Abstract
Regulatory T cells (Tregs) play a major role in the development of an immunosuppressive tumor microenvironment. Systemic Treg depletion is not favored because of the critical role of Tregs in maintaining immune homeostasis and preventing the autoimmunity. Recently, CCR8 has been identified as an important chemokine receptor expressed on intratumoral Tregs and is known to be critical for CCR8+Treg-mediated immunosuppression. However, the inherent molecular mechanisms and clinical significance of intratumoral CCR8+Tregs remain poorly understood. In this study, a retrospective analysis of 259 muscle-invasive bladder cancer (MIBC) patients from two independent clinic centers was conducted to explore the prognostic merit of CCR8+Tregs via immunohistochemistry. Eighty-three fresh MIBC samples and data from the Cancer Genome Atlas were used to evaluate the proportion and function of immune cells via flow cytometry, ex vivo intervention experiments and bioinformatics analysis. It was found that the CCR8 expression by intratumoral Tregs maintained the stability and potentiated their suppressive function by upregulating the expression of transcript factors FOXO1 and c-MAF. High level of CCR8+Tregs was associated with the immune tolerance and predicted poor survival and inferior therapeutic responsiveness to chemotherapy. Moreover, it was revealed that CCR8 blockade could destabilize intratumoral Tregs into a fragile phenotype accompanied with reactivation of antitumor immunity and augment of anti-PD-1 therapeutic benefits in MIBC. In summary, those results suggested that CCR8+Tregs represented a stable Treg subtype and a promising therapeutic target in the immunotherapy of MIBC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Muscle-invasive bladder cancer (MIBC) has a poor prognosis with a 5-year survival rate of roughly 60% [1]. The standard treatment for localized MIBC relies on radical cystectomy (RC) complemented with cisplatin-based chemotherapy in a neoadjuvant or adjuvant setting. However, the response rate to cisplatin-based regimens does not exceed 50% [2]. One of the potential difficulties is the presence of immunosuppressive cell populations, such as regulatory T cells (Tregs), which orchestrate the immune evasion to the wave of cell death following the chemotherapy [3, 4]. Therefore, there is an urgent need for elucidating the immunoregulatory mechanisms and identifying an improved stratification of MIBC for better prediction on chemotherapy treatment response.
Treg stability is defined as sustained Foxp3 expression, hypomethylation at the CNS2 locus, and maintained suppressive phenotype [5, 6]. To date, the reported dominant stabilizing factors in tumor-resident Tregs include CARMA1-BCL10-MALT1 complex [7], Helios [8, 9], Neuropilin-1 [10, 11], programmed cell death protein 1 (PD-1) [12], indoleamine 2,3-dioxygenase [13] and the PTEN phosphatase pathway [14, 15]. Under such conditions, intratumoral Tregs can potently inhibit the antitumor immunity and contribute to the development of an immunosuppressive tumor microenvironment (TME) [4,5,6]. Treg modulation strategies have been shown to increase the anti-tumor immunity and reduce the tumor burden in both preclinical and clinical settings [3, 4, 16]. However, there are some drawbacks of the strategies, such as autoimmunity and specificity of targeting [4, 16]. Therefore, a more effective strategy to selectively target intratumoral Tregs is required.
The CC-chemokine receptor CCR8 has been identified as a specific marker, which is selectively upregulated by intratumoral Tregs in several types of human cancers, such as lung, breast and colorectal cancers [17,18,19]. The increased CCR8 transcription in whole-tumor samples has indicated poor prognosis [17, 18]. Of note, CCR8+Treg has been defined as a major driver of immunosuppression in an experimental model of autoimmunity [20, 21]. Targeting CCR8 in the mouse models of cancer has shown the impaired suppressive character of the TME and enhanced antitumor immunity [22]. However, the inherent molecular mechanisms of intratumoral CCR8+Tregs and the extent of antitumor effects on human cancers resulted from targeting CCR8 remain to be determined.
In the present study, the mechanisms of CCR8 in maintaining intratumoral Tregs stability and immunosuppressive function were reported. The accumulation of CCR8+Tregs indicated immunoevasive subtype MIBC with poor prognosis and suboptimum for adjuvant chemotherapy. Furthermore, blockade of CCR8 destabilized Tregs and reprogrammed them into a fragile phenotype, which reactivated the antitumor immunity and showed a preliminary synergistic efficacy with an anti-PD1 monoclonal antibody (pembrolizumab) against malignant cells. Our findings shed light on targeting CCR8+Tregs as a promising immunotherapeutic strategy for MIBC.
Materials and methods
Patients and tumor tissue samples
This study began with two independent patient cohorts, i.e., the Zhongshan Hospital cohort (ZH cohort, n = 215) and Fudan University Shanghai Cancer Center cohort (FUSCC cohort, n = 178). All patients (n = 393) received RC initially. The exclusion criteria were followed in all the cases: (1) pathological diagnosed not as MIBC or combined with other pathological types; (2) with distant metastatic disease; (3) incomplete follow-up information; (4) preoperative chemotherapy or radiotherapy. As a result, 259 eligible MIBC patients were included (ZH cohort, n = 141; FUSCC cohort, n = 118). After RC, 119 patients (46.3%) received cisplatin-based combination chemotherapy (at least one cycle). The follow-up principle was based on the European Association of Urology guidelines for MIBC. All the follow-up data were collected from the date of surgery to July 2016. The overall survival (OS) was defined as the time from the date of surgery to death or last follow-up. The recurrence-free survival (RFS) was defined as the time from the date of surgery to the first recurrence or last follow-up. The detailed clinical-pathological characteristics of 259 patients are presented in Supplementary Table 1.
Fresh MIBC tumor tissues (n = 83) were collected from four independent clinical centers in Shanghai, China (Shanghai General Hospital, ZH, FUSCC and Ruijin Hospital). The exclusion criteria were described above. All the patients signed an informed consent.
Double immunohistochemistry and immunofluorescence
Tissue microarray (TMA) development and immunohistochemistry (IHC) were performed according to our previously described methods [23, 24]. Single staining was performed on CD8+T cells, CD4+T cells, natural killer cells, macrophages, neutrophils, mast cells and B cells. Double staining was performed on DCs and CCR8+Tregs. The details of antibodies are listed in Supplementary Table 2.
For double IHC staining, the TMAs were dewaxed and hydrated, heated with an autoclave in sodium citrate buffer for antigen retrieval and incubated with 10% normal goat serum for antigen blocking. Next, the sections were incubated with the first primary antibodies at 37 °C for 2 h, followed by HRP-labeled secondary antibody incubation and visualization using DAB reagent. Subsequently, the TMAs were incubated with the second primary antibodies overnight at 4 °C before proceeding to the secondary antibody and Vector Blue staining. Finally, the sections were washed, dehydrated and mounted.
CCR8+Treg cell counts and other stained-positive cell counts were enumerated as the mean value of 3 randomized high power magnification fields (HPF, 200× magnification) of each section. CCR8+Treg density was counted as cells/mm2. Two urologic pathologists from different medical institutes who were blinded to the clinical data reviewed the slides independently with the assistance of Image-Pro Plus (Media Cybernetics Inc.). F tests in the reliability analyses showed P = 0.640 for CCR8+Treg counting, indicating a good reliability of these two counting results. The mean value of the two counting results was adopted to conduct further analyses. To obtain the best prognostic efficacy, X-Tile Software (Yale University, version 3.6.1) was used as previously described [25].
For immunofluorescence (IF), the sections were incubated with two primary antibodies at 4 °C overnight. Then, the samples were incubated with species-appropriate rabbit/mouse secondary antibodies coupled to Alexa Fluor dyes (555, 488, Invitrogen) at 37 °C for 2 h. Finally, the slides were mounted with antifade mounting solution containing DAPI. All the antibodies and reagents are summarized in Supplementary Table 2. The slides were detected through Leica DM6000 B Microsystems.
Definition of cutoff values
For CCR8+Treg density, the values below and above 6.6 cells/mm2 were defined as low and high ones, respectively. For the CD8+T cells, the values below and above 38 cells/HPF were defined as low and high ones, respectively.
Flow cytometry
Fresh MIBC tissues were collected as soon as the samples were resected during the surgery. After digested by means of collagenase IV and lysed the red blood cells, single cell suspension was stained with the indicated monoclonal antibodies (mAbs) at 4 °C for 30 min in the dark. If necessary, staining of intracellular molecules or transcription factor was established by using Fixation/Permeabilization Solution Kit or Transcription Factor Fixation/Permeabilization buffer set (BD Biosciences) according to the manufacturer’s instructions. The stained cells were washed and re-suspended in cell staining buffer. Flow cytometry (FCM) was performed using a BD Celesta and analyzed by FlowJo software (Tree Star). All FCM antibodies and reagents are summarized in Supplementary Table 3.
Bioinformatics analysis
The Cancer Genome Atlas (TCGA) BLCA mRNA and clinical data, including RNA sequencing and clinicopathological data for 408 tumors, were downloaded from https://www.cbioportal.org on December 26, 2018, and were normalized to TPM format. The cutoff value of CCR8 expression in MIBC data was determined as median. Gene set enrichment analysis (GSEA) performed by the Molecular Signature Database (MSigDB) was used to identify the pathways that were significantly enriched in CCR8high tumor samples. If a gene set had a positive enrichment score, the majority of its members had higher expression accompanied with higher risk score, and the set was termed “enriched.”
Ex vivo intervention assay
The ex vivo intervention studies were performed according to the methods described previously [26, 27]. Briefly, fresh bladder cancer tissues were washed 3 times with RPMI-1640 medium containing 1% fetal bovine serum before being minced. The specimens were then dissociated in RPMI-1640 medium containing 1 mg/mL collagenase IV in incubator shaker at 37 °C for 2 h. After filtered through a 70 μm cell strainer (BD Labware), single cell suspensions including tumor cells, immune cells and other cells were co-cultured and randomly divided into 4 groups (isotype controls, anti-CCR8, pembrolizumab, anti-CCR8 + pembrolizumab). The cells were then cultured for 12 h in RPMI 1640 medium containing 10% FBS, corresponding isotype and neutralizing antibodies. The antibodies included in this experiment were IgG2B isotype control (0.5 μg /ml, Clone 141,945, R&D systems), anti-CCR8 (0.5 μg/ml, Clone 191,704, R&D systems) and pembrolizumab (5 µg/mL, Selleck). After overnight culture, cells were subjected to FCM analysis to examine the apoptosis of tumor cells by using FITC Annexin V Apoptosis Detection Kit I (BD Biosciences) or corresponding FCM antibodies.
Statistical analysis
The results were expressed as mean ± SD. Pearson’s χ2 test was applied for categorical variables, whereas continuous variables were analyzed by t test. The correlation analysis was made by Spearman correlation. OS and RFS were analyzed through Kaplan–Meier curves, log-rank test, and multivariate analysis based on the Cox proportional hazards method. Interaction analysis was also conducted using Cox regression. A two-tailed P value below 0.05 was considered statistically significant. Statistical analyses were conducted using SPSS, version 22.0, Graph Pad Prism Software 8.0 and Medcalc 15.
Results
CCR8+Tregs accumulate in MIBC and yielded poor prognosis in MIBC patients
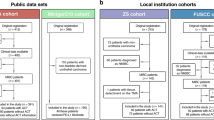
The flowchart of study population from four cohorts is illustrated in Fig. 1a. Representative IF and IHC images exhibited the accumulation of CCR8+Tregs in MIBC, respectively (Fig. 1b, c). The number of CCR8+Tregs was apparently correlated with enhanced tumor stages based on the IHC staining (Fig. 1d). Furthermore, the FCM analysis also validated that compared with peritumor tissues, MIBC samples displayed a higher percentage of Tregs expressing CCR8 (Fig. 1e, f).
CCR8+Tregs accumulate in MIBC and correlate with disease progression, a flow diagram of patients enrollment in this study. b Representative immunofluorescence staining for Foxp3 (green) and CCR8 (red) in MIBC specimens. Nuclei counterstained blue with DAPI. White arrowheads indicate CCR8+Tregs. Scale bars, 12.5 μm. c Representative immunohistochemistry images show CCR8+Treg (left) and CCR8−Treg (right) in MIBC. Scale bars, 50 μm. d The proportion of CCR8+Tregs high and low specimens in different stages of MIBC (**P < 0.01). e Flow cytometry analysis of CCR8+Tregs in MIBC and peritumor tissues. f Statistical analysis of CCR8 expression on gated CD3+CD4+Foxp3+Tregs in MIBC and peritumor tissues. **P < 0.01; ****P < 0.0001
To investigate the clinical significance of intratumoral CCR8+Tregs in MIBC, Kaplan–Meier curves and log-rank test were applied to compare OS and RFS between CCR8+Tregs low and high patients. In ZH and FUSCC cohorts, patients with high CCR8+Tregs had significantly poorer OS (P < 0.0001 and P < 0.0001; Fig. 2a, b) and RFS (P = 0.0001 and P = 0.0049; Fig. 2c, d). Next, multivariate analysis was performed to figure out whether CCR8+Tregs could serve as a potential independent prognostic factor for the survival outcomes. The analysis was conducted including the age, gender, tumor size, tumor grade, lymphovascular invasion, American Joint Committee on Cancer Staging and CCR8+Tregs accumulation. It was found that CCR8+Treg was an independent adverse prognosticator for OS and RFS according to multivariate analysis in all patients (hazard ratio [HR]: 2.976, 95% confidence interval [CI] 2.055–4.309, P < 0.001 and HR 2.244, 95% CI 1.508–3.338, P < 0.001; Fig. 2e, f). Hence, these findings suggested that CCR8+Tregs may contribute to the progression and poor prognosis of MIBC.
The prognostic value of CCR8+Tregs in patients with MIBC. a, b Overall survival curves of patients with low and high CCR8+Tregs group in two independent cohorts. c, d Recurrence-free survival curves of patients with low and high CCR8+Tregs group in two independent cohorts. Multivariate Cox proportional hazards regression analysis of the clinicopathological parameters influencing overall survival (e) and recurrence-free survival of MIBC patients (f)
CCR8+Tregs indicate suboptimum responsiveness to adjuvant chemotherapy in MIBC
The positive role of adjuvant chemotherapy (ACT) after RC has been strengthened with recent data, despite a poor level of evidence [1]. Of note, ACT application did not reach statistical significance in our study (OS: Fig. 3a; RFS: data not shown). Therefore, the association between intratumoral CCR8+Tregs and the therapeutic responsiveness to ACT was inspected. In the patients with CCR8+Tregs low group, receiving chemotherapy significantly led to better OS (Fig. 3b). However, no obvious survival improvement was observed after ACT in CCR8+Tregs high patients (Fig. 3c). As for RFS, it was found that ACT did not provide survival benefit in pT2 patients either (Fig. 3d). However, a favorable outcome was found only in pT2 patients with low CCR8+Tregs accumulation (Fig. 3e, f). The interaction test between CCR8+Tregs and ACT responsiveness revealed that CCR8+Tregs low patients exhibited far better therapeutic responsiveness to ACT than CCR8+Tregs high patients. Detailed Cox proportional hazards regression analysis is shown in Supplementary Table 4. Consequently, those results suggested that CCR8+Tregs may possibly attenuate therapeutic responsiveness to ACT in MIBC.
Predictive merit of CCR8+Tregs with response to adjuvant chemotherapy for MIBC patients. The overall survival curves for all patients (a), CCR8+Tregs high group of all patients (b) and CCR8+Tregs low group of all patients (Doi:) without or with ACT treatment. The recurrence-free survival curves for all pT2 patients (d), CCR8+Tregs high group of all pT2 patients (e) and CCR8+Tregs low group of all pT2 patients (f) without or with ACT treatment
CCR8+Tregs represent a stable Treg subtype with enhanced immunosuppressive capacity in MIBC
To further determine the role of CCR8+Tregs in MIBC progression and immunity, the phenotype difference between CCR8+Treg and its counterpart was identified. It was shown that the intratumoral CCR8+Tregs displayed a stable Treg phenotype with higher levels of stability markers, including Foxp3, CD25, CTLA-4 and PD-1, and elevated expression of suppressive and tissue resident molecules, such as LAG-3, IL-10, GZMB and CD69 (Fig. 4a). Then, the molecular mechanism of CCR8 in maintaining Treg stability was defined. By using GSEA to identify pathways that were activated in CCR8high tumors as compared with CCR8low tumors, we identified Jak-STAT signaling as the most significantly upregulated pathway (Fig. 4b). In addition, chemokine signaling pathway was significantly enriched as well. The CCL1-CCR8 axis in potentiating the suppressive function of Treg was dependent on STAT3 [20]. Furthermore, the transcript factors FOXO1 and c-MAF could be regulated by STAT3 and were reported to be involved in Treg differentiation and function [28,29,30,31,32,33,34]. Here, it was found that in TCGA cohort, the transcription level of STAT3 was highly correlated with those of FOXO1 and c-MAF (Fig. 4c). Meanwhile, the expression levels of FOXO1 and c-MAF were strongly enriched in CCR8+Tregs (Fig. 4d). Those findings indicated that CCR8+Tregs may elicit immune evasion during MIBC progression and serve as a potential intervention target for cancer immunotherapy.
CCR8+Tregs represent a stable Treg subtype with enhanced immunosuppressive capacity in MIBC. a Molecules expression in intratumoral CCR8−Tregs and CCR8+Tregs measured by flow cytometry. b Gene set enrichment analysis revealed an enrichment of Jak-STAT signaling pathway and chemokine signaling pathway involved in CCR8 high tumors. NES, normalized enrichment score. c Spearman correlation of STAT3 and the FOXO1 (left) and c-MAF (right) in TCGA cohort. d Expression of FOXO1 and c-MAF in CCR8−Tregs and CCR8+Tregs of MIBC, as measured by flow cytometry. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001
CCR8+Tregs shape immunosuppressive contexture in MIBC
Next, the influence of CCR8+Tregs on immune contexture in MIBC was discovered. Through IHC analysis, it was found that MIBC with high CCR8+Tregs exhibited higher levels of CD4+T cells, macrophages and mast cells compared with MIBC with low CCR8+Tregs accumulation (Fig. 5a). FCM analysis also validated that the level of CD4+T cells was significantly higher in MIBC samples with high CCR8+Tregs (Fig. 5b). Although no obvious association between the number proportion of CCR8+Tregs and CD8+T cells was found (Fig. 5b), CD8+T cells in MIBC specimens with high CCR8+Tregs displayed exhausted phenotype with decreased IFN-γ, TNF-α and Ki-67, yet elevated PD-1 and TIGIT expression (Fig. 5c). Th17 cells are the best-studied sources of IL-17A [35], and our current research has revealed that enriched intratumoral IL-17A+ cells indicated an antitumor immune contexture in MIBC. Of note, in CCR8+Tregs high group, the elevated CD4+T cells exhibited lower level of IFN-γ and IL-17A (Fig. 5d). To further confirm the association between CCR8+Tregs and CD8+T cell immunity in MIBC, the influence of CCR8+Tregs on the prognostic merit of CD8+T cells in MIBC was analyzed. As shown in Fig. 5e, when analyzing low and high CCR8+Tregs groups, respectively, it was notably revealed that CD8 expression predicted a highly favorable prognosis in CCR8+Tregs low group, whereas high CCR8+Tregs seemed to abrogate the beneficial effect of CD8 expression. Overall, these findings explicitly implied that CCR8+Tregs may potentially facilitate CD8+T cells immune tolerance and promote the accumulation of CD4+T cell with a less anti-tumor phenotype in MIBC.
CCR8+Tregs shape immunosuppressive contexture in MIBC a Immunohistochemistry analysis of the indicated immune cells between CCR8+Tregs low and high group in MIBC TMAs (n = 141). b Flow cytometry analysis to determine the CD8+T and CD4+T cells frequency of CD45+cells in CCR8+Tregs low and high MIBC specimens. c Upper: the proportion of effector molecule and Ki-67-positive CD8+T cells to total CD8+T cells between CCR8+Tregs low and high MIBC specimens; down: the ratio of inhibitory receptor-positive CD8+T cells in total CD8+T cells between CCR8+Tregs low and high MIBC specimens. d The ratio of indicated proteins positive CD4+T cells to total CD4+T cells between CCR8+Tregs low and high MIBC specimens. e The overall survival curves of CD8+T cells low and high groups in low and high CCR8+Tregs-infiltrating patients. *P < 0.05; **P < 0.01; CD8, CD8+T cells; CD4, CD4+T cells; NK, nature killer cells; DC, dendritic cells; M0, M0 macrophages; Neu, neutrophils; Mast, mast cells; B, B cells
CCR8 blockade promotes Treg destabilization, reactivates the antitumor activity and improves the efficacy of PD-1 inhibitor
The effects of blocking CCR8 on immune microenvironment in MIBC were investigated using a CCR8 neutralizing antibody. According to the methods described by the recent literature [26, 27], the tumor culture system in vitro was established to simulate the in vivo tumor immune system of patients in our study, which included tumor cells, immune cells and other cells. This system included antigen-presenting cells, such as dendritic cells and macrophages, which were responsible for the activation of CD8+T cells. In addition, a transcriptome analysis of human cancer specimens has revealed that tumor-infiltrating Treg cells assume an activated phenotype that is distinct from Treg cells in peripheral tissues, supporting the notion that Treg cells in the TME are activated and have a strong immunosuppressive capacity [17].
After incubating with the CCR8 neutralizing antibody for 12 h, single MIBC cell suspensions were subjected to FCM analysis. As shown in Fig. 6a, the proportion and intensity of Foxp3 expression in CD4+T cells were remarkably downregulated, as well as transcript factors and immunosuppressive molecules mentioned above, indicating the Treg destabilization after CCR8 blockade. Furthermore, the characters of CD8+ and CD4+T cells were investigated. Compared with the control group, blockade of CCR8 led to significant upregulation of IFN-γ, TNF-α, Ki-67 in CD8+T cells and IFN-γ, IL-17A in CD4+Foxp3−T cells, whereas the numbers of PD-1+ CD8+T and TIGIT+ CD8+T cells declined markedly (Fig. 6b, c). This suggested that blockade of CCR8 may promote the anti-tumor activity of CD8+T cells and repolarize CD4+T cells toward an anti-tumor phenotype. Of note, it was found that CCR8 blockade significantly upregulated the expression of IL-17A in Tregs compared with that in the control group (Fig. 6c). As the overall objective response rates of five immune-checkpoint inhibitors (ICIs) targeting PD-1 or PD-L1 were relatively low in MIBC(< 30%) [36], the potential synergistic role of CCR8 blockade was investigated with pembrolizumab in mediating the tumor cell elimination. Consequently, it was demonstrated that the combination of targeting CCR8 and pembrolizumab could significantly hamper the proliferation of tumor cells and trigger their apoptosis, as compared with single treatment groups (Fig. 6d, e). Taken together, these findings implied that blockade of CCR8 may elicit a preliminary synergistic effect with pembrolizumab to facilitate the clearance of tumor cells.
CCR8 blockade promotes Treg destabilization, reactivates the antitumor activity and improves the efficacy of PD-1 inhibitor a MIBC tissues were digested, incubated with control or CCR8-neutralizing antibody, and subjected to flow cytometry analysis to determine the molecules expression in Tregs. b Following the incubation with isotype or CCR8-neutralizing antibody, MIBC single cell suspension was subjected to flow cytometry analysis to determine the positive ratios of the indicated molecules in CD8+T cells. c Flow cytometry analysis to determine the positive ratios of the indicated molecules in CD4+FOXP3− cells and Tregs. d The synergistic effect of CCR8-neutralizing antibody and pembrolizumab on the frequency of Ki67+ epithelial cells in MIBC samples. e The synergistic effect of CCR8-neutralizing antibody and pembrolizumab on the frequency of apoptotic epithelial cells in MIBC specimens. *P < 0.05; **P < 0.01
Discussion
In this study, variable CCR8 expression was revealed by intratumoral Tregs in MIBC. CCR8+Treg represented a stable Treg subtype with enhanced immunosuppressive capacity, and its frequency increased with disease progression. High amounts of intratumoral CCR8+Tregs indicated an inferior OS and RFS and may predict ACT irresponsiveness and immune tolerance in MIBC patients. Targeting CCR8 using neutralizing antibody destabilized Tregs into a fragile phenotype, resultantly reactivating the anti-tumor immunity in MIBC. Those findings highlighted the importance of targeting CCR8 in the immunotherapy of MIBC.
Remarkably, our study was the first to reveal the mechanism of CCR8 in maintaining the stability of intratumoral Tregs in MIBC. Through a STAT3-dependent pathway, CCR8+Tregs elevated the expression of transcript factors FOXO1 and c-MAF, which contributed to the increased expression of Foxp3 and subsequent suppressive molecules, leading to the development of immunosuppressive TME in MIBC. FOXO1 is the key for Foxp3 upregulation during Treg development and controls the expression of a subset of Treg-associated genes [28,29,30]. It was reported that the interaction between CCL1 and CCR8 induced the phosphorylation of STAT3, which could increase the expression and translocation of FOXO1 into the nucleus [31, 32]. The transcription factor c-MAF has been recognized as a critical regulator of IL-10 production in Treg cells [33, 34]. What is more, it was reported that the intestinal Treg cells required c-MAF to regulate the abundance of TH17 cells [34], which was consistent with our findings that CD4+T cells in MIBC specimens with high amounts of CCR8+Tregs secreted a low level of IL-17A. In addition, while constitutive activation of the PI3K/Akt/mTOR pathway could prevent the induction of Foxp3 by means of phosphorylation and inactivation of FOXO1 [5], c-MAF could in turn prevent the excessive activation of the PI3K/Akt/mTOR pathway [34], indicating the complex regulatory networks between transcript factors and the PTEN phosphatase pathway. Those findings implicated that upregulation of FOXO1 and c-MAF induced by CCR8 has a pivotal role in keeping Tregs stable and enhancing their immunosuppressive function in MIBC.
Treg fragility is defined as the retention of Foxp3 expression with loss of suppressive function, accompanied by upregulation of proinflammatory markers such as IFN-γ and IL-17A [6]. Early studies have indicated that induction of a fragile Treg phenotype showed not only the loss of suppressive function but the gain of effector activity that initiates tumor control in mouse models of cancer [7,8,9]. Interestingly, using fresh human tumor specimens, our study revealed that blockade of CCR8 within the TME reprogrammed intratumoral Tregs into a fragile phenotype, leading to elevated secretion of IL-17A by Tregs. Recent data have reported that c-MAF is required for the differentiation of naïve T cells specific to the pathobiont Helicobacter hepaticus into RORγt+Foxp3+Tregs [34]. In the absence of c-MAF, the differentiation of these cells was diverted to Foxp3−Th17 effector cells, which may partly explain the upregulation of IL-17A by Tregs in our study. In addition, given that Tregs have been implicated in resistance to ICIs, sensitizing Tregs to become fragile may be an effective strategy to utilize alongside PD-1 blockade [7, 11]. It was found that combination of CCR8 blockade and pembrolizumab significantly increased the apoptosis and death proportion of tumor cells than pembrolizumab alone. Furthermore, MIBC with high CCR8+Tregs accumulation showed irresponsiveness to ACT in our study, which indicated that these patients were likely to benefit from therapy combining CCR8 blockade with chemotherapy.
Our present study also has some limitations. First, the design was retrospective, and the number of patients was relatively small. In addition, it lacked researches in vivo to further characterize the role of CCR8+Tregs in MIBC. Further confirmation of our findings within the framework of larger, multi-centered and randomized clinical trials is warranted, associated with in vivo and in vitro studies.
Conclusions
This study identified CCR8+Tregs as a stable Treg subtype and an independent prognostic indicator for survival and responsiveness to ACT in patients with MIBC. Most importantly, therapeutic targeting of CCR8 provided a more specific modulation of Tregs and a potentially robust approach to MIBC immunotherapy.
Abbreviations
- ACT:
-
Adjuvant chemotherapy
- CI:
-
Confidence interval
- FCM:
-
Flow cytometry
- FUSCC:
-
Fudan University Shanghai Cancer Center
- GSEA:
-
Gene set enrichment analysis
- HR:
-
Hazard ratio
- ICIs:
-
Immune-checkpoint inhibitors
- IF:
-
Immunofluorescence
- IHC:
-
Immunohistochemistry
- MIBC:
-
Muscle-invasive bladder cancer
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death protein 1
- RC:
-
Radical cystectomy
- RFS:
-
Recurrence-free survival
- TCGA:
-
The Cancer Genome Atlas
- TMA:
-
Tissue microarray
- TME:
-
Tumor microenvironment
- Treg:
-
Regulatory T cell
- ZH:
-
Zhongshan Hospital
References
Alfred Witjes J, Lebret T, Comperat EM, Cowan NC, De Santis M, Bruins HM, Hernandez V, Espinos EL, Dunn J, Rouanne M, Neuzillet Y, Veskimae E, van der Heijden AG, Gakis G, Ribal MJ (2017) Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol 71(3):462–475. https://doi.org/10.1016/j.eururo.2016.06.020
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18(17):3068–3077. https://doi.org/10.1200/jco.2000.18.17.3068
Schneider AK, Chevalier MF, Derre L (2019) The multifaceted immune regulation of bladder cancer. Nat Rev Urol 16(10):613–630. https://doi.org/10.1038/s41585-019-0226-y
Togashi Y, Shitara K, Nishikawa H (2019) Regulatory T cells in cancer immunosuppression: implications for anticancer therapy. Nat Rev Clin Oncol 16(6):356–371. https://doi.org/10.1038/s41571-019-0175-7
Munn DH, Sharma MD, Johnson TS (2018) Treg destabilization and reprogramming: implications for cancer immunotherapy. Cancer Res 78(18):5191–5199. https://doi.org/10.1158/0008-5472.Can-18-1351
Overacre-Delgoffe AE, Vignali DAA (2018) Treg fragility: a prerequisite for effective antitumor immunity? Cancer Immunol Res 6(8):882–887. https://doi.org/10.1158/2326-6066.Cir-18-0066
Di Pilato M, Kim EY, Cadilha BL, Prussmann JN, Nasrallah MN, Seruggia D, Usmani SM, Misale S, Zappulli V, Carrizosa E, Mani V, Ligorio M, Warner RD, Medoff BD, Marangoni F, Villani AC, Mempel TR (2019) Targeting the CBM complex causes Treg cells to prime tumours for immune checkpoint therapy. Nature 570(7759):112–116. https://doi.org/10.1038/s41586-019-1215-2
Kim HJ, Barnitz RA, Kreslavsky T, Brown FD, Moffett H, Lemieux ME, Kaygusuz Y, Meissner T, Holderried TA, Chan S, Kastner P, Haining WN, Cantor H (2015) Stable inhibitory activity of regulatory T cells requires the transcription factor Helios. Science 350(6258):334–339. https://doi.org/10.1126/science.aad0616
Nakagawa H, Sido JM, Reyes EE, Kiers V, Cantor H, Kim HJ (2016) Instability of Helios-deficient Tregs is associated with conversion to a T-effector phenotype and enhanced antitumor immunity. Proc Natl Acad Sci U S A 113(22):6248–6253. https://doi.org/10.1073/pnas.1604765113
Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J, Workman CJ, Vignali DA (2013) Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature 501(7466):252–256. https://doi.org/10.1038/nature12428
Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA (2017) Interferon-gamma drives treg fragility to promote anti-tumor immunity. Cell 169(6):1130–1141.e1111. https://doi.org/10.1016/j.cell.2017.05.005
Boussiotis VA (2016) Molecular and biochemical aspects of the PD-1 checkpoint pathway. N Engl J Med 375(18):1767–1778. https://doi.org/10.1056/NEJMra1514296
Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH (2007) Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest 117(9):2570–2582. https://doi.org/10.1172/jci31911
Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, Wolchok JD, Mautino MR, Celis E, Sharpe AH, Francisco LM, Powell JD, Yagita H, Mellor AL, Blazar BR, Munn DH (2015) The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci Adv 1(10):e1500845. https://doi.org/10.1126/sciadv.1500845
Merkenschlager M, von Boehmer H (2010) PI3 kinase signalling blocks Foxp3 expression by sequestering Foxo factors. J Exp Med 207(7):1347–1350. https://doi.org/10.1084/jem.20101156
Tanaka A, Sakaguchi S (2017) Regulatory T cells in cancer immunotherapy. Cell Res 27(1):109–118. https://doi.org/10.1038/cr.2016.151
De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola ML, Panzeri I, Moro M, Crosti M, Mazzara S, Vaira V, Bosari S, Palleschi A, Santambrogio L, Bovo G, Zucchini N, Totis M, Gianotti L, Cesana G, Perego RA, Maroni N, Pisani Ceretti A, Opocher E, De Francesco R, Geginat J, Stunnenberg HG, Abrignani S, Pagani M (2016) Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating t regulatory cells. Immunity 45(5):1135–1147. https://doi.org/10.1016/j.immuni.2016.10.021
Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY (2016) Regulatory T cells exhibit distinct features in human breast cancer. Immunity 45(5):1122–1134. https://doi.org/10.1016/j.immuni.2016.10.032
Wang L, Simons DL, Lu X, Tu TY, Solomon S, Wang R, Rosario A, Avalos C, Schmolze D, Yim J, Waisman J, Lee PP (2019) Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. Nat Immunol 20(9):1220–1230. https://doi.org/10.1038/s41590-019-0429-7
Barsheshet Y, Wildbaum G, Levy E, Vitenshtein A, Akinseye C, Griggs J, Lira SA, Karin N (2017) CCR8(+)FOXp3(+) Treg cells as master drivers of immune regulation. Proc Natl Acad Sci U S A 114(23):6086–6091. https://doi.org/10.1073/pnas.1621280114
Karin N (2018) Chemokines and cancer: new immune checkpoints for cancer therapy. Curr Opin Immunol 51:140–145. https://doi.org/10.1016/j.coi.2018.03.004
Villarreal DO, L'Huillier A, Armington S, Mottershead C, Filippova EV, Coder BD, Petit RG, Princiotta MF (2018) Targeting CCR8 induces protective antitumor immunity and enhances vaccine-induced responses in colon cancer. Cancer Res 78(18):5340–5348. https://doi.org/10.1158/0008-5472.Can-18-1119
Fu H, Zhu Y, Wang Y, Liu Z, Zhang J, Xie H, Fu Q, Dai B, Ye D, Xu J (2018) Identification and validation of stromal immunotype predict survival and benefit from adjuvant chemotherapy in patients with muscle-invasive bladder cancer. Clin Cancer Res 24(13):3069–3078. https://doi.org/10.1158/1078-0432.Ccr-17-2687
Hu B, Wang Z, Zeng H, Qi Y, Chen Y, Wang T, Wang J, Chang Y, Bai Q, Xia Y, Wang Y, Liu L, Zhu Y, Dai B, Guo J, Xu L, Zhang W, Xu J (2020) Blockade of DC-SIGN+ tumor-associated macrophages reactivates anti-tumor immunity and improves immunotherapy in muscle-invasive bladder cancer. Cancer Res. https://doi.org/10.1158/0008-5472.Can-19-2254
Camp RL, Dolled-Filhart M, Rimm DL (2004) X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 10(21):7252–7259. https://doi.org/10.1158/1078-0432.Ccr-04-0713
Lin C, He H, Liu H, Li R, Chen Y, Qi Y, Jiang Q, Chen L, Zhang P, Zhang H, Li H, Zhang W, Sun Y, Xu J (2019) Tumour-associated macrophages-derived CXCL8 determines immune evasion through autonomous PD-L1 expression in gastric cancer. Gut 68(10):1764–1773. https://doi.org/10.1136/gutjnl-2018-316324
Adeegbe DO, Liu Y, Lizotte PH, Kamihara Y, Aref AR, Almonte C, Dries R, Li Y, Liu S, Wang X, Warner-Hatten T, Castrillon J, Yuan GC, Poudel-Neupane N, Zhang H, Guerriero JL, Han S, Awad MM, Barbie DA, Ritz J, Jones SS, Hammerman PS, Bradner J, Quayle SN, Wong KK (2017) Synergistic immunostimulatory effects and therapeutic benefit of combined histone deacetylase and bromodomain inhibition in non-small cell lung cancer. Cancer Discov 7(8):852–867. https://doi.org/10.1158/2159-8290.Cd-16-1020
Ouyang W, Beckett O, Ma Q, Paik JH, DePinho RA, Li MO (2010) Foxo proteins cooperatively control the differentiation of Foxp3+ regulatory T cells. Nat Immunol 11(7):618–627. https://doi.org/10.1038/ni.1884
Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, Peng M, Chan P, Ma Q, Mo Y, Meijer D, Zhao K, Rudensky AY, Atwal G, Zhang MQ, Li MO (2012) Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature 491(7425):554–559. https://doi.org/10.1038/nature11581
Luo CT, Liao W, Dadi S, Toure A, Li MO (2016) Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature 529(7587):532–536. https://doi.org/10.1038/nature16486
Oh HM, Yu CR, Golestaneh N, Amadi-Obi A, Lee YS, Eseonu A, Mahdi RM, Egwuagu CE (2011) STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J Biol Chem 286(35):30888–30897. https://doi.org/10.1074/jbc.M111.253500
Oh HM, Yu CR, Dambuza I, Marrero B, Egwuagu CE (2012) STAT3 protein interacts with Class O Forkhead transcription factors in the cytoplasm and regulates nuclear/cytoplasmic localization of FoxO1 and FoxO3a proteins in CD4(+) T cells. J Biol Chem 287(36):30436–30443. https://doi.org/10.1074/jbc.M112.359661
Xu M, Pokrovskii M, Ding Y, Yi R, Au C, Harrison OJ, Galan C, Belkaid Y, Bonneau R, Littman DR (2018) c-MAF-dependent regulatory T cells mediate immunological tolerance to a gut pathobiont. Nature 554(7692):373–377. https://doi.org/10.1038/nature25500
Neumann C, Blume J, Roy U, Teh PP, Vasanthakumar A, Beller A, Liao Y, Heinrich F, Arenzana TL, Hackney JA, Eidenschenk C, Galvez EJC, Stehle C, Heinz GA, Maschmeyer P, Sidwell T, Hu Y, Amsen D, Romagnani C, Chang HD, Kruglov A, Mashreghi MF, Shi W, Strowig T, Rutz S, Kallies A, Scheffold A (2019) c-Maf-dependent Treg cell control of intestinal TH17 cells and IgA establishes host-microbiota homeostasis. Nat Immunol 20(4):471–481. https://doi.org/10.1038/s41590-019-0316-2
Stockinger B, Omenetti S (2017) The dichotomous nature of T helper 17 cells. Nat Rev Immunol 17(9):535–544. https://doi.org/10.1038/nri.2017.50
Felsenstein KM, Theodorescu D (2018) Precision medicine for urothelial bladder cancer: update on tumour genomics and immunotherapy. Nat Rev Urol 15(2):92–111. https://doi.org/10.1038/nrurol.2017.179
Acknowledgements
We thank Dr. Lingli Chen (Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai, China) and Dr. Peipei Zhang (Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China) for their excellent pathological technology help.
Funding
This study was funded by grants from National Natural Science Foundation of China (81570607, 31770851, 81702496, 81702497, 81702805, 81772696, 81872082, 81902556, 81902563, 81902898, 81974393), three-year action plan for promoting clinical skills and clinical innovation in municipal hospitals of Shanghai Shenkang (16CR2003A), National Natural Science Foundation for Young Scholars of China (81902566), Shanghai Jiaotong University Medical-Engineering Cross Research Fund (YG2019QNA53), National Key R&D Program of China (2017YFC0114303), Shanghai Municipal Natural Science Foundation (16ZR1406500, 17ZR1405100, 19ZR1431800), Guide Project of Science and Technology Commission of Shanghai Municipality (17411963100), Shanghai Sailing Program (18YF1404500, 19YF1407900, 19YF1427200), Shanghai Municipal Commission of Health and Family Planning Program (20174Y0042, 201840168, 20184Y0151), Fudan University Shanghai Cancer Center for Outstanding Youth Scholars Foundation (YJYQ201802) and Shanghai Cancer Research Charity Center. All these study sponsors have no roles in the study design, in the collection, analysis and interpretation of data.
Author information
Authors and Affiliations
Contributions
TW, QZ, HZ and HZ for acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript; ZL, JS, ZW, YX, JW, QB, YX, YW, LL, YZ, LX, BD and JG for technical and material support; YC, XW and JX for study concept and design, analysis and interpretation of data, drafting of the manuscript, obtained funding and study supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have declared that they have no conflict of interest.
Ethics approval and consent to participate
Written informed consent was obtained from each patient included, and the protocol of all study cohorts was approved by the Clinical Research Ethics Committee of Zhongshan Hospital and the Ethics Committee of Fudan University Shanghai Cancer Center.
Consent for publication
All authors provide their consent for publication of the manuscript.
Availability of data and materials
All data generated that are relevant to the results presented in this article are included in this article. Other data that were not relevant for the results presented here are available from the corresponding author Dr. Xu upon reasonable request.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, T., Zhou, Q., Zeng, H. et al. CCR8 blockade primes anti-tumor immunity through intratumoral regulatory T cells destabilization in muscle-invasive bladder cancer. Cancer Immunol Immunother 69, 1855–1867 (2020). https://doi.org/10.1007/s00262-020-02583-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02583-y