Abstract
Background
Immune-related adverse events (irAEs) comprise a distinct spectrum of auto-inflammatory manifestations triggered due to immune checkpoint inhibitors (ICI). Current data on the association of irAEs with outcomes in NSCLC treated with nivolumab are limited.
Methods and objectives
We pooled data from 531 metastatic NSCLC patients from five centers treated with nivolumab after failing platinum-based chemotherapy. The primary objective was to investigate the relationship between irAEs with clinical benefit to nivolumab as well as to elucidate patterns of irAE-related ICI discontinuations and their impact on survival.
Results
33.0% (173/531) of patients treated with nivolumab were noted to have an irAE. Patients with irAEs had a significantly longer median PFS [6.1 vs. 3.1 months, HR 0.68 95% CI (0.55–0.85); p = 0.001] and OS [14.9 vs. 7.4 months, HR 0.66 95% CI (0.52–0.82); p < 0.001)] compared to those without irAEs. In multivariate analysis, the presence of irAEs showed a significantly better PFS [HR 0.69, 95% CI (0.55–0.87); p = 0.002] and a trend for better OS [HR 0.62, 95% CI (0.55–1.03); p = 0.057]. Patients with permanent ICI discontinuation secondary to index irAE had a significantly shorter median PFS [2.3 vs. 6.6 months, HR 1.74 95% CI (1.06–2.80); p = 0.02] and median OS [3.6 vs. 17.6 months; HR 2.61 95% CI (1.61–4.21); p < 0.001] compared to those that did not have permanent ICI discontinuation.
Conclusions
Our pooled exploratory analysis demonstrates improved clinical benefit to nivolumab in NSCLC patients experiencing irAEs. We also observed negative impact of irAE-related treatment discontinuation on survival in this group of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immune checkpoint inhibitors (ICIs) comprise of a broad spectrum of monoclonal antibodies that modulate the functioning of checkpoint molecules such as programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein-4 (CTLA-4) and thus derive anti-tumor activity primarily by potentiating a T cell-mediated attack [1]. The advent of ICI has revolutionized the treatment landscape for many cancers. Non-small cell lung cancer (NSCLC) especially has witnessed a paradigm shift with significant improvements in survival, response rates, and durability of disease control, both in the upfront and second-line settings [2]. Based on results from Checkmate-017 and 057, nivolumab was the first ICI approved as a single agent in the second-line setting for patients with advanced NSCLC progressing after platinum-based chemotherapy [3].
Compared to conventional chemotherapy, ICIs are known to have a distinct toxicity profile that can often mimic autoimmune conditions. These autoimmune toxicities that are speculated to arise from ICI-induced disruption in the immunological homeostasis normally maintained by PD-1 and CTLA-4 are categorized as immune-related adverse events (irAEs) [4]. IrAEs can involve a multitude of organ systems, some more commonly than others [5, 6]. The organ specificity, timing, and severity of irAEs are highly idiosyncratic and often depend on the type of checkpoint inhibition and the underlying malignancy [5].
Based on toxicity and response data of ICIs within the last decade, we have come to understand that achieving maximal benefit from ICIs involves maintaining a delicate balance between promoting anti-tumor immunity and preventing autoimmunity. The clinical benefit from ICIs can often be offset due to irAE-related morbidity and occasional mortality. Based on consensus guidelines, one can consider rechallenging patients with ICI once irAEs revert to ≤ grade-1 after appropriate management involving either watchful waiting off treatment, immunosuppression, or endocrine replacement [7]. However, if severe (≥ grade 3), these irAEs can result in permanent ICI discontinuation and also increase the risk of potential hospitalizations [8]. Recent data pooled from two clinical trials in melanoma indicated a lack of influence of irAE-related early treatment discontinuations on overall outcomes [9]. However, there are limited studies that have evaluated the safety of rechallenging ICI following irAEs or elaborated on the influence of irAE-related ICI discontinuations on outcomes in NSCLC. Thus, due to their propensity to impact treatment decisions, understanding the role of irAEs in influencing short-term and long-term outcomes in NSCLC patients treated outside of clinical trials from large datasets needs to be explored.
Another emerging aspect of significant interest is the putative relationship between anti-tumor efficacy and irAEs. There is an ongoing effort to understand whether the development of irAEs in NSCLC correlates with an effective anti-tumor immune response. One explanation for this phenomenon could be the potential mechanism of molecular mimicry of antigens shared between tumor and healthy tissue [10, 11]. However, current data regarding the association of clinical benefit with irAEs is not consistent. Some studies published recently have shown a direct relationship between irAEs and response to immunotherapy in NSCLC [12, 13], while others do not report the same relationship when accounting for the duration of treatment [14, 15], given the time-dependent nature of irAEs. The reason for this discrepancy is not clear but could be related to the measurement of different efficacy outcomes such as response versus survival as well as differences in the methodology of accounting for the duration of treatment as a confounding variable (i.e., landmark analysis).
To further investigate the relationship between irAEs with clinical benefit to nivolumab as well as to elucidate patterns of irAE-related ICI discontinuations and their impact of survival, we conducted a pooled exploratory analysis of metastatic NSCLC-treated with single-agent nivolumab in a multicenter, global cohort.
Patients and methods
We conducted a pooled analysis of 531 patients with metastatic NSCLC derived from five retrospective cohorts. Among the participating institutions, three were located in the United States, one in Italy and one in Japan. All contributors were trained in good clinical practice. Patients ≥ 18 years of age with stage-IV NSCLC who had received at least one cycle of nivolumab were included. Out of 531 patients, 97% (514) were treated with second-line single-agent nivolumab as part of the standard of care practice after they progressed on at least one line of platinum-based chemotherapy. Seventeen patients did not receive nivolumab as part of the standard of care approach. Eleven patients received nivolumab in a clinical trial setting; 5 of 11 patients were treated with nivolumab as part of a trial after failing platinum-based chemotherapy; the other 6 of 11 patients received upfront nivolumab again as part of a clinical trial. Six patients received nivolumab as part of an expanded access program following progression on platinum chemotherapy. To ensure uniformity with respect to prognostic outcomes, only metastatic NSCLC patients treated with nivolumab at each center were selected for the study.
Side effects with a high probability of having an underlying immunological basis, as documented by the treating provider and warranting frequent monitoring or potential intervention, were labeled as irAEs. We categorized irAEs primarily as pneumonitis, colitis, thyroid dysfunction, hepatitis, skin-related irAE, musculoskeletal (myositis or arthritis), or others (Supplementary Fig. 1). Fatigue and infusion reactions were not included as irAE. Clinical and molecular data (Supplementary Table 1) obtained from electronic medical records were used to derive correlations. Index irAE was defined as the first irAE after starting nivolumab. Secondary irAE was defined as an irAE manifesting after the index irAE while on nivolumab or after nivolumab discontinuation. Secondary irAEs also incorporated recurrent irAEs, i.e., irAEs that were similar to the index irAE but occurred as a subsequent event. Index irAEs occurring in ≤ 12 weeks were labeled as early irAEs. This time point was based on the fact that most early irAEs occur within the first 3 months of ICI initiation [16]. Also, several other studies used a 12-week landmark assessment to evaluate the influence of irAEs [15, 17]. Patients that had permanent treatment discontinuation due to the index irAE were labeled as the “group with IrAE-related permanent ICI discontinuation.” Patients who either did not have ICI interruptions due to irAE or resumed ICI after a temporary interruption were labeled as the “group without IrAE-related permanent ICI discontinuation.” Time to resolution of irAE was defined as the time from diagnosis of irAE to symptom recovery independent of steroid use or ICI discontinuation. The number of nivolumab cycles was used to calculate the approximate weeks on therapy. Progression-free survival (PFS) was defined as the time from the start of immunotherapy to the date of disease progression or death, whichever occurred first. Patients who were alive without disease progression were censored on the date of their last scan. Overall survival (OS) was defined as the time from the start of immunotherapy to death. Patients who were still alive were censored at the date of the last contact. Toxicity grading (G) was assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.1. Performance status (PS) was categorized based on the Eastern Cooperative Oncology Group (ECOG) classification [18]. Responses were graded based on Response Evaluation Criteria for Solids tumor (RECIST1.1) and categorized as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) based on Computed Tomography assessment by the treatment team. The best overall response rates (ORR) were based on combining CR and PR disease response. The primary objective was to ascertain the relationship of irAEs with clinical outcomes, including immunotherapy discontinuations.
Statistical analysis
Demographic data and characteristics were summarized using median and range for continuous outcomes and percentages for categorical outcomes. Chi-square/Fisher’s exact test (as appropriate) was used to determine associations between the categorical covariates of interest. Mann–Whitney test was used to derive a correlation between a categorical variable and continuous variables. Unadjusted Kaplan–Meier survival curves with log-rank testing were generated to compare the PFS and OS. Hazard ratios and confidence intervals in univariate and multivariate analyses were derived using Cox proportional model. p < 0.05 was considered significant. The analysis was conducted using IBM SPSS (version 22.0, SPSS, Chicago, IL, USA).
Results
Patient characteristics and irAE profiles
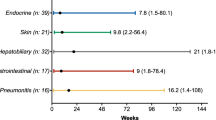
The baseline clinical and molecular characteristics of the study cohort are shown (Supplementary Table 1). The median duration of ICI was 12.8 weeks (range: 0–195). In our cohort of 531 patients, 33.0% (n = 173/531) of patients treated with nivolumab were noted to have an irAE. The grading, time to onset, and resolution of index irAEs are summarized (Supplementary Table 2). There was no difference in baseline clinical or molecular characteristics for patients with and without irAEs (Supplementary Table 3). 30.1% (n = 52/173) of patients with irAE had ≥ 2 irAEs (Fig. 1). 75.7% (n = 131/173) patients had an index irAE ≤ 12 weeks from treatment initiation of which 15.3% (n = 20/131) were ≥ G3. Index pneumonitis constituted 60% (12/20) of all ≥ G3 irAEs presenting in the first 12 weeks. 69.9% (121/173) had documented data on whether steroids were used to manage the index irAE. In this cohort, 45.5% (n = 55/121) of patients were initiated on a steroid dose of 1–2 mg/kg for any index irAE. Pneumonitis (30.9%; n = 17/55) was the most common index irAE requiring steroids with 41.2% (n = 7/17) patients having ≥ G3 index pneumonitis requiring steroid use. A significantly higher proportion of patients that were not started on steroids did not have permanent ICI discontinuation (94.5 vs. 65.6%; p < 0.001. In the group not started on steroids for index irAE, around 70% were G1 events. The index irAE distribution for the 16 patients with G2 irAEs not started on steroids was: joint involvement (1/16), skin involvement (4/16), and thyroid involvement (11/16). Out of the four patients with G3 index irAEs in this group, three were due to thyroid involvement and one was an NSTEMI.
Index irAE distribution in patients with ≥ 2 irAEs: In patients with ≥ 2 irAEs during treatment course (n = 52), thyroid dysfunction (26.9%; n = 14/52), skin involvement (17.3%; n = 9/52) and pneumonitis (13.5%; n = 7/52) were the three most commonly documented index irAEs. Pneumonitis was the most frequent secondary irAE following index thyroiditis (64.4%; n = 9/14) and index dermatitis (55.6%; n = 5/9). A majority, i.e., 88.5% (46/52) patients in this group with ≥ 2 irAEs had index irAEs ≤ G2. Data on index irAE was not available (N/A) in 28.8% (15/52) patients. Distribution of irAEs classified as “other” is provided in Supplementary Fig. 1
IrAEs and ICI efficacy
RECIST assessment was available in 80% (425/531). Patients with irAEs had a higher objective response rate (ORR) compared with patients with no-irAEs 40.1% (4.9% CR + 35.2% SD) versus 14.1% [(2.1% CR +12.1% PR); p < 0.01]. Patients with irAEs had a significantly longer median PFS [6.1 vs. 3.1 months, HR 0.68 95% CI (0.55–0.85); p = 0.001] and OS [14.9 vs. 7.4 months, HR 0.66 95% CI (0.52–0.82); p < 0.001] compared to those without irAEs (Fig. 2). Best ORR were noted to be higher in patients with ≥ 2 irAEs vs. < 2 irAEs during treatment course (55.5 vs. 33.0%; p = 0.01). No difference in median PFS or OS was identified when comparing steroid vs. no steroid for index irAEs (14.8 vs. 16.8 months; p > 0.1).
IrAEs and survival. A 83.1% (n = 441) had documented data on PFS. Median PFS for the cohort was 3.57 months (95% CI 3.0–4.0). Patients with irAEs had a longer median PFS versus patients with no irAEs {6.1 vs. 3.1 months, p = 0.001; HR 0.68, 95% CI 0.55–0.85). b Median OS for the entire cohort (n = 531) was 9.6 months (95% CI 8.2–11.1). The median OS for patients with irAEs was significantly higher compared to patients with no irAEs [14.9 vs. 7.4 months; HR 0.66, 95% CI (0.52–0.82); p < 0.001]
Using Cox regression analysis, we compared clinically relevant factors to determine association with PFS and OS (Tables 3, 4). After adjusting for factors having significant association using multivariate analysis, ECOG < 2 and ICI duration ≥ 3 months were found to be associated with better PFS and OS. Presence of irAEs showed a significantly better PFS [HR 0.69, 95% CI (0.55–0.87); p = 0.002] and a trend for better OS [HR 0.62, 95% CI (0.55–1.03); p = 0.057].
Group without irAE-related permanent ICI discontinuation
79.7% (n = 138/173) of patients either continued ICI without interruption or restarted ICI after temporary treatment discontinuation due to the index irAE (Fig. 3). Only 7.9% (n = 11/138) patients in this group were noted to have index irAE ≥ G3. IrAEs involving thyroid (n = 45/138) and skin (n = 35/138) constituted the most common index irAEs in the non-ICI discontinuation group. Secondary or recurrent irAEs were observed in 32.6% (n = 45/138) of patients without permanent ICI discontinuation. 8.9% (n = 4/45) of recurrent irAEs were ≥ G3. In the entire cohort, pneumonitis was the most frequent secondary irAE in patients that did not have permanent ICI discontinuation (40%, n = 18/45). Secondary irAE occurrence for thyroid was 28.9% (n = 13/45) and skin was 20.0% (n = 9/45). Use of steroids for index irAE did not appear to reduce the incidence of secondary irAEs (69.9 vs. 69.6%; p ≥ 0.1).
Nivolumab treatment status in relation to index irAEs: 33% (n = 173/531) of patients had an index irAE. Among this group, a majority of patients (79.7%) did not have permanent nivolumab discontinuation (Non-ICI discontinuation group) after index irAE. A total of 16.7% of patients (n = 29/173) discontinued nivolumab permanently (ICI discontinuation group) due to the index irAE. A majority i.e. 65.2% (n = 19/29) of irAE-related permanent ICI discontinuations, were due to index pneumonitis. Twenty-four patients had time to index irAE ≤ 12 weeks (Supplementary table-4). In the early irAE-related ICI discontinuation group (≤ 12 weeks), 41.7% (n = 10/24) were G2, 50% (n = 12/24) were G3 and 8.3% (n = 2/24) were G4
In patients that did not have permanent ICI discontinuation, 65.9% (n = 91/138) had data on time to irAE resolution. Within this cohort, 50.5% (n = 46/91) had time to index irAE resolution of > 4 weeks. Secondary irAEs in patients that did not discontinue ICI permanently were more frequent when the time to index irAE symptom resolution was > 4 weeks (63.6 vs. 42.1%; p = 0.049). A majority of irAEs with time to resolution > 4 weeks had index irAE ≤ G2 (95.2%, n = 44/46). Grade of index irAE and time to index irAE onset did not correlate with the occurrence of secondary irAEs in the non-ICI discontinuation group.
Group with IrAE-related permanent ICI discontinuation
Permanent discontinuation of nivolumab due to index irAE occurred in 16.7% of patients (n = 29/173; Fig. 3). Recurrent irAEs were observed in 17.2% (n = 5/29) patients with irAE-related ICI discontinuation. IrAEs leading to permanent ICI discontinuations were more common for index irAE ≥ G3 (58.6 vs. 8.03%; p < 0.001). 82.8% (n = 24) of all discontinuations were due to index irAE occurring ≤ 12 weeks (Supplementary Table 4). 75.7% (n = 131/173) patients had an index irAE ≤ 12 weeks from treatment initiation. The overall frequency of ICI discontinuations did not differ in early versus late irAEs (18.6 vs. 13.1%, p > 0.1). Distribution of index irAE ≥ G3 leading to early versus late ICI discontinuation was similar (58.3 vs. 60%; p > 0.1).
Best ORR was higher in patients who did not have ICI discontinuation compared to patients with irAE-related ICI discontinuations (43.9 vs. 20.0%; p = 0.052). Patients with ICI discontinuation secondary to index irAE had a significantly shorter median PFS [2.3 vs. 6.6 months, HR 1.74 95% CI (1.06–2.80); p = 0.02] and median OS [3.6 vs. 17.6 months, HR 2.61 95% CI (1.61–4.21); p < 0.001; Fig. 4] compared to those that either continued ICI without interruption or restarted ICI after temporary treatment discontinuation due to the index irAE. Multivariate Cox regression analysis showed that late irAEs (> 12 weeks) and non-ICI discontinuation was associated with better PFS and OS in patients with irAEs (Tables 3, 4). In addition, among all irAE subtypes, skin-related irAE was the only predictor associated with improved PFS but not OS.
Correlation of nivolumab status after index irAE with survival: Patients with irAE associated permanent nivolumab discontinuation (nivolumab not restarted) had: a Inferior median PFS [2.3 vs. 6.6 months, HR 1.74 95% CI (1.06–2.80); p = 0.02] and b Inferior median OS survival [3.6 vs. 17.6 months, HR 2.61 95% CI (1.61–4.21); p < 0.001]
Discussion
There is a paucity of literature adequately addressing questions related to irAEs and their impact on NSCLC outcomes. Our study represents the most extensive retrospective multi-institutional patient-level data exploring the influence of irAEs on outcomes in metastatic NSCLC treated with nivolumab, a majority of whom were standard of care. We observed an improved PFS and OS in patients that had irAEs during the treatment course. Also, our data provide insight into the distinct patterns of index and secondary irAEs with nivolumab. We identified pneumonitis as the most common index irAE responsible for ICI discontinuations. In our attempt to elucidate irAE outcomes, we observed inferior survival in patients that had permanent ICI discontinuations due to index irAEs. Our findings are unique, given that the data represents a patient cohort treated outside of clinical trials across several centers from an international cohort.
Immune checkpoints are vital components of the molecular machinery involved in maintaining peripheral immune tolerance. Conceivably ICI-induced blockade of these regulatory pathways has the potential to disrupt this balance, which is considered as one of the prime mechanisms that can trigger irAEs [5]. Thus, irAEs are more likely to reflect an exaggerated host immune function. Given that recognizing biomarkers unique to irAEs represents an unmet need, ongoing efforts have been gaining momentum to understand the immunological underpinnings that predispose some patients to irAEs. Early data suggest the aberrant presentation of antigens shared between tumor and healthy tissue with concomitant T-cell-mediated cross-reactivity [4, 19] or alterations in various B-cell subsets [20] as the most plausible mechanisms. However, these data lack prospective validation and remain exploratory for now.
A majority of irAEs in our study were identified within the first 12 weeks following treatment initiation, with 15.3% of these being ≥ G3. Interestingly, we identified that low-grade index irAEs were more likely to present with ≥ 2 irAEs where the secondary irAE was distinct from the index irAE (Fig. 1). Pneumonitis was the most frequent secondary irAE following index thyroid or skin irAEs. Thus, subtle symptoms of cough and mild shortness of breath that could represent early stages of pneumonitis should not be ignored, especially in patients that present with a low-grade index irAEs involving the thyroid and skin. Of note, patients that did not have permanent ICI discontinuation following an index irAE, time to index irAE resolution of > 4 weeks correlated with a higher incidence of secondary irAEs. Index irAE ≤ G2 accounted for 95.2% of patients that had time to index irAE symptom recovery of > 4 weeks. These findings suggest that patients with low-grade index irAEs where clinicians may not favor permanent ICI discontinuation may benefit from close monitoring of signs and symptoms of secondary irAEs during the treatment course. Importantly, educating patients about patterns of secondary irAEs and encouraging early reporting of suspicious irAEs symptoms needs to be considered. Alternatively, timely and aggressive symptom management of these low-grade irAEs could mitigate time off treatment and potentially improve outcomes. Furthermore, from a management standpoint, the involvement of multi-disciplinary care teams to promote cross-communication can facilitate timely diagnosis and management of certain complex multi-system irAEs, thereby potentially improving outcomes [21].
Steroid use for index irAE was associated with a higher incidence of patients that did not have a permanent ICI discontinuation. This is explained by the fact that most of these patients had G1 index irAEs. However, the use of steroids did not have an influence on decreasing the incidence of secondary irAEs. This suggests that there are other interactions involving intrinsically activated host factors and perhaps the persistence of activated autoimmunity that may not be entirely suppressed despite the use of adequate dose and duration of steroids. Due to the limited number of patients with available steroid data, these findings are not without limitations and warrant larger datasets aimed at studying immune mechanisms implicated in the development of irAEs.
The overall incidence of pneumonitis in our cohort was 9.1%, which is somewhat consistent with what has been reported in the initial trials of nivolumab in previously treated advanced NSCLC [22]. The influence of previous radiation on pneumonitis, which could represent a potential confounder, was not captured in our study. Despite being the most common irAE requiring immunosuppression with steroids, 65.5% of permanent ICI discontinuations were due to index pneumonitis, predominantly occurring in the first 3 months of starting treatment (Supplementary Table 4). This is not surprising as data reported to date have shown that ICI-related pneumonitis can lead to significant morbidity with frequent hospitalizations often warranting permanent treatment cessation [8, 23].
In our analysis, we did not identify clinical or molecular factors unique to patients with irAEs (Supplementary Table 3). More importantly, our data demonstrated higher ORR as well as improved PFS and OS in patients with irAEs (Fig. 2, Tables 1, 2). These findings are consistent with recent reports of clinical benefit in patients with NSCLC [17, 24] and melanoma [25] that had irAEs secondary to ICIs. Some of these findings have also been reproduced using pooled clinical trial data in patients with urothelial carcinoma where the increase in OS was related to the occurrence of irAEs but did not seem to correlate with duration of ICI exposure [26]. Although no precise mechanisms have been established, these emerging data suggest that the occurrence of irAEs could reflect better anti-tumor efficacy due to the proposed mechanism of persistent and robust immune activation. Thus, abnormal ICI induced immune cross-reactivity due to antigens shared between normal and tumor tissue that manifest as irAEs could be one of the useful predictive/prognostic factors to identify patients that may display a durable treatment response to ICI.
To date, there is limited evidence defining the optimal duration of ICI in NSCLC. Most outcomes data with nivolumab in the metastatic setting are based on treatment until the patient experiences disease progression or encounters unacceptable toxicity. Our finding of the impact of the duration of ICI on survival (Tables 3, 4) is akin to results from the Checkmate -153, which is an ongoing phase IIIB/IV trial. In this trial, patients with continuous treatment of nivolumab for > 1 year had a better PFS than those with fixed-duration treatment [27]. Interestingly, 35% of patients who were retreated after stopping nivolumab in the trial had disease progression, indicating that tumor biology could be an important determinant of response. Thus, while some aspects of these results favor a more extended treatment with nivolumab to improve survival, due to the paucity of randomized studies with adequate follow-up, the optimal duration of ICI in advanced NSCLC remains debatable.
The use of ICIs in NSCLC patients with poor performance status is not well defined [28]. Similar to our analysis, other retrospective reports have shown an ECOG of ≥ 2 to be associated with poor outcomes in NSCLC treated with ICIs [29]. Inferior survival in an individual with an ECOG of ≥ 2 can be explained based on the poor physiological reserve either due to the nature of the tumor itself or other unrelated medical comorbidities. These can, in turn, pose restrictions in the ability to tolerate therapy as well as therapy-related side effects resulting in treatment interruptions or discontinuations [30]. Conversely, poor patient performance status in advanced cancers may be a surrogate for aggressive tumor biology with intrinsic resistance to ICI. Validating these findings prospectively can be challenging due to a lack of enrollment of patients with ECOG > 2 into clinical trials employing ICIs. This is where the role of robust retrospective datasets from routine clinical practice ICIs for select patients with poor performance status and limited treatment options can provide vital additional information to assist in the optimal selection of patients for ICIs.
Decisions regarding continuing ICIs for ≤ G2 irAE symptoms or timing of readministration of ICIs after ≥ G3 irAEs in select patients with limited treatment options can often pose a significant dilemma. In our dataset, we observed 33% of patients had secondary or recurrent irAEs in the non-ICI discontinuation group, a majority of (> 90%), which were low grade. Most of these secondary irAEs did not resemble the index irAE. A recently reported study for NSCLC demonstrated 52% recurrent irAEs in patients that restarted ICI, with 60% recurrent events labeled as low grade [31]. Another study evaluating the safety of rechallenging ICIs for ≥ G2 irAEs in multiple tumor types reported an irAE recurrence of 55%; however, with 62% of these being ≥ G3 [32]. One explanation for the high-grade secondary irAEs in this study compared to our experience and that reported by Santini et al. [31] could be due to the confounding effect of evaluating multiple tumor types. It is also important to note that in contrast to the studies by Santini et al. [31] and Simonaggio et al. [32], where most of the evaluated patients had ≥ G2 irAEs, we accounted for all grade irAEs (including G1). Due to data limitations, we were unable to stratify patients who were rechallenged after temporary treatment interruption or continued ICI without any treatment interruption. These variations make it particularly challenging to derive cross-study comparisons. Although none of these studies were conducted prospectively, they provide important insights into the feasibility of restarting ICI and elaborate on the patterns of secondary or recurrent irAEs in patients that do not have permanent ICI discontinuation. Based on the observations derived from these studies, we propose that the decision to rechallenge ICI after irAEs should be based on the clinical judgment of the treating physician taking into context the patient’s state of physical health, comorbidities, initial response to ICI, disease prognosis, and the of risk exacerbating residual irAE symptoms if not completely resolved.
We have reported on the negative impact of ICI discontinuations after index irAEs on PFS and OS (Fig. 4a, b; Tables 3, 4). These findings can be an extrapolation of similar results published recently outlining the influence of irAE-related treatment delays or interruptions on OS [33]. In our understanding, the findings of improved survival in this group potentially correspond to a longer duration of exposure to the ICI. Also, findings from others have revealed poor outcomes with irAE-related ICI discontinuations in NSCLC, albeit in patients who had not achieved an objective response rate before ICI discontinuations [31]. Although early irAEs (≤ 12 weeks) had inferior survival (Tables 3, 4) and accounted for 82.8% ICI discontinuations (Supplementary Table 4) in our study, we did not find an association between time to index irAE and rate of ICI discontinuation. Also, the distribution of index irAE ≥ G3 was not different for the early versus late irAE group. These results allude to the fact that there could be other important clinical factors that influence the decision on treatment discontinuations in patients with early irAEs, which can eventually impact survival. We acknowledge that our current data analysis precludes us from delineating whether the poor outcomes observed in patients is due to permanent ICI discontinuations per se or due to clinical deterioration arising as a consequence of irAE-related morbidity and the prolonged recovery associated with these events. Understanding these factors, as well as patterns of discontinuations, could serve as an essential guide to better define subsequent management algorithms in the setting of early irAEs.
Our study has several limitations inherent to a retrospective analysis. Although having data from multiple institutions allows for better generalizability of our findings, identification of irAEs was dependent on the treating physician at the respective institution. To ensure uniformity, we only assessed nivolumab-associated irAE outcomes in metastatic NSCLC after having failed chemotherapy. However, this precludes us from making similar conclusions about irAEs in the context of first-line ICIs alone or with chemo-immunotherapy, which currently is the standard practice in NSCLC, especially across North America and Europe. Due to the lack of centralized data entry, attributing causation can be challenging in the presence of potential confounders. We have tried to overcome this by using multivariate analysis in justifying our findings. We were not able to capture data on the number of previous lines of treatment, history of radiation, use of other immunosuppressive strategies aside from steroids, and site of metastasis that could potentially impact our results. As mentioned above we did not have information on RECIST responses at the time of irAEs. Also, due to limitations in the clinically annotated data provided by the participating institutions, our interpretation of secondary irAEs in the non-ICI discontinuation group needs to be viewed with caution. This is because we did not compartmentalize results for ≤ G2 index irAEs based on continuous nivolumab treatment versus temporary nivolumab interruption.
Conclusions
Given that the majority of our data is based on real-world experience of patients treated with standard of care nivolumab after failing chemotherapy, it adds important information to the current body of evidence for irAEs in patients treated outside of clinical trials. The occurrence of nivolumab induced irAEs in our study conferred a numerically longer PFS and OS. Also, most of the early permanent nivolumab discontinuations were due to index pneumonitis which was also the most common secondary irAE in patients with > 1 irAE.
In summary, findings from our data emphasize the importance of developing novel biomarkers that can help in identifying patients at risk for early toxicities, treatment discontinuation, and predisposition to secondary irAEs. We acknowledge that retrospective studies have several limitations which in most cases preclude them in influencing clinical decision making. Nevertheless, cumulative observations from studies similar to ours could potentially serve as a useful guide in devising optimal treatment approaches in NSCLC patients experiencing irAEs secondary to ICIs.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
28 April 2020
The original version of this article unfortunately contained a mistake. The second sentence of the section “irAEs and ICI efficacy” should read as.
Abbreviations
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein-4
- ECOG:
-
Eastern cooperate oncology group
- G:
-
Grade
- ICI:
-
Immune checkpoint inhibitors
- IrAE:
-
Immune-related adverse events
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death-1
- PFS:
-
Progression-free survival
- PS:
-
Performance status
References
Wei SC, Duffy CR, Allison JP (2018) Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 8(9):1069–1086. https://doi.org/10.1158/2159-8290.cd-18-0367
Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL et al (2019) Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res 25(15):4592–4602. https://doi.org/10.1158/1078-0432.CCR-18-1538
Vokes EE, Ready N, Felip E, Horn L, Burgio MA et al (2018) Nivolumab versus docetaxel in previously treated advanced non-small-cell lung cancer (CheckMate 017 and CheckMate 057): 3-year update and outcomes in patients with liver metastases. Ann Oncol 29(4):959–965. https://doi.org/10.1093/annonc/mdy041
Young A, Quandt Z, Bluestone JA (2018) The balancing act between cancer immunity and autoimmunity in response to immunotherapy. Cancer Immunol Res 6(12):1445–1452. https://doi.org/10.1158/2326-6066.CIR-18-0487
Postow MA, Sidlow R, Hellmann MD (2018) Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378(2):158–168. https://doi.org/10.1056/NEJMra1703481
Johnson DB, Chandra S, Sosman JA (2018) Immune checkpoint inhibitor toxicity in 2018 Immune checkpoint inhibitor toxicity immune checkpoint inhibitor toxicity. JAMA 320(16):1702–1703. https://doi.org/10.1001/jama.2018.13995
Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ et al (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: american society of clinical oncology clinical practice guideline. J Clin Oncol 36(17):1714–1768. https://doi.org/10.1200/jco.2017.77.6385
Reynolds KL, Cohen JV, Durbin S, Thomas M, Dougan M et al (2018) Inpatient admissions related to immune-related adverse effects (irAE) among patients treated with immune checkpoint inhibitors for advanced malignancy: a tsunami is coming, but are we ready? J Clin Oncol 36(5_suppl):127. https://doi.org/10.1200/jco.2018.36.5_suppl.127
Schadendorf D, Wolchok JD, Hodi FS, Chiarion-Sileni V, Gonzalez R et al (2017) Efficacy and safety outcomes in patients with advanced melanoma who discontinued treatment with nivolumab and ipilimumab because of adverse events: a pooled analysis of randomized phase II and III trials. J Clin Oncol 35(34):3807–3814. https://doi.org/10.1200/jco.2017.73.2289
Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J et al (2016) Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375(18):1749–1755. https://doi.org/10.1056/NEJMoa1609214
Berner F, Bomze D, Diem S, Ali OH, Fassler M et al (2019) Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol 5(7):1043–1047. https://doi.org/10.1001/jamaoncol.2019.0402
Haratani K, Hayashi H, Chiba Y, Kudo K, Yonesaka K et al (2018) Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 4(3):374–378. https://doi.org/10.1001/jamaoncol.2017.2925
Cortellini A, Chiari R, Ricciuti B, Metro G, Perrone F et al (2019) Correlations between the immune-related adverse events spectrum and efficacy of anti-PD1 immunotherapy in NSCLC patients. Clin Lung Cancer 20(4):237–247. https://doi.org/10.1016/j.cllc.2019.02.006
Kothari S, Bagley S, Aggarwal C, Bauml J, Alley E et al (2017) P3.02c-029 immune-related adverse events and their effect on outcomes in patients (pts) with non-small cell lung cancer (NSCLC) treated with nivolumab: topic: IT. J Thorac Oncol 12(1):1290. https://doi.org/10.1016/j.jtho.2016.11.1824
Owen DH, Wei L, Bertino EM, Edd T, Villalona-Calero MA et al (2018) Incidence, risk factors, and effect on survival of immune-related adverse events in patients with non–small-cell lung cancer. Clin Lung Cancer 19(6):e893–e900. https://doi.org/10.1016/j.cllc.2018.08.008
Zimmermann S, Peters S, Owinokoko T, Gadgeel SM (2018) Immune checkpoint inhibitors in the management of lung cancer. Am Soc Clin Oncol Educ Book 38:682–695. https://doi.org/10.1200/edbk_201319
Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG et al (2019) Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 145(2):479–485. https://doi.org/10.1007/s00432-018-2805-3
Oken MM, Creech RH, Tormey DC, Horton J, Davis TE et al (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5(6):649–655
Rapisuwon S, Izar B, Batenchuk C, Avila A, Mei S et al (2019) Exceptional response and multisystem autoimmune-like toxicities associated with the same T cell clone in a patient with uveal melanoma treated with immune checkpoint inhibitors. J Immuno Ther Cancer 7(1):61. https://doi.org/10.1186/s40425-019-0533-0
Das R, Bar N, Ferreira M, Newman AM, Zhang L et al (2018) Early B cell changes predict autoimmunity following combination immune checkpoint blockade. J Clin Invest 128(2):715–720. https://doi.org/10.1172/JCI96798
Naidoo J, Cappelli L, Lipson EJ, Forde PM, Sharfman WH et al (2018) A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Clin Oncol 36(15_suppl):6538. https://doi.org/10.1200/jco.2018.36.15_suppl.6538
Nishino M, Hatabu H, Hodi FS, Ramaiya NH (2017) Drug-related pneumonitis in the era of precision cancer therapy. JCO Precis Oncol 1:1–12. https://doi.org/10.1200/po.17.00026
Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS et al (2018) Pneumonitis in non–small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 13(12):1930–1939. https://doi.org/10.1016/j.jtho.2018.08.2035
Hofman P (2019) Is the onset of adverse effects of immunotherapy always bad news for the patients…?-certainly not. Ann Transl Med 7(1):5. https://doi.org/10.21037/atm.2019.01.14
Indini A, Di Guardo L, Cimminiello C, Prisciandaro M, Randon G et al (2019) Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 145(2):511–521. https://doi.org/10.1007/s00432-018-2819-x
Maher VE, Fernandes LL, Weinstock C, Tang S, Agarwal S et al (2019) Analysis of the association between adverse events and outcome in patients receiving a programmed death protein 1 or programmed death ligand 1 antibody. J Clin Oncol 37(30):2730–2737. https://doi.org/10.1200/JCO.19.00318
Spigel DR, McLeod M, Hussein MA, Waterhouse DM, Einhorn L et al (2017) 1297ORandomized results of fixed-duration (1-year) vs continuous nivolumab in patients (pts) with advanced non-small cell lung cancer (NSCLC). Ann Oncol. https://doi.org/10.1093/annonc/mdx380.002
Passaro A, Spitaleri G, Gyawali B, de Marinis F (2019) Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol 37(22):1863–1867. https://doi.org/10.1200/JCO.18.02118
Park W, Kwon D, Saravia D, Desai A, Vargas F et al (2018) Developing a predictive model for clinical outcomes of advanced non-small cell lung cancer patients treated with nivolumab. Clin Lung Cancer 19(3):280–288. https://doi.org/10.1016/j.cllc.2017.12.007
Passaro A, Spitaleri G, Gyawali B, Marinis F (2018) Immunotherapy in non-small-cell lung cancer patients with performance status 2: clinical decision making with scant evidence. J Clin Oncol. https://doi.org/10.1200/jco.18.02118
Santini FC, Rizvi H, Plodkowski AJ, Ni A, Lacouture ME et al (2018) Safety and efficacy of re-treating with immunotherapy after immune-related adverse events in patients with NSCLC. Cancer Immunol Res 6(9):1093–1099. https://doi.org/10.1158/2326-6066.CIR-17-0755
Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M et al (2019) Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.1022
Ksienski D, Wai ES, Croteau N, Fiorino L, Brooks E et al (2019) Efficacy of nivolumab and pembrolizumab in patients with advanced non-small-cell lung cancer needing treatment interruption because of adverse events: a retrospective multicenter analysis. Clinical Lung Cancer 20(1):e97–e106. https://doi.org/10.1016/j.cllc.2018.09.005
Acknowledgements
We thank the contributing centers as well as ECU IRB. We also thank the clinical research coordinators from ECU (Ms. Sue Ann Joyner and Ms. Susan Eubanks) for facilitating data sharing.
Funding
No relevant funding was required in conducting this study.
Author information
Authors and Affiliations
Contributions
ARN was involved in the acquisition, analysis, interpretation of data acquired from all institutions and drafting of the manuscript. ARN, CC, and MH were involved in the clinical annotation of data from East Carolina University. BR was involved in assisting in some aspects of the conceptual design of the study and data interpretation. BR, DHO, and VF were involved in critical revisions of the manuscript content. BR, AG, YT, JS, DHO, SKP, JB, VF, and WP were involved in collection of patient data from respective institutions. GAO, RC, SS, GDLL, CC, and PW were involved in the clinical care of patients from respective institutions. MM and GAO assisted in revising the manuscript drafts. ARN had full access to data from all participating institutions and assumes full responsibility for the integrity and accuracy of the analysis. All authors had access and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no potential conflicts of interest directly or indirectly relevant to the conduct or analysis of this study.
Ethical approval
The primary IRB approval was obtained from East Carolina Univ IRB (UMCIRB 15-001400). All participating centers had secured their respective IRB approval to collect their institutional data. To permit the sharing of de-identified data, a data use agreement was signed between ECU and the outside institution providing de-identified data. Patient consent was not required as part of the IRB approved studies since no identifiable information was shared or used for analysis or publication. All this was done in accordance with the regulations covered by the respective institutional IRBs.
Informed consent
No identifiable patient information has been published warranting individual consent from patients. The requirement for individual patient informed consent for the purpose of data collection and publication was waived as per the regulations covered under the respective institutional IRBs (East Carolina Univ, Ohio State Univ, Sendai Kousei Hospital, Univ of Perugia, University of Miami).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Naqash, A.R., Ricciuti, B., Owen, D.H. et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother 69, 1177–1187 (2020). https://doi.org/10.1007/s00262-020-02536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02536-5