Abstract
Introduction
A proteomic analysis of hepatocellular carcinoma (HCC) has revealed that Heat Shock Protein 70 (HSP70) is among the cancer antigen proteins of HCC. Moreover, we confirmed that HSP70 was highly expressed in HCC by immunohistochemical staining. Based on these results, we developed an HSP70 mRNA-transfected dendritic cell (DC) therapy for treating unresectable or recurrent HCC, and the phase I trial was completed successfully. Thus, we aimed to investigate the safety and efficacy of this therapy as a postoperative adjuvant treatment after curative resection for HCC to prevent recurrence by conducting a phase I/II randomized controlled clinical trial.
Methods
Patients (n = 45) with resectable HCC of stages II–IVa were registered and randomly assigned into two groups (DC group: 31 patients, control group: 14 patients) before surgery. The primary endpoint was disease-free survival (DFS), and the secondary endpoints were safety and overall survival. The DC therapy was initially administered at approximately 1 week after surgery, and twice every 3–4 weeks thereafter.
Results
No adverse events specific to the immunotherapy were observed in the DC group. There was no difference in DFS between the DC and control groups (p = 0.666). However, in the subgroup with HSP70-expressing HCC, DFS of the DC group tended to be better (p = 0.090) and OS of the DC group was significantly longer (p = 0.003) than those of the control group.
Conclusion
The HSP70 mRNA-transfected DC therapy was performed safely as an adjuvant therapy. The prognosis of HSP70-expressing HCC cases could be expected to improve with this therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The recurrence rate of hepatocellular carcinoma (HCC) is still high even after its curative resection; thus, an adjuvant therapy for preventing the recurrence after the curative resection of HCC has long been desired. In the recent years, the efficacy of sorafenib, lenvatinib, regorafenib, and ramucirumab have been reported for unresectable recurrent liver cancer [1], but the postoperative adjuvant therapy has not been established [2]. Chemotherapy and targeted therapy after hepatectomy may be burdensome for some patients because of their adverse events [3].
However, recently, it has been reported that there are some HCC patients who can benefit from immunotherapy [4,5,6,7]. Ia addition, immunotherapy after hepatectomy has fewer adverse events, and treatment completion may be easier than that of chemotherapy and targeted therapy [8]. Previously, we have reported that proteomic and immunohistochemical staining experiments in our department demonstrated that heat-shock protein 70 (HSP70) is highly and specifically expressed by HCC cells [9, 10], and based on these results, we developed an HSP70 mRNA-transfected dendritic cell (DC) therapy for treating unresectable or recurrent HCC. The phase I trial confirming the safety and efficiency of this therapy has been completed [11]. We aimed to investigate the safety and efficacy of the HSP70 mRNA-transfected DC therapy as a postoperative adjuvant treatment after curative resection for HCC to prevent recurrence by conducting a phase I/II randomized controlled clinical trial with stratification for clinical stage and operative method.
Materials and methods
Patients
Eligible patients were aged ≥ 20 years; with radiographically diagnosed completely resectable HCC of stage II, III, or IVa in accordance with the classification of the Liver Cancer Study Group of Japan (LCSGJ) 5th edition [12]; without pretreatment for HCC 30 days prior to the surgical resection; with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; and without active infection, serious illness, or concomitant nonmalignant disease that was difficult to control by medication.
The following exclusion criteria were applied: other malignant diseases; acute coronary syndrome that developed within 6 months; severe interstitial pneumonia, pulmonary fibrosis, and emphysema; continuous administration of corticosteroid or immunosuppressive agents; pregnancy; severe allergy; contraindicated to undergo contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI) due to renal dysfunction, iodine allergy, or other reasons; severe mental symptoms that make their participation in this trial difficult; and inappropriateness as a subject for other reasons.
Study design and treatment
Written informed consent was obtained from all patients. The protocol was approved by the institutional review board of Yamaguchi University (IRB number: H24-40), and was registered with the UMIN Clinical Trials Registry (registration no. UMIN000010691). The study was conducted in accordance with the Good Clinical Practice guidelines and the Declaration of Helsinki.
This single-center, randomized-controlled, phase I/II clinical trial evaluated the adjuvant immunotherapy with HSP70 mRNA-transfected DC therapy in patients with HCC. The phase I study was conducted to evaluate the safety of DC therapy in nine patients. The phase II study was conducted to assess the efficacy and safety of DC therapy. All eligible participants were registered and assigned randomly before surgery to the following two groups in a 2:1 ratio: DC group who received the adjuvant DC therapy and the control group who had no adjuvant treatment. Patients were stratified according to clinical stage as II, III, or IVa and operative method as partial or anatomic resection. Patients were randomized using a computer-generated randomization chart.
Peripheral blood mononuclear cells (PBMCs) were harvested with the COBE Spectra Apheresis System (COBE BCT, Inc., Lakewood, CO) from the patients in the DC group. The first harvest was 1–5 days before surgery. The second harvest was 4–9 weeks after surgery, and the third harvest was 8–15 weeks after surgery. The PBMCs from 3000 ml of blood were enriched by density gradient centrifugation with Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden). The PBMCs were incubated for 45 min in a 5% CO2 atmosphere at 37 °C in a serum-free AIM-V medium (Gibco, Paisley, Scotland). Plastic-adherent cells were cultured in an AIM-V medium containing 800 U/mL of granulocyte–macrophage colony-stimulating factor (GM-CFS) (Osteogenetics GmbH, Wurzburg, Germany) and 500 U/mL of interleukin-4 (IL-4) (Osteogenetics GmbH). On day 6, immature DCs were cultured in the AIM-V medium containing 300 U/mL of tumor necrosis factor-α (TNF-α; R&D Systems, Minneapolis, MN). Cultures were checked for endotoxins, mycoplasma, and bacterial contamination prior to administration. On day 10, floating and loosely adherent cells were collected as mature DCs. Maturated DCs (2 × 106 cells/400 µL) and 10 µg of HSP70 mRNA were mixed and electroporated for 500 µs with 400 V (Harvard Apparatus, Holliston, MA). DCs were washed three times with saline and suspended in 2-mL saline. Patients in the DC group received a DC agent intradermally in the inguinal region. They were scheduled to receive the DC agent three times (first administration: 5–9 days after surgery; second administration: 5–10 weeks after surgery, third administration: 9–16 weeks after surgery). After confirmation of no bacterial contamination, patients were administered dendritic cells. Cytokines, such as interferon, chemotherapy agents, other immunotherapy agents, hormonal therapy, and stem cell therapy were contraindicated during the study.
End points and assessments
The primary endpoints were 1- and 3-year disease-free survival (DFS) from curative hepatectomy, and secondary endpoints were safety and overall survival (OS). OS was measured from the date of curative hepatectomy until death from any cause.
Tumor assessments were performed using dynamic CT or MRI every 4–8 weeks from baseline in both groups. All scans were reviewed by two independent radiologists at each site who were blinded to the group assignment. The adverse events (AEs), which were classified and graded according to the Common Terminology Criteria for Adverse Events, version 4.0, were assessed from the time the patient provided written informed consent until the end of the study or until they dropped out from the study.
Immunohistochemical staining of HSP70
Immunohistochemical staining (IHS) was performed using an EnVision + kit according to the manual provided by Dako (Carpinteria, CA). Anti-Hsp70 mAb (Abcam, Cambridge, UK) was used for the staining. IHC results for HSP70 were rated negative, borderline, positive, or strongly positive. Cases wherein scores were 10% ≥ positive were defined as expression-positive cases. IHC staining was analyzed by two certified Japanese pathologists who were blinded to the clinicopathological parameters.
Immunomonitoring
PBMCs were used in surface marker expression assessments. The surface markers included exhaustion markers [programmed death-1 (PD-1), T-cell immunoglobulin mucin-3 (Tim-3)] and immunosuppressive (regulatory T (Treg) cells identified as CD4 + CD25 + CD45RA- cells and myeloid-derived suppressor (MDSC) cells identified as CD11b + CD33 + cells. Monoclonal antibodies used herein were purchased from BioLegend (San Diego, CA, USA), Beckton Dickinson (Franklin Lakes, NJ, USA), Beckman Coulter (Brea, CA, USA), or Miltenyi Biotec (Auburn, CA, USA), unless otherwise specified, comprising anti-CD8 (clone HIT8a), anti-CD4 (clone VIT4), anti-PD-1 (clone EH12.2H7), anti-TiM-3 (clone F38-2E2), anti-CD45RA (clone HI100), anti-CD25 (clone B1.49.9), anti-CD11b (clone Bear1), and anti-CD33 (clone WM53) antibodies. Flow cytometry was performed using a MACSQuant Analyzer 10 (Miltenyi Biotec, Auburn, CA, USA), and the output was analyzed using FlowJo (FlowJo LLC, Ashland, OR, USA). We investigated whether the parameters, such as clinicopathological status, biochemical examination, lymphocyte subset, and tumor markers are biomarkers of the therapeutic effect in the DC group. The cut-off values of the clinicopathological status, biochemical examination data, and tumor markers were defined according to the facility standard. The cut-off value of the lymphocyte subset was determined by using the receiver-operating characteristic curve. Using these cut-off values to further analysis– we determined the prognostic factors.
Analysis of DC subsets
Induced DC subsets were analyzed with mAbs against surface antigens. All mAbs were purchased from Coulter (Hialeah, FL, USA). FITC-conjugated anti-CD80 (B7-1), − CD83 (HB-15) were used. PE-conjugated anti-CD86 (B7-2) and -CD40 were also used according to the manufacturer's instructions. Samples were analyzed with an EPICS Flow Cytometer (Coulter Electronics, Inc., Hialeah, FL, USA) at a fluorescence excitation wavelength of 488 nm at 200–500 mW. For each sample, 5000 DCs were analyzed.
Statistical analysis
The sample size for the study was determined on the basis of the primary end point of 1-year recurrence rate. In this randomized-controlled trial, the expected 1-year recurrence rate was 10%. We estimated that we would require the enrollment of 45 patients to justify proceeding with a follow-up randomized controlled trial. We noted that the 1-year recurrence rate of 160 patients who had undergone surgery was 30% in our institution in the past. The study would have at least 80% power to detect a relative reduction in the risk of recurrence compared to a historical control, using a two-sided level of 0.05.
The efficacy outcomes were assessed according to the intention-to-treat principle. The Kaplan–Meier curves were generated for DFS and OS, and the log-rank test was used for group comparisons. In addition, because of the preoperative assignment, as a result of postoperative pathological diagnosis, it was assumed that some cases would be found to be other than HCC. Therefore, we planned to perform a subgroup analysis of only pure HCC or HSP-expressing HCC. The Chi square test or Fisher’s exact test was performed for categorical variables. The Wilcoxon rank sum test was performed for quantitative comparisons. p < 0.05 was considered statistically significant. All statistical analyses were carried out with JMP version 12.0 (SAS Institute Japan, Tokyo, Japan).
Results
Patients
Between July 2012 and February 2015, 46 participants were screened. All eligible participants were assigned randomly to either the DC (n = 31) or control (n = 15) group (Fig. 1). Among these randomized patients, 44 (30 in the DC group and 14 in the control group) were included in the efficacy analysis and safety population. One patient in the DC group who only received the DC therapy twice because of her mental problem was also included in the efficacy analysis and safety population. Two patients were excluded from the efficacy analysis, including one patient in the DC group who withdrew his study participation after group assignment and one patient in the control group who was found to be inoperable after group assignment. No patients were lost to follow up in each group. The median follow-up duration was 50.8 and 45.9 months in the DC and control groups, respectively.
None of the differences in the baseline characteristics between the two study groups were statistically significant (Table 1).
Safety
Among the safety population cohort, AEs were reported in 39 patients (88.6%); of these, 35 patients (79.5%) had mild to moderate AEs (grade 1 or 2). The frequency of all AEs between the two groups was comparable. Most of these AEs were affected by background liver disease. In the DC group, two patients had grade 3 anemia and two patients had grade 3 thrombocytopenia. However, there was no significant change in liver function in these patients. Furthermore, although these patients met the inclusion criteria, anemia and thrombocytopenia were more prominent before DC treatment than in other cases. So, these AEs were unlikely to be associated with DC therapy and likely to be related to liver cirrhosis or splenomegaly, which developed during the chronic course of the disease. The frequency of grade 3 AEs between the two groups was also comparable. No patient in the DC group developed local skin reactions. Two patients of the DC group experienced transient fever, one patient of the DC group developed a grade 2 rash and one patient of the DC group developed grade 1 pruritus. These immune-related AEs were well tolerated (Table 2).
We also evaluated AEs associated with apheresis, but no AEs of Grade 2 or higher, including hypotension, bleeding, and air embolism were observed.
Survival analysis
Disease-free survival
The 1- and 3-year DFS of the DC group were 76.7% and 50.0%, respectively, and those of the control group were 92.9% and 50.0%, respectively. Among 44 patients in the efficacy population cohort, eight patients experienced tumor recurrence at 1 year, including seven out of the 30 patients in the DC group and one of the 14 patients in the control group. A total of 22 patients experienced tumor recurrence at 3 years, including 15 out of the 30 patients in the DC group and seven out of the 14 patients in the control group. We also evaluated the median DFS, which was measured from the date of curative hepatectomy to the first recurrence. The median DFS was 34.7 and 40.2 months in the DC and control groups, respectively (Fig. 2a), There was no significant difference in the DFS between the two groups (p = 0.666).
The Kaplan–Meier estimates of disease-free survival (DFS) and overall survival (OS). The Kaplan–Meier estimates of disease-free survival (DFS) and overall survival (OS). a DFS of per protocol population. b OS of per protocol population. c DFS of the subgroup of HCC patients. d OS of the subgroup of HCC patients. e DFS of the subgroup of HCC-expressing HSP70. f OS of the subgroup of HCC-expressing HSP70
In the subgroup analysis of pure HCC, excluding seven cases that were revealed after surgery to be non-pure HCC such as combined HCC, sarcomatoid change and cholangiocellular carcinoma (CCC), the 1- and 3-year DFS of the DC group were 85.2% and 55.6%, respectively, and those of the control group were 90.0% and 40.0%, respectively. There was no significant difference in the DFS between the two groups (p = 0.473). (Fig. 2c). There was also no difference in the characteristics between the two groups (Supplementary Table 1). Contrarily, we also performed a subgroup analysis of the non-HCC patients. In this subgroup, the median DFS was significantly shorter in the DC group than in the control group (Supplementary Fig. 1 and Supplementary Table 2).
A subgroup analysis according to the HSP70 expression of the tumor in the pure HCC group was also carried out (Supplementary Table 3). The 1- and 3-year DFSof the DC group were 85.7% and 47.6%, respectively, and those of the control group were 85.7% and 14.3%, respectively. However, in this subgroup, median DFS in the DC group waslonger than those of the control group (median 33.2 vs. 17.9 months) although the difference was not statistically significant (p = 0.090) (Fig. 2e). There was no difference in the characteristics between the two groups.
Overall survival
During the data cut-off date, the efficacy population cohort had 14 deaths, including eight patients in the DC group and six in the control group. In the DC group, five patients died from recurrent HCC and three patients died from other causes (rupture of varix in 1, renal failure in 1, and pneumonia in 1 patient). In the control group, six patients died from recurrent HCC. There was no difference in the OS between the two groups.
In the subgroup analysis of pure HCC, median OS was significantly longer in the DC group than in the control group (p = 0.044) (Fig. 2d). The 3-year OS rate was 96.3% and 70.0% in the DC and control groups, respectively. Contrarily, in the subgroup of non-HCC patients, median OS was significantly shorter in the DC group than in the control group (Supplementary Fig. 1, Supplementary Table 2).
In the subgroup analysis according to the HSP70 expression of the tumor in the pure HCC group, the OS was significantly longer in the DC group than in the control group (Fig. 2f). The OS of all individual HSP70-expressing HCC patients is illustrated in a swimmer plot (Supplementary Fig. 2). In addition, the site of the first recurrence and management after recurrence are shown in Table 3. There was no difference in the first recurrence site between the two groups. The median number of surgery or local–regional therapy, such as radiofrequency ablation (RFA) or transcatheter arterial chemoembolization (TACE), after recurrence in the DC and control groups was two (1–7 times and 1–4 times, respectively). However, the median duration from the first hepatectomy to becoming refractory to surgery or local–regional therapy was longer in the DC group than in the control group (DC group: not reached; control group: 38.7 months; p = 0.039).
Immunological analyses
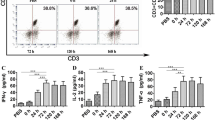
Changes in the exhaustion marker expression in PBMCs before and after DC therapy.
To determine whether DC therapy re-invigorated the exhausted T cells and improved the systemic immunosuppressive microenvironment, we analyzed the surface marker expression in PBMCs before and after DC therapy in the DC group. No significant changes were observed in the other cell exhaustion markers before and after the DC therapy in the DC group (Fig. 3). The exhaustion markers did not also change significantly over time in the HCC patients alone (Supplementary Fig. 3).
Further analysis in the DC group
The DC group of HCC patients was divided into 3-year recurrence-free groups and early recurrence group (DFS < 1 year) to identify the prognostic factors. The clinical, pathological, and immunological pretreatment feature associated with early HCC recurrence was the high percentage of serum Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein (AFP-L3) (p = 0.003). Moderate differentiation (p = 0.070) and high level of Tregs (p = 0.078) tended to be prognostic factors of early HCC recurrence (Supplementary Table 4).
We also evaluated the difference in surface markers of PBMCs before and after DC therapy by dividing the DC group of HCC patients into the early recurrence group and 3-year recurrence-free groups. The MDSC levels after DC therapy were significantly higher in the early recurrence group (Supplementary Fig. 4).
Profiles of surface markers of induced DCs
Surface marker expression of DCs before and after administration of TNF-α was shown (Supplementary Fig. 5). The analysis was not possible in all cases to ensure a DC for administration to patients because the number of DCs was low in some cases. However, samples were available, evaluable, and analyzed in 24 cases. The median expression level of each antigen in DCs before administration of TNF-α was found in 7.6% (2.6–61.2%) (CD80), 68.1% (14.9–94.9%) (CD86), 1.6% (0.1–11.1%) (CD83), and 97.9% (48.4–99.7%) (CD40). The median expression level of each antigen in DCs after administration of TNF-α was found in 90.3% (53.2–99.2%) (CD80), 95.0% (63.4–99.7%) (CD86), 71.7% (8.9–93.4%) (CD83), 99.8% (96.9–100%) (CD40). The percentage of CD80 + , CD83 + , CD86 + , and CD40 + after administration of TNF-α were high enough to show high-quality DC with immunogenic maturation.
Discussion
In this study, we assessed the safety and efficacy of the adjuvant immunotherapy with HSP70-DC in patients with resectable HCC. We previously confirmed the safety of HSP70 mRNA-transfected DC therapy as a treatment for unresectable or recurrent HCC [11]. In this study, we performed DC therapy as an adjuvant treatment after curative hepatectomy for HCC, and the toxicity in this study observed was manageable. HSP70 was mildly expressed not only in HCC but also in normal hepatocytes, and DCs possibly attacked normal hepatocytes; however, such a phenomenon was not observed in this study. Most of the AEs were likely to be affected by background liver disease, and all patients recovered with appropriate treatment. Overall, the HSP70 mRNA-transfected DC therapy as an adjuvant treatment after curative hepatectomy was safe and well tolerated in this cohort.
No statistical difference was found in the DFS or OS rate between the DC and control groups. The seven cases were revealed to be non-pure HCC, including combined HCC, sarcomatoid change, and CCC, after surgery through the preoperative imaging examination. Something might go wrong with the trial design. The reason why we designed clinical trials to be preoperatively assigned was to perform apheresis in the best preoperative immune condition and to administer DCs immediately after surgery when the cell-mediated immunity was suppressed [13]. We might have to confirm the pre-operative pathological diagnosis in this study. However, it has been reported that the accuracy of imaging examination for HCC is high. In addition, preoperative needle biopsy carries the risk of needle track seeding and bleeding. It is reported that needle track seeding following biopsy of HCC occurs in 1.6–3.4% of cases [14,15,16]. Most patients with HCC may have hemorrhage due to liver cirrhosis, and there is also a concern of hemorrhage after a needle biopsy. For these reasons, we assigned the patients into groups even without obtaining a definitive histologic diagnosis. However, combined HCC, sarcomatoid change, and CCC are biologically different from pure HCC, and the prognosis of these hepatic malignant diseases is worse than that of pure HCC [17,18,19]. Advanced cases tended to be unevenly distributed in the DC group, and the prognosis for non-HCC in this study was worse in the DC group than in the control group, which may influence the results among the two groups.
The non-HCC cases should not have been the subject of this study. Thus, we performed a subgroup analysis with only pure HCC patients and confirmed that there was no difference in DFS between the DC and control groups, but OS was significantly longer in the DC group. However, there are some limitations to these results. One problem is that the prognosis of the control group is poor compared to general reports. In recent years, it was reported that the 3-year OS and recurrence rates after curative hepatectomy in HCC patients in Japan were 85% and 43%, respectively [20]. The OS of the DC group with pure HCC did not improve compared to that in the previous report. Given that this clinical trial included a small number of patients, the patients with a poor prognosis might have been unevenly distributed in the control group; thus, the OS between the two groups might have been different. We also performed a subgroup analysis on HSP70-expressing HCC and found that the OS and DFS were significantly longer in the DC group than in the control group. It was previously reported that the prognosis was worse in the HSP70-expressing HCC than in the HSP70-non-expressing HCC [21]. In the subgroup analysis of HSP70-expressing HCC, the prognosis of the control group was also slightly poorer than that of a previous report, but the prognosis in the treatment group is longer than the that report. In addition, there is no statistically significant difference in the background characteristics between the DC and control groups. These results indicated the HSP70-specific efficacy of this therapy.
The recurrence of HCC is broadly divided into the following two types: intrahepatic metastasis and multicentric occurrence. However, the type of recurrence that could be prevented by the DC therapy is still unknown. Sakon et al. reported that DFS curves obtained by the Kaplan–Meier method for HCC patients in the early (within 2 years) and late (4 years after surgery) follow-up periods were approximated by two regression lines, which represent both residual intrahepatic metastasis and multicentric liver carcinogenesis and only multicentric liver carcinogenesis [22]. They also reported that the difference in the coefficients between the early and late periods indicated the annual rate of residual intrahepatic metastasis. In this study, because the difference in the slope between the early and late periods in the DC group was small, DC therapy might prevent residual intrahepatic metastasis. Furthermore, in the subgroup analysis of HSP70-expressing HCC, there was no difference in the site of first recurrence and frequency of local–regional therapy, but there were more local regional therapy-refractory cases that were shifted to systemic therapy in the control group than in the DC group. Thus, DC therapy might prevent not only intrahepatic metastasis but also systemic metastasis, because it allowed the performance of local–regional therapy for a long time after recurrence.
Immunologically, MDSC was significantly higher at 1 month after the DC therapy in the early recurrence group with HCC. MDSC amplifies the immune suppressive activity of DCs via crosstalk [23]. Therefore, it was suggested that the involvement of MDSC might have affected the therapeutic effect of DC therapy. Also, it is well known that the MDSC amplifies the immune suppressive activity of patients with several types of cancers and that might lead to poor prognosis of patients [24,25,26]. Treatment with DC therapy alone might be inadequate for such patients who require strong immunosuppression.
Several clinical studies of adjuvant therapy after hepatectomy for HCC using the oral multikinase inhibitor sorafenib, a representative anti-tumor drug for HCC, were conducted. The result of a previous retrospective study that utilized sorafenib after resection of BCLC stage C HCC was positive [27, 28], but the result of a large phase III, double-blind, placebo-controlled study involving patients with HCC with a complete radiological response after surgical resection or local ablation was negative [3]. Thus, the positive role of sorafenib as an adjuvant therapy for HCC after surgery is still controversial. In addition, it was reported that sorafenib as an adjuvant therapy for HCC was feasible, but some severe AEs caused by sorafenib administration were also reported [3]. Some clinical trials on postoperative adjuvant chemotherapy have also been conducted, but only few of their patients had improved postoperative prognosis [29, 30]. The postoperative adjuvant therapy with new molecular targeted drugs, such as lenvatinib, is also expected in the future, but none has been established so far.
On the contrary, it was reported that adjuvant immunotherapy for HCC, including DC therapy, cytokine-induced killer cell therapy, and tumor-associated antigen-derived peptide vaccine therapy, might improve patients’ survival and be less toxic than the therapy with sorafenib.[31,32,33,34]. The result of the present clinical trial was comparable to those of other previous clinical trials in terms of efficacy and safety. In particular, a specific therapeutic effect can be expected for HSP70-expressing HCC, which has a poor prognosis [21], when compared with other adjuvant immunotherapies. Thus, the expression of HSP70, which is an immunological target, on the tumor cell may be a biomarker of this therapy. In other words, the HSP70 expression of tumor cells may be used as an accompanying diagnostic method for selecting the cases that would most benefit from the DC therapy. Furthermore, compared to other DC therapies, such as those using peptides [35], the DC therapy used in the present study is more advantageous, as it is not affected by human leukocyte antigen (HLA) class-I because of the transfection of HSP70 mRNA by electroporation. However, as mentioned previously, this therapy is affected by suppressive immunity, such as MDSC, and the development of combination therapy to eliminate this suppressive immunity is necessary in the future.
This study has several limitations. One of the most important limitations was that this study has a small number of patients. In addition, some of host and tumor immunological factor might not be fully examined; thus, our findings need to be further validated in future appropriate phase III clinical trials.
In conclusion, HSP70 mRNA-transfected DC therapy is safe as an adjuvant treatment after hepatectomy for HCC. It was suggested that this therapy may be effective for HSP70 expressing cases. In the future, it is necessary to verify the efficacy of this treatment in clinical trials limited to HSP70-expressing HCC. To improve the effectiveness of this therapy, the identification of optimal biomarkers and development of combination therapy for restoration of suppressive immunity are necessary.
Change history
05 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00262-020-02819-x
References
Ikeda M, Morizane C, Ueno M, Okusaka T, Ishii H, Furuse J (2018) Chemotherapy for hepatocellular carcinoma: current status and future perspectives. Jpn J Clin Oncol 48:103–114
Ban D, Ogura T, Akahoshi K, Tanabe M (2018) Current topics in the surgical treatments for hepatocellular carcinoma. Ann Gastroenterol Surg 2:137–146
Bruix J, Takayama T, Mazzaferro V et al (2015) Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol 16:1344–1354
Nishida N, Kudo M (2018) Immune checkpoint blockade for the treatment of human hepatocellular carcinoma. Hepatol Res 48(8):622–634
Hazama S, Tamada K, Yamaguchi Y, Kawakami Y, Nagano H (2018) Current status of immunotherapy against gastrointestinal cancers and its biomarkers: Perspective for precision immunotherapy. Ann Gastroenterol Surg 2(4):289–303
Matsui H, Hazama S, Tamada K et al (2019) Identification of a promiscuous epitope peptide derived from HSP70. J Immuno 42:244
Nakajima M, Hazama S, Tamada K et al (2020) A phase I study of multi-HLA-binding peptides derived from heat shock protein 70/glypican-3 and a novel combination adjuvant of hLAG-3Ig and Poly-ICLC for patients with metastatic gastrointestinal cancers: YNP01 trial. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-020-02518-7
Takayama T, Sekine T, Makuuchi M et al (2000) Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. The Lancet 356:802–807
Takashima M, Kuramitsu Y, Yokoyama Y, Iizuka N, Toda T, Sakaida I, Okita K, Oka M, Nakamura K (2003) Proteomic profiling of heat shock protein 70 family members as biomarkers for hepatitis C virus-related hepatocellular carcinoma. Proteomics 3:2487–2493. https://doi.org/10.1002/pmic.200300621
Yoshida S, Hazama S, Tokuno K, Sakamoto K, Takashima M, Tamesa T, Torigoe T, Sato N, Oka M (2009) Concomitant overexpression of heat-shock protein 70 and HLA class-I in hepatitis C virus-related hepatocellular carcinoma. Anticancer Res 29:539–544
Maeda Y, Yoshimura K, Matsui H et al (2015) Dendritic cells transfected with heat-shock protein 70 messenger RNA for patients with hepatitis C virus-related hepatocellular carcinoma: a phase 1 dose escalation clinical trial. Cancer Immunol Immunother 64:1047–1056. https://doi.org/10.1007/s00262-015-1709-1
Okabayashi T, Yamamoto J, Kosuge T, Shimada K, Yamasaki S, Takayama T, Makuuchi M (2001) A new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variables. Cancer 92:2374–2383
Ogawa K, Hirai M, Katsube T, Murayama M, Hamaguchi K, Shimakawa T, Naritake Y, Hosokawa T, Kajiwara T (2000) Suppression of cellular immunity by surgical stress. Surgery 127:329–336
Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Bt T, Moutardier V, Farges O, Valla D (2001) Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol 35:254–258
Huang G-T, Sheu J-C, Yang P-M, Lee H-S, Wang T-H, Chen D-S (1996) Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma—a study based on 420 patients. J Hepatol 25:334–338
Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, Ohtomo K, Makuuchi M (1999) Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology 30:889–893
Chen MF (1999) Peripheral cholangiocarcinoma (cholangiocellular carcinoma): clinical features, diagnosis and treatment. J Gastroenterol Hepatol 14:1144–1149
Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D (2002) Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer 94:2040–2046
Liao SH, Su TH, Jeng YM, Liang PC, Chen DS, Chen CH, Kao JH (2019) Clinical manifestations and outcomes of patients with sarcomatoid hepatocellular carcinoma. Hepatology 69:209–221
Hasegawa K, Kokudo N, Makuuchi M et al (2013) Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 58:724–729
Shin E, Ryu HS, Kim S-H, Jung H, Jang J-J, Lee K (2011) The clinicopathological significance of heat shock protein 70 and glutamine synthetase expression in hepatocellular carcinoma. J Sci 18:544–550
Sakon M, Umeshita K, Nagano H et al (2000) Clinical significance of hepatic resection in hepatocellular carcinoma: analysis by disease-free survival curves. Arch Surg 135:1456–1459
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in cancer biology Elsevier, Newyork
Limagne E, Euvrard R, Thibaudin M et al (2016) Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX–bevacizumab drug treatment regimen. Can Res 76:5241–5252
Jiang H, Gebhardt C, Umansky L, Beckhove P, Schulze TJ, Utikal J, Umansky V (2015) Elevated chronic inflammatory factors and myeloid-derived suppressor cells indicate poor prognosis in advanced melanoma patients. Int J Cancer 136:2352–2360
Gonda K, Shibata M, Ohtake T, Matsumoto Y, Tachibana K, Abe N, Ohto H, Sakurai K, Takenoshita S (2017) Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett 14:1766–1774
Li J, Hou Y, Cai X-B, Liu B (2016) Sorafenib after resection improves the outcome of BCLC stage C hepatocellular carcinoma. World J Gastroenterol 22:4034
Xia F, Wu L-L, Lau W-Y, Huan H-B, Wen X-D, Ma K-S, Li X-W, Bie P (2016) Adjuvant sorafenib after heptectomy for Barcelona Clinic Liver Cancer-stage C hepatocellular carcinoma patients. World J Gastroenterol 22:5384
Lai EC, Lo C-M, Fan S-T, Liu C-L, Wong J (1998) Postoperative adjuvant chemotherapy after curative resection of hepatocellular carcinoma: a randomized controlled trial. Arch Surg 133:183–188
Ono T, Yamanoi A, Nazmy El Assal O, Kohno H, Nagasue N (2001) Adjuvant chemotherapy after resection of hepatocellular carcinoma causes deterioration of long-term prognosis in cirrhotic patients: Metaanalysis of three randomized controlled trials. Cancer 91:2378–2385
Sawada Y, Yoshikawa T, Ofuji K et al (2016) Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology 5:e1129483
Shimizu K, Kotera Y, Aruga A et al (2014) Postoperative dendritic cell vaccine plus activated T-cell transfer improves the survival of patients with invasive hepatocellular carcinoma. Hum Vac Immunothera 10:970–976
Lee J-H, Tak WY, Lee Y et al (2017) Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology 6:e1328335
Lee JH, Lee J-H, Lim Y-S et al (2015) Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 148(1383–91):e6
Butterfield LH, Ribas A, Dissette VB et al (2006) A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four α-fetoprotein peptides. Clin Cancer Res 12:2817–2825
Acknowledgments
The authors thank Ms. Akiko Sano and Mrs. Kaori Kaneyasu for their excellent technical assistance with this work. The authors also thank Dr. Kohei Sakai for the generation of HSP70 mRNA.
Funding
No relevant funding was used for this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study design, data interpretation and review of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflict of interest.
Ethics approval
The protocol was approved by the institutional review board of Yamaguchi University (IRB number: H24-40) and was registered with the UMIN Clinical Trials Registry (registration no. UMIN000010691). The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
Informed consent
All patients provided written informed consent prior to undergoing any study-related procedure.
Consent for publication
Consent for publication was obtained from all patients. In addition, all authors agree with the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Immunotherapy targeting HSP70 as a tumor-associated antigen (TSA) using mRNA-transfected DC was safe and effective for curatively resected HSP70-expressing HCC. This study revealed HSP70 could be a TSA for the immunotherapy against HCC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Matsui, H.M., Hazama, S., Nakajima, M. et al. Novel adjuvant dendritic cell therapy with transfection of heat-shock protein 70 messenger RNA for patients with hepatocellular carcinoma: a phase I/II prospective randomized controlled clinical trial. Cancer Immunol Immunother 70, 945–957 (2021). https://doi.org/10.1007/s00262-020-02737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02737-y