Abstract
Purpose
Aspirating bone marrow from the iliac crest using small volumes of 1–4 ml with a 10-ml syringe has been historically proposed for harvesting adult mesenchymal stem cells and described as a standard technique to avoid blood dilution. The disadvantage of repeated small aspirations is that there is a significantly increased time to harvest the bone marrow. However, it is not known if a large volume syringe can improve the rate of bone marrow aspiration without increasing blood dilution, thus reducing the quality of the aspirate. We compared the concentrations of mesenchymal stem cells obtained under normal conditions with two different size syringes.
Methods
Thirty adults (16 men and 14 women with a mean age of 49 ± 14 years) underwent surgery with aspiration of bone marrow from their iliac crest. Bilateral aspirates were obtained from the iliac crest of the same patients with a 10-ml syringe and a 50-ml syringe. Cell analysis determined the frequencies of mesenchymal stem cells (as determined by the number of colonies) from each size of syringe. The cell count, progenitor cell concentration (colonies/ml marrow) and progenitor cell frequency (per million nucleated cells) were calculated. All bone marrow aspirates were harvested by the same surgeon.
Results
Aspirates of bone marrow demonstrated greater concentrations of mesenchymal stem cells with a 10-ml syringe compared with matched controls using a 50-ml syringe. Progenitor cell concentrations were on average 300 % higher using a 10-ml syringe than matched controls using a 50-ml syringe (p < 0.01).
Conclusions
In normal human donors, bone marrow aspiration from 30 patients demonstrated a reduced mesenchymal stem cell number in aspirates obtained using a larger volume syringe (50 ml) as compared with a smaller volume syringe (10 ml).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the 1960s, Owen and Friedenstein discovered multipotent progenitors of conjunctive tissue by cultivating bone marrow cells of rabbits at low density in a liquid medium containing serum, which resulted in the presence of colonies of spindle-shaped, plastic-adherent cells with a fibroblastic appearance [14]. These colonies were derived from one cell type in the bone stromal marrow and they were named colony-forming unit-fibroblasts. Bone marrow stromal cells (BMSCs) were named “mesenchymal stem cells” (MSCs) in the 1990s [1, 8] and this term MSC will be used in the text. Due to the BMSC capacity for ex vivo expansion, BMSCs frequently are used in innovative therapeutic approaches [3] such as tissue engineering. The therapeutic potential of these cells has drawn considerable interest in the treatment of various orthopaedic conditions, ranging from spinal fusions, treatment of non-unions following traumatic injury, avascular necrosis as well as in challenging soft tissue sports medicine applications.
Since their first description mesenchymal stem cells have been found to reside in all tissues, and of course in bone [10, 11, 17]. The most extensively studied source of mesenchymal stem cells is bone marrow. MSCs are present in the mononuclear fraction of medullary cells. Quantification of MSCs provides an estimate for the number of MSCs in bone marrow to be between 1 per 104 and 1 per 106 mononuclear cells [1, 2]. In a normal human (in the absence of disease) a significant factor affecting this frequency is the age of the donor [2, 6, 12, 13]. The frequency of MSCs in the bone marrow aspirate also depends on the technique of culture and on the technique of aspiration because of the risk of blood dilution [13]. Blood dilution artificially increases the number of mononuclear cells (MNCs), but does not increase the number of MSCs (because MSCs are present in very restricted numbers in the blood [9]).
Small volumes of aspirate from 1 to 4 ml obtained with a 10-ml syringe have been proposed and described as a standard technique to avoid blood dilution [2, 5, 12, 13], and this technique is frequently reported in the literature. However, a disadvantage of repeated small aspirations is the longer aspiration time required to obtain a volume of sufficient bone marrow. Some surgeons use a larger volume syringe to improve the rate of bone marrow aspiration. The thinking behind the use of a larger volume syringe is that it can generate a stronger negative pressure and therefore aspirate more MSCs with a larger overall aspirate volume. To our knowledge, no data are available to guide the surgeon in selecting the optimum size syringe for bone marrow aspirations. No report has studied the influence of the size of the syringe and the influence of aspirated volume on the number of bone marrow MNCs (BM-MNCs) and the number of MSCs that can be obtained during bone marrow aspiration.
This study had the following goals: to determine (1) blood dilution as reflected by the number of nucleated cells coming from blood in the bone marrow aspirates, (2) the number of BM-MNCs in bone marrow aspirates (3) and the number of BM-MSCs in bone marrow aspirates obtained during elective orthopaedic operations in normal patients. The study also defined how the number and concentration of BM-MSCs are influenced by aspiration with a 10-ml or 50-ml volume syringe as well as assessing the influence of aspiration volume in each syringe size.
Materials and methods
Patient demographics
Thirty adult patients scheduled for surgery were enrolled in this prospective, Institutional Review Board-approved study. Patients provided informed consent before being included in the study. The patients included 16 men and 14 women (mean age 49 ± 14 years) who were scheduled to undergo an elective orthopaedic procedure with bone marrow treatment. All patients were fully informed with respect to the rationale of the study and the associated risks, in accordance with a protocol and a signed consent form approved by the Henri Mondor Hospital. Patients who were more than 18 years of age were eligible. Patients were excluded from the study if they had an active infection or had previous iliac crest harvest. Patients also were excluded if they suffered from any myeloproliferative disorder or if they were being managed with chronic steroid medication or chemotherapy.
Study design
Each patient had percutaneous aspiration of bone marrow from both the left and the right anterior iliac crest after induction of anaesthesia. With use of an established technique that had been validated in previous studies [6], bone marrow cells were aspirated directly into 10- or 50-ml syringes that had been preloaded with heparinised saline solution (1,000 units of sodium heparin in 1.0 ml of saline solution). A smear was made at the time of each aspiration to confirm the presence of nucleated cells and the adequacy of the aspirate. The schematic drawing of Fig. 1 demonstrates the insertion of the Jamshidi needle used in this study. Under sterile conditions, a fenestrated Jamshidi needle was pushed through a skin opening into the anterior aspect of the iliac crest at site 1. The heparin-treated 10-ml syringe was attached to the needle. Bone marrow was harvested, and the syringe and needle were removed. A new needle was inserted into the iliac crest 2 cm posterior to the previous insertion site (site 2). A distance of 2 cm was selected as aspirations within 1 cm of the previous draw site demonstrated significantly reduced cell yields (data not shown). Another heparin-treated 10-ml syringe was attached and 2 ml of bone marrow was aspirated. The process was repeated to obtain draws of 4 and 10 ml with 10-ml syringes (sites 3–4). The aspiration procedure was moved to the contralateral side to harvest 5, 10, 20 and 50 ml volumes of aspirate with 50-ml syringes (sites 7–10). To confirm equivalent cell yields from the right and left iliac crest, identical test draws of 4 ml of bone marrow were performed with a 20-ml syringe (intermediate size between 10 and 50 ml) with the needle at the same level (both in the crest and in depth) in the right and left iliac bone of each patient (Fig. 1, sites 5 and 6). After each patient we changed the order of right and left iliac crest. For each patient bone marrow samples from each of the 10-ml syringes were compared to samples of marrow aspirated from the contralateral iliac crest taken with a 50-ml syringe.
Four parameters were measured or calculated from the cell analysis of each of the bone marrow aspirate samples. The first parameter was the dilution of nucleated cells coming from the bone marrow by cells in the peripheral blood. Blood was collected on a Vacutainer® plastic blood collection tube (whole blood tube with spray-coated K2ethylenediaminetetraacetate). This number was calculated according to the haematocrit of the bone marrow compared to the haematocrit of the peripheral blood. Haematocrit was determined by centrifuging blood in a capillary tube. The second parameter was the bone marrow nucleated cell count (the number of bone marrow nucleated cells per 1.0 ml of marrow aspirate). To assess cell recovery in each syringe, the number of total nucleated cells (TNCs) was determined by counting marrow cells on a haemocytometer. The number of nucleated cells from the peripheral blood was subtracted. The result was normalised as BM-MNCs to the volume of marrow aspirated as a percentage of the total syringe volume filled for each of the 30 patients in this study. The third parameter was the concentration of connective tissue progenitor cells (the number of connective tissue progenitor cells per millilitre of aspirate) in the bone marrow. The frequency of connective tissue progenitor cells (the number of connective tissue progenitor cells per million nucleated cells) was assessed by determining the number of colony-forming units (MSCs) present in the cultured sample. The MSC assay was performed by plating 2 million cells from the bone marrow aspirate into 25 cm2 tissue culture flasks. Following ten days under standard growth conditions as previously described [6], the number of colonies containing at least 50 cells was evaluated (Fig. 2). The concentration of osteogenic connective tissue progenitor cells was calculated for each sample as the product of the nucleated cell count and the prevalence of osteogenic connective tissue progenitor cells. The fourth parameter was the concentration of connective tissue progenitor cells (the number of connective tissue progenitor cells per millilitre of volume) in the peripheral blood. This was calculated in the same manner as the concentration of connective tissue progenitor cells in bone marrow.
Statistical analysis
Data are reported as mean ± 1 standard deviation and the significance level was set at a probability value of less than 0.05. The independent factors included the identity of the patient and the data from the individual aspirates (ten per patient). Results from the fitting of the statistical model are reported as model-based means. Ninety-five per cent confidence intervals were calculated for the mean numbers of colony-forming units and of nucleated cells. Variance components are reported as the proportion of total variability attributable to the patients and the aspirates. A multivariate analysis was conducted to evaluate the relationship between the set of variables of the groups (group of data with a syringe of 10 ml and group of data with a syringe of 50 ml). The non-parametric Mann–Whitney U test was used to identify the significance of the differences between groups. The chi-square test was used to identify trends within groups with categorical variables.
Results
Left vs right iliac crest bone marrow aspiration
Despite the inherent variability of bone marrow aspiration due to differences in marrow architecture within the iliac crest, the statistical analysis demonstrated no significant (p = 0.52) difference in MSC concentrations in the 4-ml aspirates with the 20-ml syringe in the right and left side of the patients (393 ± 150/ml versus 413 ± 152 MSC/ml).
Number of nucleated cells coming from blood in the bone marrow aspirates
The mean number of nucleated cells in blood was 7.5 ± 2.1 million cells/ml. To calculate the fraction and concentration of blood-derived cells, we made the assumption that the nucleated cell count coming from peripheral blood in the bone marrow was correlated to the haematocrit of the bone marrow. For example, if the cell count in the peripheral blood was 8 million cells/ml with a haematocrit at 45 %, and the haematocrit of the bone marrow was 30 %, we evaluated that the nucleated cells coming from the peripheral blood was 5.33 million cells/ml [(8/45) × 30]. Therefore, if a mean of 50 million cells were obtained from a 1-ml aspirate, then approximately 44.7 million cells (50−5.3 = 44.7), or approximately 89.7 % (44.7/50), were bone marrow-derived and 11.3 % were blood cells. This approach was used to calculate the data shown in Table 1. In aspirations performed with a 50-ml syringe, the fraction of peripheral blood cell increases more dramatically, resulting in dilution of the bone marrow samples, compared with a 10-ml syringe. Also, each syringe type showed a rapid increase in the fraction of peripheral blood when a larger volume of bone marrow was collected.
Bone marrow nucleated cell count
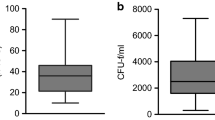
Bone marrow nucleated cells were calculated by subtracting the total number of nucleated cells from the peripheral blood as described above. Higher concentrations of BM-MNCs were achieved when using the smaller 10-ml syringe when compared to the 50-ml syringe (Table 2). The total number of nucleated cells per millilitre of aspirated marrow was always higher (Fig. 3) in the 10-ml syringe with mean values (Table 2) ranging from 65 ± 52 to 20 ± 9 (depending on the volume of aspiration) as compared with the mean values of the 50-ml syringe ranging from 14 ± 3 to 9 ± 1 (depending on the volume of aspiration). Likewise, the aspiration of only 10 % of the full syringe volume resulted in a greater BM-MNC concentration than syringes filled with progressively higher percentages of the full volume for either syringe size (Fig. 4). When roughly equivalent volumes are examined (i.e. 4 ml in a 10-ml syringe and 5 ml in a 50-ml syringe) the BM-MNC yields in smaller syringes were consistently higher (Table 2). The cell count for a collection volume of 4 ml in a 10-ml syringe was, on average, about 300 % higher than the paired (i.e. roughly similar volume of bone marrow) aspirates obtained with the 50-ml syringe (p = 0.001). But when the collection volume of 10 ml in a 10-ml syringe was compared with a collection volume of 10 ml in a 50-ml syringe, aspirates with the 10-ml syringe were, on average, only 150 % higher (p = 0.05).
There was significant variation between patients in the number of BM-MNCs (Table 2). When these variations were analysed (with the same volume of aspirate in the same size syringe), the variations between patients of harvest were greater with the 10-ml syringe compared with the variations observed with the 50-ml syringe (Table 2). This may be explained by the fact that with a 10-ml syringe most of the variation is related to bone marrow variability compared to the variation in the 50-ml syringe that is related to the peripheral blood count variability (between 41 and 86 % of nucleated cells come from peripheral blood). While there was variation from site to site of aspiration and patients, the same order of variations in bone marrow cell count could be related to individual differences as to the size of the syringe and to the per cent of volume filled in each syringe size (Table 2).
Concentration of mesenchymal stem cells in bone marrow
No patient demonstrated any MSC counts in the cultures from the peripheral blood samples; therefore the MSC colonies were assumed to come from bone marrow. MSC numbers showed a similar distribution to that seen with BM-MNC concentrations, showing a similar dependence on syringe size and aspiration volume (Fig. 4a). In the cultures of normal bone marrow from our adult patients, the number of MSC (Table 3) obtained by each syringe varied from 84 to 7,581/ml aspirate (ratio of variation on the order of 100). The maximum MSC number (mean 2,062 ± 1,552) was obtained with an aspiration of 1 ml with a 10-ml syringe and the minimum MSC number (mean 95 ± 8) with an aspiration of 50 ml with a 50-ml syringe (Table 3).
Despite the inherent variability of bone marrow aspiration, aspirates of marrow with the 10-ml syringe demonstrated greater concentrations of connective tissue progenitor cells, as determined by MSC, at all aspirated volumes compared with aspirates obtained with the 50-ml syringe (Fig. 4b; Table 3). Also, aspiration of only 10 % of the full syringe volume resulted in a greater MSC concentration compared to syringes filled with progressively higher percentages of the full volume for either syringe size. Additionally, when roughly equivalent volumes are examined (i.e. 4 ml in a 10-ml syringe and 5 ml in a 50-ml syringe) the MSC yields were consistently higher. On average, the concentration of osteogenic BM-MSCs in the 10-ml syringes was 300 % higher than in the paired (roughly same volume of bone marrow) in 50-ml syringes (p = 0.01). In no patient was the concentration of BM-MSCs in the 50-ml syringes significantly higher than that in the 10-ml syringes.
While there was variation from site to site of aspiration and patients, the same ratio of variations in MSC counts as related to individual differences could be related to the size of the syringe and to the per cent of volume filled in each syringe size (Table 3).
Discussion
The vacuum pressure exerted in harvesting bone marrow is one of the factors that regulates bone marrow aspiration since MSCs are attached to bone and some vacuum pressure is necessary to release them. The magnitude of the pressure exerted within the marrow will depend on the volume which has been created within the syringe when the plunger is retracted. Clearly, a larger syringe can create a larger maximum negative pressure (MNP). Some surgeons believe that the use of large volume syringes improves the quality of bone marrow aspiration. In theory, a larger volume syringe should generate a stronger negative pressure and therefore harvest more MSCs with a larger volume of aspiration. Therefore this study evaluated the numbers of BM-MNCs and BM-MSCs in bone marrow aspirates obtained during elective orthopaedic operations in normal patients to define how their number and concentration are influenced by aspiration with a 10- or a 50-ml volume syringe as well as to assess the influence of aspiration volume in each syringe size.
We found that for both 10- and 50-ml syringe sizes, the aspiration of only 10–20 % of the full syringe volume resulted in a higher MSC concentration compared to syringes that were filled to a greater percentage of the syringe’s full volume. This means that high-quality harvesting of MSCs needs strong aspiration to create a negative pressure that decreases as the syringe is filled. This observation conforms to previous studies [1, 13]. The augmentation of the volume of aspiration when filling the syringe leads to dilution by peripheral blood. The dilution is the result of blood flow in the iliac crest, and the rate at which dilution occurs is likely a function of the rate of local blood flow in relation to the number and size of local vessels and sinusoids. This rate increases when the volume of aspirated bone marrow increases in the syringe, with the consequence that more nucleated cells come from blood rather than from bone marrow. The number of nucleated cells in blood is significant, but there are not significant numbers of MSCs in blood [9]. The existence of circulating MSCs remains a subject of debate [15]. Their presence in blood of adult animals has been discussed [4, 7, 15, 16]; but MSCs are present in very low numbers in the blood and not found in all tested individuals. Observations of MSCs in the blood of humans were invalidated by experiments [9] and attributed to contamination from fragments of conjunctive tissue. Since there are few MCSs in the peripheral blood as observed in our data, blood dilution must decrease the total MSC concentration in bone marrow aspirates.
We also found that a higher concentration of MSCs was observed when bone marrow aspirate was obtained with a 10-ml syringe as compared with a 50-ml syringe. It may appear contradictory that a smaller aspiration volume can result in a higher concentration of MSCs than a larger aspiration volume. However, this may be explained by some physical concepts: According to the equation, pressure = force/area, with the same force a smaller diameter generates higher pressures and one can generate a more negative pressure with a syringe with a smaller diameter. For an equivalent force of a draw, the negative pressure created by the syringe is stronger with a small diameter plunger than with a large diameter plunger, as is the case with the plunger diameter in a small vs large volume syringe. In the situation of bone marrow aspiration in the operating room it also is easier to draw the plunger of a small syringe at a higher speed as compared with a large syringe due to reduced drag. The ease of drawing a small syringe allows greater transmission of force to the plunger during the aspiration. Friction also will slow down the speed that the plunger can be pulled. This friction will have two main components: friction of the plunger seal as it moves in the barrel and the drag caused by the fluid itself. This second component can be significant if the fluid is as viscous as bone marrow. A smaller diameter plunger minimises frictional resistance as it moves by providing a smaller area for the frictional forces to exert resistance. These physical concepts may explain what appeared to be paradoxical results in bone marrow aspiration: “less volume is more” to get stem cells.
Our study has some limitations. Bone marrow aspiration from only 30 patients was examined. Aspirates with the 10- and 50-ml syringes were harvested on different sides of the iliac crest. However, the aspiration with the 20-ml control syringes demonstrated that the pelvic bone marrow is not altered by a regional (right or left side) process in the same patient. The number and function of BM-MSCs in bone marrow from the iliac crest probably reflect the systemic physiological status of the donor, despite the inherent variability of bone marrow aspiration due to differences in marrow architecture within the iliac crest at different sites. We used the number of BM-MSCs that formed under the conditions of culture as an estimate of the number of progenitor cells. There are various methods to identify MSCs, but the literature related to bone marrow MSCs supports the concept that bone marrow-derived colonies giving rise to colony-forming units are derived from mesenchymal stem cells. There are other factors in addition to syringe size and aspiration volume that may affect the harvesting of MSCs from bone marrow. For example, the number of nucleated cells increases if the local bone marrow is highly cellular and loosely connected, allowing bone marrow cells in the marrow space to easily flow into the aspiration needle. Conversely, the number of nucleated cells decreases if the marrow cavity is hypocellular or relatively fibrotic. Some pathologies also may change the number of MSCs.
In conclusion, if the primary goal is to obtain bone marrow-derived cells with the minimum number of aspirates, and if the concentration of progenitors cells is not critical (because higher concentrations can be obtained with a point of care processing system), then aspiration volume with a 50-ml syringe or an intermediate size (20 or 30 ml) is feasible. We estimated that a 5-ml aspirate with a 50-ml syringe will yield 30 % of the bone marrow cells at a given site compared with a 10-ml syringe. This may be sufficient to treat certain pathologies and at this moment the critical number of MSCs that are necessary to treat a specific pathology is not well known. Successful treatment of some pathologies (i.e. non-unions [6]) has been shown to depend both on the total number of bone marrow cells and on the concentration of cells. Consequently, the recommendation is to use a small syringe with a large number of aspiration sites when a large number of cells is needed, because larger volume aspirates contribute little to the overall number of bone marrow progenitor cells and can result in unnecessary blood dilution. The recommendation is also to avoid filling the syringe to full volume with each aspiration.
References
Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9:641–650
Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenzie S, Broxmeyer HE, Moore MA (1980) Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood 56:289–301
Djapic T, Kusec V, Jelic M, Vukicevic S, Pecina M (2003) Compressed homologous cancellous bone and bone morphogenetic protein (BMP)-7 or bone marrow accelerate healing of long-bone critical defects. Int Orthop 27(6):326–330
Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S (2005) Circulating osteoblast-lineage cells in humans. N Engl J Med 352:1959–1966
Hernigou P, Beaujean F, Lambotte JC (1999) Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br 81:349–355
Hernigou P, Poignard A, Beaujean F, Rouard H (2005) Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am 87:1430–1437
He Q, Wan C, Li G (2007) Concise review: multipotent mesenchymal stromal cells in blood. Stem Cells 25:69–77
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7:393–395
Kuznetsov SA, Mankani MH, Leet AI, Ziran N, Gronthos S, Robey PG (2007) Circulating connective tissue precursors: extreme rarity in humans and chondrogenic potential in guinea pigs. Stem Cells 25:1830–1839
Lee HS, Huang GT, Chiang H, Chiou LL, Chen MH, Hsieh CH, Jiang CC (2003) Multipotential mesenchymal stem cells from femoral bone marrow near the site of osteonecrosis. Stem Cells 21:190–199
Loeffler M, Potten CS (1997) Stem cells and cellular pedigrees – a conceptual introduction. In: Potten CS (ed) Stem cells. Academic, New York, pp 119–146
McLain RF, Fleming JE, Boehm CA, Muschler GF (2005) Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentrations from the vertebral body and iliac crest. J Bone Joint Surg Am 87:2655–2661
Muschler GF, Boehm C, Easley K (1997) Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am 79:1699–1709
Owen M, Friedenstein AJ (1988) Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp 136:42–60
Rochefort GY, Delorme B, Lopez A, Hérault O, Bonnet P, Charbord P, Eder V, Domenech J (2006) Multipotential mesenchymal stem cells are mobilized into peripheral blood by hypoxia. Stem Cells 24:2202–2208
Roufosse CA, Direkze NC, Otto WR, Wright NA (2004) Circulating mesenchymal stem cells. Int J Biochem Cell Biol 36:585–597
Tuli R, Tuli S, Nandi S, Wang ML, Alexander PG, Haleem-Smith H, Hozack WJ, Manner PA, Danielson KG, Tuan RS (2003) Characterization of multipotential mesenchymal progenitor cells derived from human trabecular bone. Stem Cells 21:681–693
Acknowledgments
We thank Ted Sand and the other members of Celling Biosciences for the review of the final manuscript and their help in translation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hernigou, P., Homma, Y., Flouzat Lachaniette, C.H. et al. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. International Orthopaedics (SICOT) 37, 2279–2287 (2013). https://doi.org/10.1007/s00264-013-2017-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-013-2017-z