviewer.
Bone marrow collection and processing
Bone marrow aspirate (BMA, 55 ml) was collected over acid citrate dextrose-anticoagulant (ACD-A, 5 ml) from the patient’s posterior iliac crest. The procedure was performed with IV sedation consisting of Versed and Fentanyl. Positioning of the Jamshidi needle in the iliac wing was confirmed by fluoroscopy. BMA was collected in a 60-ml syringe in a series of discrete pulls on the plunger (targeting a collection of 5–10 ml per pull), with repositioning of the needle tip between pulls based on the reported enrichment of progenitor cells by Hernigou et al. [13]. The BMA was processed using the ART bone marrow concentration system (Celling Biosciences, Austin, TX, USA) to produce a bone marrow concentrated cell preparation. Typically, a BMC volume of 7 ml (6 ml for injection and 1 ml for cell analysis) was drawn from the processed device. Cell analysis included total nucleated cell (TNC) concentration and standard 10-day in vitro colony forming unit-fibroblast (CFU-F) assay at dilutions of 50,000 to 1 million TNC per well in 12-well plates.
Intradiscal injection
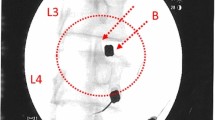
With the patient in a prone position, the injection site(s) was treated with local anaesthetic (1 % buffered lidocaine). BMC was percutaneously injected into the symptomatic disc(s) through a standard posterior lateral discogram approach with a two-needle technique. The injection point of the 22-gauge needle was verified with fluoroscopy. Approximately 2–3 ml of BMC was used per symptomatic lumbar disc injection. Patients were prescribed pain medicine to be used as needed for three days and put on restricted physical activity for two weeks.
Clinical outcomes determination and statistical analysis
ODI and VAS scores were collected from patients by non-investigator personnel employed by the clinic. Pre-treatment and 12-month MRI were analysed by a blinded, independent radiologist. Univariable data comparisons were analysed by two-tailed Student’s t test with a 95 % confidence interval (α = 0.05, Microsoft Excel). Multivariable data were determined with analysis of variance (ANOVA) using JMP 9 statistical analysis software (SAS Institute, Cary, NC, USA). A p < 0.05 was considered to be statistically significant.
Results
Cellular analysis
The average TNC concentration in BMC of non-surgery patients was 129.6 million/ml. CFU-F assay indicated an average frequency of 0.0021 % among TNC, corresponding to 2,702 CFU-F (MSC)/ml.
Injection results
There were no complications associated with the injection of BMC into the nucleus pulposus. Two year follow-up data were obtained for all 26 patients (21 non-surgical, 5 surgical). Post-injection pain relief and decreased impairment measurements (VAS and ODI, respectively) for non-surgical patients prior to treatment as well as at three, six, 12- and 24-month follow-up visits are illustrated in Fig. 1. It was previously observed that pain relief was linked to MSC (or CFU-F) concentration in the BMC, with a natural segregation of patients with greater or less than 2,000 CFU-F/ml. This trend was consistent through 24 months (Fig. 2), although only statistically significant at three and six month time points (p < 0.01). The percentage of ODI and VAS reduction from baseline is reported in Table 2. For each patient cohort, the reduction in pain at every follow-up time point was statistically significant from average baseline scores (p < 0.001).
Clinical results
Two patients progressed to a lumbar fusion between six and 12 months following their initial injection. Between 18 and 24 months, three additional patients elected to proceed with lumbar fusion or artificial disc replacement. Only one of these five patients has reported improvement in VAS and/or ODI following the surgical intervention. The other four patients report ODI and VAS numbers similar to their pre-injection pain and disability scores. Of 24 surviving patients, 20 received MRI at 12 months. The modified Pfirrmann scale [1 (radiographically normal) to 8 (significant darkening of disc and loss of disc height)] was used by a blinded independent reviewer to assign quantitative scores for injected discs and compared to pretreatment scores. Eight patients improved by one grade, while the remaining twelve patients maintained their previous score (discs did not worsen over 12 months after injection). None of the eight patients who improved by MRI at 12 months went on to surgery through 24 months. There was no correlation between the patients' pretreatment Pfirrmann grade or discography and their progression to surgery. Of the five patients who opted for surgery, four were in the MSC concentration range of < 2,000 CFU-F/ml.
Discussion
Discogenic low back pain is a major public health issue [2]. Chiropractic care, physical therapy, activity modifications and medications have shown efficacy in the treatment of acute low back pain [14–16]. There is, however, limited evidence to show their efficacy to treat chronic discogenic low back pain [17–21]. Phillips et al. published an excellent systematic review on the treatment of chronic discogenic low back pain [7]. After establishing strict inclusion and exclusion criteria for the available publications, they reviewed 26 studies. Six papers reported on prospective randomised studies comparing fusion versus non-surgical therapy in patients with moderate to severe low back pain and disability that had persisted for one year or more [22–25]. The results of the six papers showed an average of 35.3 % improvement in the surgical group (547 patients) and a 20 % improvement in the non-surgical group (372 patients). Twelve prospective randomised studies were reviewed comparing various fusion techniques [6]. The minimum follow-up in every study was two years. The weighted average results in the 12 studies were a 43.3 % improvement in back pain (1,420 patients) with a re-operation rate of 12.5 %.
Biologic approaches to treating discogenic pain are appealing due to their less invasive and financially costly nature compared to surgery. Prior to the study, the authors were encouraged by early published results of cell therapies for disc treatment, including juvenile chondrocyte therapies [26]. However, any cell preparations not meeting the US Food and Drug Administration (FDA) guidelines of Section 361 (autologous, minimal manipulation, single procedure, etc.) are classified as a drug (or 351 product) and are not currently available for treatment outside of registered clinical trials. This includes allogeneic cells, in vitro culture-expanded cells, enzymatically or ultrasound-digested adipose or stromal vascular fraction cells, cadaveric bone marrow cells and placental/amniotic products. Platelet-rich plasma (PRP) offers autologous growth factors for regenerative applications and can be prepared by several FDA-cleared devices, but PRP does not provide any stem or progenitor cells as it is derived from whole blood [27]. As the disc is an avascular or poorly vascularised tissue and therefore contains few pericytes/MSCs, it was hypothesised that any biologic injectate should contain progenitor cells to participate in the healing event either directly or through paracrine functions [11]. Autologous BMC concentrated at the point-of-care was identified as an FDA-compliant source of stem and progenitor cells for discogenic pain therapy. This study was initiated to establish safety and feasibility of the procedure while gathering preliminary data to support a larger clinical investigation. The magnitude and sustainment of pain relief and the correlation of clinical outcomes with CFU-F/MSC concentration was not expected.
The current authors previously published the minimum one year follow-up data from the present study [28]. Key findings from the 12-month study include the following: average reduction in disability was 58 % based on the ODI and average reduction of pain was 61 % based on the VAS. Most of this improvement occurred within three months of the disc injection, but was sustained through 12 months. Mesenchymal cell count concentration was linked to pain and disability mitigation. Patients with greater than 2,000 MSC/ml averaged 70 % reduction in ODI and 69 % reduction in VAS, versus patients with less than 2,000 MSCs/ml having a 52 % reduction in ODI and 60 % reduction in VAS. Only two of 26 patients progressed to surgery between six and 12 months. As patient questionnaire-based pain scores can be viewed as subjective, quantifiable results were measured via MRI [29]. Of the 20 patients with a 12-month MRI scan, eight improved at least one Pfirrmann grade, as determined by a blinded reader experienced in the modified Pfirrmann scoring rubric.
The results of this study, in which patients suffering from discogenic low back pain (similar to that recently reported on by Phillips et al.) received an intradiscal injection of autologous BMC obtained from the iliac wing under IV sedation in a 45-minute outpatient procedure, have shown durable pain and ODI improvements out to two years. These results are markedly better than fusion or no treatment in terms of pain and disability mitigation [7]. The overall improvement in ODI was 67 % and 72 % for VAS (p < 0.001) in the 21/26 patients who had not undergone surgery at the two year follow-up. Only five patients elected to proceed with surgery (spinal fusion or artificial disc replacement). Only one of these five patients reported any significant improvement in pain or quality of life following the surgery. The other four patients’ ODI and VAS scores are similar to those from before the intradiscal BMC injection. The remaining 21 patients have improved such that none reported contemplating surgery at the 24-month follow-up point. No patient was made worse from the BMC injection and there were no serious complications associated with the procedure. Overall, a majority of patients enrolled in the study have reported a durable improvement in VAS and ODI levels following a single intradiscal injection of BMC at the two year milestone.
Limitations of this study include: the small population (26 patients), the lack of randomisation with a control group and that MRIs were only obtained in 20 of 24 remaining patients at the 12-month follow-up point. Processing disposables were provided at no cost by Celling Biosciences (Austin, TX, USA) without any further financial contributions to the study or principal investigator. Future studies should include a prospective randomised control group. The authors are not aware of a comparable, published study in which a patient’s autologous concentrated BMA has been injected intradiscally to treat degenerative disc disease (DDD) and also are unaware of other cell-based therapies that have achieved the degree of improvement in VAS and ODI scores shown in this study at the two year milestone. The lack of adverse events during the course of the study, whether at the site of aspiration or the disc injection, strongly supports the clinical feasibility for using autologous BMC for treating patients suffering from DDD. The reported correlation of the degree of durable VAS and ODI improvements with patients receiving > 2,000 MSC/ml of injectate further emphasises the need to provide BMC with a greater concentration of the mononuclear cell fraction, including progenitor cells like MSCs.
These preliminary results strongly suggest that the intradiscal injection of a patient’s autologous BMC into a pathogenic disc has the potential to provide a non-surgical option for treating discogenic back pain after conventional therapy has failed, prior to progressing to surgical fusion. The morbidity and cost of this percutaneous procedure (performed in a treatment room with fluoroscopy) are substantially less than surgical resolution, and the clinical results appear to be superior [28] so far for the majority (approximately 80 %) of patients still enrolled at the two year milestone compared to those who progressed to a surgical procedure.
References
Dagenais S, Caro J, Haldeman S (2008) A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 8:8–20. doi:10.1016/j.spinee.2007.10.005
Luo X, Pietrobon R, Sun SX et al (2004) Estimates and patterns of direct health care expenditures among individuals with back pain in the United States. Spine 29:79–86. doi:10.1097/01.BRS.0000105527.13866.0F
Schnitzer TJ, Ferraro A, Hunsche E, Kong SX (2004) A comprehensive review of clinical trials on the efficacy and safety of drugs for the treatment of low back pain. J Pain Symptom Manage 28:72–95. doi:10.1016/j.jpainsymman.2003.10.015
White AP, Arnold PM, Norvell DC et al (2011) Pharmacologic management of chronic low back pain: synthesis of the evidence. Spine 36:S131–S143. doi:10.1097/BRS.0b013e31822f178f
Gornet MF, Burkus JK, Dryer RF, Peloza JH (2011) Lumbar disc arthroplasty with Maverick disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine 36:E1600–E1611. doi:10.1097/BRS.0b013e318217668f
Zigler J, Delamarter R, Spivak JM et al (2007) Results of the prospective, randomized, multicenter Food and Drug Administration investigational device exemption study of the ProDisc-L total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine 32:1155–1162. doi:10.1097/BRS.0b013e318054e377, discussion 1163
Phillips FM, Slosar PJ, Youssef JA et al (2013) Lumbar spine fusion for chronic low back pain due to degenerative disc disease: a systematic review. Spine (Phila Pa 1976) 38:E409–E422. doi:10.1097/BRS.0b013e3182877f11
Deyo RA, Gray DT, Kreuter W et al (2005) United States trends in lumbar fusion surgery for degenerative conditions. Spine 30:1441–1445. doi:10.1097/01.brs.0000166503.37969.8a, discussion 1446–1447
Cheng JS, Lee MJ, Massicotte E et al (2011) Clinical guidelines and payer policies on fusion for the treatment of chronic low back pain. Spine 36:S144–S163. doi:10.1097/BRS.0b013e31822ef5b4
Bederman SS (2012) Commentary: the degenerative lumbar spine: a chronic condition in search of a definitive solution. Spine J 12:98–100. doi:10.1016/j.spinee.2012.01.005
Murphy MB, Moncivais K, Caplan AI (2013) Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45:e54. doi:10.1038/emm.2013.94
Buchanan RM, Blashki D, Murphy MB (2014) Stem cell therapy for regenerative medicine. Chem Eng Prog 110:55–58
Hernigou P, Homma Y, Flouzat Lachaniette HC et al (2013) Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int Orthop 37:2279–2287. doi: 10.1007/s00264-013-2017-z
Mehling WE, Gopisetty V, Bartmess E et al (2012) The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine 37:678–684. doi:10.1097/BRS.0b013e318230ab20
Weber H, Holme I, Amlie E (1993) The natural course of acute sciatica with nerve root symptoms in a double-blind placebo-controlled trial evaluating the effect of piroxicam. Spine 18:1433–1438
Carey TS, Garrett J, Jackman A et al (1995) The outcomes and costs of care for acute low back pain among patients seen by primary care practitioners, chiropractors, and orthopedic surgeons. The North Carolina Back Pain Project. N Engl J Med 333:913–917. doi:10.1056/NEJM199510053331406
Steele J, Bruce-Low S, Smith D et al (2013) A randomized controlled trial of limited range of motion lumbar extension exercise in chronic low back pain. Spine 38:1245–1252. doi:10.1097/BRS.0b013e318291b526
Becker A, Held H, Redaelli M et al (2012) Implementation of a guideline for low back pain management in primary care: a cost-effectiveness analysis. Spine 37:701–710. doi:10.1097/BRS.0b013e31822b01bd
Soer R, Reneman MF, Vroomen PCAJ et al (2012) Responsiveness and minimal clinically important change of the Pain Disability Index in patients with chronic back pain. Spine 37:711–715. doi:10.1097/BRS.0b013e31822c8a7a
Gillard DM, Corenman DS, Dornan GJ (2014) Failed less invasive lumbar spine surgery as a predictor of subsequent fusion outcomes. Int Orthop 38:811–815. doi:10.1007/s00264-013-2167-z
Kanayama M, Oha F, Hashimoto T (2015) What types of degenerative lumbar pathologies respond to nerve root injection? A retrospective review of six hundred and forty one cases. Int Orthop 39:1379–1382. doi:10.1007/s00264-015-2761-3
Brox JI, Nygaard ØP, Holm I et al (2010) Four-year follow-up of surgical versus non-surgical therapy for chronic low back pain. Ann Rheum Dis 69:1643–1648. doi:10.1136/ard.2009.108902
Fairbank J, Frost H, Wilson-MacDonald J et al (2005) Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. BMJ 330:1233. doi:10.1136/bmj.38441.620417.8F
Fritzell P, Hägg O, Wessberg P, Nordwall A et al (2001) 2001 Volvo Award winner in Clinical Studies: lumbar fusion versus nonsurgical treatment for chronic low back pain: a multicenter randomized controlled trial from the Swedish Lumbar Spine Study Group. Spine 26:2521–2532, discussion 2532–2534
Ohtori S, Koshi T, Yamashita M et al (2011) Surgical versus nonsurgical treatment of selected patients with discogenic low back pain: a small-sized randomized trial. Spine 36:347–354. doi:10.1097/BRS.0b013e3181d0c944
Coric D, Pettine KA, Sumich A, Boltes MO (2013) Prospective study of disc repair with allogeneic chondrocytes presented at the 2012 Joint Spine Section Meeting. J Neurosurg Spine 18:85–95. doi: 10.3171/2012.10.SPINE12512
Murphy MB, Blashki D, Buchanan RM et al (2012) Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials 33:5308–5316. doi:10.1016/j.biomaterials.2012.04.007
Pettine KA, Murphy MB, Suzuki RK, Sand TT (2015) Percutaneous injection of autologous bone marrow concentrate cells significantly reduces lumbar discogenic pain through 12 months. Stem Cells 33:146–156. doi:10.1002/stem.1845
Fischer CA, Neubauer E, Adams HS et al (2014) Effects of multidisciplinary pain treatment can be predicted without elaborate questionnaires. Int Orthop 38:617–626. doi:10.1007/s00264-013-2156-2
Acknowledgments
The authors thank Mr. Tyler Santomaso and Ms. Melissa Samano for their contributions in data collection and review. Celling Biosciences (Austin, TX) provided concentration devices for the processing of each patient’s bone marrow aspirate.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pettine, K., Suzuki, R., Sand, T. et al. Treatment of discogenic back pain with autologous bone marrow concentrate injection with minimum two year follow-up. International Orthopaedics (SICOT) 40, 135–140 (2016). https://doi.org/10.1007/s00264-015-2886-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-015-2886-4