Abstract
Adaptive immune responses contribute to the pathogenesis of melanoma by facilitating immune evasion. V-domain Ig suppressor of T-cell activation (VISTA) is a potent negative regulator of T-cell function and is expressed at high levels on monocytes, granulocytes, and macrophages, and at lower densities on T-cell populations within the tumor microenvironment. In this study, 85 primary melanoma specimens were selected from pathology tissue archives and immunohistochemically stained for CD3, PD-1, PD-L1, and VISTA. Pearson’s correlation coefficients identified associations in expression between VISTA and myeloid infiltrate (r = 0.28, p = 0.009) and the density of PD-1+ inflammatory cells (r = 0.31, p = 0.005). The presence of VISTA was associated with a significantly worse disease-specific survival in univariate analysis (hazard ratio = 3.57, p = 0.005) and multivariate analysis (hazard ratio = 3.02, p = 0.02). Our findings show that VISTA expression is an independent negative prognostic factor in primary cutaneous melanoma and suggests its potential as an adjuvant immunotherapeutic intervention in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Melanoma is a malignant proliferation of melanocytes with a high rate of metastasis compared to other skin cancers. Patients with metastatic disease have a poor prognosis with a median survival time of 8 months [1]. Despite growing awareness of risk factors for melanoma [2], the incidence appears to be on the rise across all age groups with increasing mortality among older persons, particularly for thin lesions [3]. Prognosis is correlated with depth of invasion (Clark level, Breslow’s depth), ulceration, mitotic index, and lymphocytic infiltration of the tumor [4,5,6]. In particular, the density of immune cell infiltration into the tumor has been shown to be an independent prognostic factor with 5- and 10-year survival rates as high as 77 and 55%, respectively, for those with a brisk infiltrate, compared to 37 and 27%, respectively, for those with absence of tumor-infiltrating lymphocytes (TIL) [5, 7].
In some human cancers, tumor-cell-surface expression of immune checkpoint ligands results in downregulation of TIL, facilitating tumor cell proliferation and potentially reversing the otherwise favorable prognosis of TIL response. Programmed death ligand 1 (PD-L1) is expressed on a subset of melanomas and colocalizes with its conjugate receptor, programmed cell death protein 1 (PD-1), on lymphocytes [8]. The PD-1–PD-L1 complex inhibits the immune system through a decrease in T-cell function (e.g., reduced secretion of IFN-gamma, IL-2, and IL-10) and increased T-cell apoptosis [9]. V-domain Ig suppressor of T-cell activation (VISTA; also known as PD-1H, Dies1, Gi24) was recently identified as another potent negative regulator of T-cell function [10,11,12], but with a somewhat different expression and activation pattern as compared to the PD-l–PD–L1 axis. In contrast to PD-L1, which is primarily found on activated and exhausted T cells, VISTA is more highly expressed on myeloid and granulocytic cells as well as naïve T-cells [13]. As a negative checkpoint regulator with a different expression pattern than those previously identified, VISTA could have important novel implications in cancer immunology [14].
Little published data on VISTA exist from either human primary or metastatic melanoma tumor samples and the relationship between VISTA, PD-1, PD-L1, and other tumor features remains poorly characterized. The goal of our study was to elucidate (1) VISTA expression in primary human melanoma lesions: both on the tumor cells and on the tumor-infiltrating inflammatory cells, (2) the relationship of VISTA expression to the expression of PD-1 and PD-L1 within the melanoma tumor microenvironment, and (3) the association between VISTA expression and patient survival.
Materials and methods
Case selection
Eighty-five archived cutaneous melanoma cases with adequate tissue and follow-up information were obtained from the Department of Pathology and Laboratory Medicine at Dartmouth–Hitchcock Medical Center. The average clinical follow-up time of these patients was 7.6 years (ranging from 7 months to 18 years). All tumors were reviewed by a dermatopathologist (SY) to confirm accuracy of the original diagnosis and pathological stage. Tumors were classified according to the American Joint Committee on Cancer (AJCC) staging system 7th edition.
Patient demographics and follow-up data were extracted from the electronic medical record. This study was approved by the Dartmouth College Committee for the Protection of Human Subjects/Institutional Review Board.
Immunohistochemistry
Immunohistochemical (IHC) stains were performed on freshly prepared 4-μm paraffin sections with the following primary antibodies: PD-L1 (clone E1L3N, 1:100, Cell Signaling Danvers, MA), PD-1 (clone NAT105, 1:100, Abcam Cambridge, MA), CD3 (clone LN10, 1:200, Leica Microsystems, Buffalo Grove, IL), and VISTA (clone GG8, 1:1000 ImmuNext, NH) [11]. IHC stains were performed using Leica Bond Max or RX Automated stainer (Leica Microsystems), Bond Epitope Retrieval 1 or 2 (Leica Microsystem and Bond polymer Red refine detection kit Cat # DS9390) according to the manufacturers’ instructions. Omission of primary antibodies was performed as a negative control.
All slides were evaluated and scored using light microscopy. Tumor-infiltrating myeloid cells (mainly neutrophils) were identified based on morphology. IHC staining was measured by quantitative and semi-quantitative assessment of positive staining in tumor cells and TIIC. Membranous staining of PD-L1 was evaluated for melanoma cells and TIIC. The percentage of tumor cells displaying PD-L1 membrane staining out of 1000 tumor cells was counted, and tumors were defined as PD-L1+ if at least 1% of the melanoma cells had membranous staining. TIIC were defined as PD-L1+ or VISTA+ if there was any PD-L1 staining or VISTA staining in the TIIC, respectively. Evaluation of PD-1 positive inflammatory cells was based on average counts from five representative high-power fields evaluated at 400× magnification above an imaginary line, which separates the tumoral area from the dermal tissue below as described by Piras et al [15]. The density of TIL was semi-quantified using an H&E and CD3 immunostained section based on a scale of 0–3 (absent, mild, moderate, and strong). Positive and negative IHC staining patterns of all antibodies tested were observed in older and newer cases. No effect of age of blocks on staining pattern was seen.
The cancer genome atlas melanoma data
The cancer genome atlas (TCGA) project seeks to categorize cancers, including malignant melanoma, based on common genetic mutations identified by high-throughput sequencing. Detailed methodology with regard to melanoma has been described previously [16]. Gene expression was evaluated by RNA sequencing, where total RNA was converted to mRNA libraries using the Illumina mRNA TruSeq kit. RNA-seq data have been generated for 103 primary melanoma samples and 366 metastatic melanoma samples. Clinical data available as part of the TCGA include age at initial melanoma diagnosis, sex, AJCC stage at initial diagnosis, and survival data which were curated from the medical record for each patient.
Statistical analysis
Statistical analysis system (SAS 9.4) (SAS Institute Inc., Cary, NC) software was used for data analyses. Correlation between the expression of immune markers and the pathologic parameters was calculated based on Pearson’s correlation coefficients. All tests are two sided with significance level set to 0.05. Disease-specific survival data were analyzed using the Kaplan–Meier method, and estimated hazard ratios (HR) and 95% confidence intervals (CI) of prognostic factors were derived from univariate and multivariate Cox’s proportional hazard models. Mantel–Cox log-rank score was used to assess statistical significance. Unadjusted and adjusted multivariate analyses using TCGA data according to tumor sample type (primary or metastatic) were performed with age, sex, and pathologic stage at diagnosis included as confounding factors.
Results
Study sample
The study sample was comprised of specimens of primary melanoma from 85 patients for whom follow-up data were available (Table 1). Forty-nine of the 85 patients were male (57.6%). The most common anatomic location was the extremities (41, 48.2%), followed by the trunk (30, 35.3%), and head and neck (14, 16.5%). Histologic subtypes included superficial spreading (34, 40.0%), nodular (24, 28.2%), lentigo maligna (4, 4.7%), acral lentiginous (4, 4.7%), desmoplastic (5, 5.9%), and unclassified (14, 16.5%). Specimens were approximately evenly distributed by T stage and 51 (60%) patients developed metastatic disease at follow-up. TIL density ranged from 0 to 3 (Table 1).
TCGA data from a total of 403 melanoma patients, included PD-1, PD-L1, and VISTA mRNA expression for 103 primary melanoma samples and 366 metastatic melanoma samples, as some patients, have multiple metastases included in the data set.
Immunophenotype of the tumor microenvironment
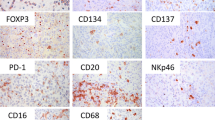
Representative IHC staining patterns of the antibodies evaluated are shown in Fig. 1. T lymphocytes are highlighted by CD3 immunostain (Fig. 1a). VISTA staining was essentially negative in melanoma cells, consistent with the previous unpublished findings of VISTA positivity in only one of the 27 melanoma cell lines (data not shown). Occasional staining was observed in pigmented melanoma cells, which was interpreted as nonspecific staining. The majority of VISTA IHC positivity was observed in neutrophils, which were usually seen in association with tumor ulceration. Rare VISTA expression was also observed in some mononuclear cells (which could include lymphocytes, monocytes and macrophages) within the overall TIIC. In this study, any VISTA positive staining within the observed TIIC was designated as a positive staining pattern (Fig. 1b). Among the 85 cases examined, 53 specimens (62.4%) showed focal VISTA positivity in morphologically identified tumor inflammatory infiltrate. In contrast, PD-L1 expression was found in both melanoma cells and TIIC (Fig. 1c, d). Thirty-one melanomas (36.5%) showed PD-L1 membrane staining in ≥ 1% tumor cells and 28 (32.9%) showed focal PD-L1 expression in TIIC (Table 2). PD-1 was negative in tumor cells, but was more widely expressed in TIIC compared to VISTA and PD-L1 (Fig. 1e). The average number of PD-1 positive cells in TIIC per 5 high-powered fields was 18, ranging from 0 to 120.
Representative immunohistochemical staining patterns. a CD3 expression in TIIC. b VISTA expression in tumor-infiltrating neutrophils and rare mononuclear cells. c PD-L1 expression in melanoma cells. d PD-L1 expression in TIIC. e PD-1 expression in TIIC. f Negative control (original magnification ×400). Arrows indicate positively stained cells
Pearson’s correlation coefficients were used to evaluate immunophenotypic relationships between melanoma tumor cells and TIIC (Table 3). Tumor PD-L1 expression significantly correlated with the presence of PD-L1 expression in TIIC (r = 0.35, p < 0.001), the presence of ulcer (r = 0.33, p = 0.002) and TIL density (r = 0.29, p = 0.008). The presence of PD-L1+ TIIC positively correlated with the density of PD-1+ cells in TIIC (r = 0.22, p = 0.047), TIL density (r = 0.34, p = 0.002), and the presence of ulcer (r = 0.25, p = 0.024), in addition to its correlation with PD-L1 expression in tumor (r = 0.35, p < 0.001). The presence of VISTA expression in tumor inflammatory infiltrate positively correlated with the presence of neutrophilic infiltrate (r = 0.28, p = 0.009) and the density of PD-1+ cells in TIIC (r = 0.31, p = 0.005). Furthermore, TIL density negatively correlated with neutrophilic infiltrate (r = − 0.32, p = 0.003) and positively correlated with PD-L1 expression in melanoma (r = 0.29, p = 0.008), the PD-L1 expression in TIIC (r = 0.34, p = 0.002), and the density of PD-1+ cells in TIIC (r = 0.48, p < 0.001). Ulceration significantly correlated with tumor depth (r = 0.5, p < 0.001), the presence of neutrophilic infiltrate (r = 0.37, p < 0.001), PD-L1 expression in tumor (r = 0.33, p = 0.002), and PD-L1 expression in TIIC (r = 0.25, p = 0.024), but did not correlate with VISTA expression in TIIC (r = 0.14, p = 0.2).
Survival and multivariate analysis
To further evaluate the impact of these immune markers on survival, Kaplan–Meier disease survival curves were generated based on follow-up clinical data. Patients with any level of VISTA expression in the TIIC of their primary melanoma had a lower disease-specific survival compared to patients with negative VISTA expression in their tumor inflammatory infiltrate (p = 0.003) (Fig. 2). Other factors, including PD-L1 expression in tumor, PD-L1 expression in TIIC, the density of PD-1 expression in TIIC, and the density of TIL, showed no significant impact on melanoma-specific survival. We further investigated the potential prognostic significance of tumor depth, ulceration, and PD-L1 expression in tumor, expression of PD-1, PD-L1, and VISTA in TIIC, as well as TIL density using a Cox proportional hazard model for univariate and multivariate models, respectively (Table 4). In univariate analysis, short disease-specific survival was significantly associated with thicker tumor (HR 1.11, p < 0.001), the presence of ulcer (HR 5.62, p < 0.0001) and the presence of VISTA expression in tumor inflammatory infiltrate (HR 3.57, p = 0.005) (Table 4). In multivariate analysis, the presence of ulcer (HR 4.74, p = 0.006) and the presence of VISTA expression within TIIC (HR 3.02, p = 0.02) continue to be significant independent prognostic factors, and tumor depth predicted worse survival (HR 1.08, p = 0.06).
Prognostic significance was further explored using TCGA melanoma data in unadjusted and adjusted multivariate analyses. Among primary melanoma samples, there were no statistically significant correlations between survival and mRNA expression of PD-L1 (HR 0.46, p = 0.14), PD-1 (HR 0.86, p = 0.06), or VISTA (HR 0.61, p = 0.3) (Table 5). In contrast, among metastatic melanoma samples, mRNA expression of all three immune checkpoint proteins appeared to be associated with improved survival (PD-L1 HR 0.82, p < 0.001; PD-1 HR 0.87, p < 0.001, VISTA HR 0.82, p = 0.003) (Table 5).
Discussion
In this retrospective review of primary melanoma cases, we sought to characterize VISTA expression within the TME and explore its potential as a prognostic factor. Focal VISTA expression on TIIC was observed in a majority of tumor specimens and was positively associated with myeloid cell infiltration and PD-1 expression on TIIC. VISTA positive TIICs were correlated with worse disease-specific survival. A recent study in early stage head and neck squamous cell carcinoma showed that VISTA expression was a poor prognostic factor [17] and are in accordance with our similar findings in those with cutaneous melanoma. The importance of immune check point inhibition in the treatment of early stage melanoma has been demonstrated in CheckMate 238, a trial comparing adjuvant immunotherapy with nivolumab versus ipilimumab that demonstrated the superiority of nivolumab in relapse free survival for stage III disease [18]. In addition, high-dose ipilimumab was found to prolong survival in stage III disease over observation [19]. The correlation we found between VISTA expression on TIIC, neutrophilic infiltrate and the density of PD-1 on TIIC in the TME combined with the correlation of VISTA on TIIC and poorer survival, provide strong support that VISTA may be a novel target for future immunotherapy in early stage disease.
Immune checkpoint receptors and their ligands play an important physiologic role by both preserving self-tolerance and attenuating the inflammatory response [20, 21]. The aberrant expression of PD-L1 on tumor cells downregulates TILs, promoting immune evasion and malignant progression [22, 23]. In the metastatic setting, PD-1+ melanoma-specific T-cells within the TME show decreased levels of cytokine production compared to PD-1 negative T-cells from normal tissue and peripheral blood of the same patient [24]. The heterogeneity of PD-L1 expression on tumor cells and TILs across a range of tumors types, and the associated variation in findings of prognostic value, illustrates that constitutive and adaptive mechanisms are not mutually exclusive [21]. The role of PD-L1+ tumor cells in melanoma, and a possible association with clinical outcome, remains poorly characterized. Oba et al. found PD-L1+ tumor cells to be associated with increased vertical growth and shorter survival times [25]. Among those with metastatic disease, PD-L1 expression appears to be highest along the tumor margin and is a positive predictor of response to anti-PD-1 therapy [26], suggesting a role in metastatic progression. Our study provides further evidence of an important but complex relationship between melanoma and the PD-1–PD-L1 axis. We identified positive associations between PD-L1+ melanoma cells, PD-L1+ TIIC, and TIL density in the TME of primary melanoma, but there was no association between either PD-1 or PD-L1 and survival in multivariate analysis in our cohort. In a previous univariate analysis, Madore, et al. found that patients with PD-L1+ melanoma in locoregional metastases had improved survival compared to patients with PD-L1 negative disease, but this was not observed when examining expression in the primary lesion [27]. Data from our study support a lack of association between PD-L1 expression on either tumor cells or TIIC within primary lesions and melanoma-specific survival.
VISTA, an immunoglobulin superfamily ligand which has an extracellular domain with distant sequence similarity to that of PD-L1, represents a possible alternative immune checkpoint pathway [10]. VISTA overexpression downregulates the immune system through suppressing T-cell proliferation, blunting T-cell cytokine secretion (e.g., IL-10, TNF-alpha, IFN-gamma), inducing FoxP3 expression, and stabilizing the persistence of the regulatory T-cell pool [10, 28]. In support of its immunomodulatory function, VISTA blockade slows tumor growth in murin cancer models and increases the propensity for autoimmune encephalomyelitis in mice, a model for multiple sclerosis [10], while a VISTA agonistic antibody prevents graft versus host disease [11]. Molecular methods in murine experiments have shown that VISTA is expressed exclusively on hematopoietic cells, most prominently in the myeloid lineage including neutrophils, macrophages, and dendritic antigen presenting cells (APCs) [10]. In both mouse and human tissue samples, VISTA potently suppressed T-cell proliferation and function as measured by decreased levels of IL-10, TNF-alpha, and IFN-gamma [11, 13]. A role for VISTA in cancer immunosurveillance was evaluated in mice transplanted with melanoma, in which VISTA blockade resulted in increased numbers of tumor-specific CD4+ and CD8+ T cells and decreased FoxP3+ regulatory T cells within the TME, in addition to delayed tumor progression in genetically predisposed mice [14]. Combination therapy with anti-VISTA and anti-PD-1 in murine melanoma models has proven to be more effective than that of monotherapy with anti-VISTA [29]. To begin to expand on the results of murine models, our study can now confirm a likely role for VISTA in the melanoma TME of primary tumors in humans and suggest that VISTA may play a role in other early stage tumors.
Potential relationships between VISTA and other immune checkpoint pathways, including PD-1/PD-L1, have not yet been fully elucidated. In contrast to PD-L1, VISTA is expressed on naïve T-cells and is upregulated more strongly and specifically on tumor-infiltrating myeloid cells [10, 13]. Single knockout mice lacking either PD-1 or VISTA showed increased levels of chronic inflammation, while mice lacking both showed higher levels of immune-related phenomena, supporting the non-redundancy of these pathways [29]. Nonetheless, we found strong positive associations between TIIC VISTA expression and TIIC PD-1 expression within the melanoma TME, providing evidence that both likely play an important role in melanoma pathogenesis and contribute simultaneously to avoidance of immune recognition.
TIIC VISTA expression in our study was related to decreased survival in univariate analysis and remained an independent prognostic factor in multivariate analyses. This is consistent with the previously discussed immune suppression function of VISTA within the TME. Tumor characteristics known to stratify patient survival such as tumor depth and ulceration were also included in our multivariate analysis and were related to poorer outcomes as expected. Interestingly, PD-1 and PD-L1 expressions were not prognostic factors in our study. In combination, the data from primary melanoma tumors suggest that VISTA may be a key relevant pathway in driving cancer progression. While on the whole, PD-L1 expression has generally been considered a poor prognostic factor in melanoma [20], and there remains conflicting data both in melanoma and in other types of cancer [23, 25, 30,31,32,33]. Our research offers one possible explanation for these differing results, since VISTA may have been a previously unidentified confounder. Interestingly, in a recent publication, negative checkpoint regulation by VISTA has now also been shown to be a mechanism of acquired resistance to anti-PD-1 therapy [34].
In contrast to the IHC results from the primary melanoma specimens in our series, PD-L1, PD-1, and VISTA mRNA expression from the TCGA melanoma samples correlated with improved survival, but only among metastatic lesions. Overall, there were high levels of PD-1, PD-L1, and VISTA expression associated with metastatic lesions, which may indicate a relatively “hot” immune environment. In this case, higher gene expression levels of immune checkpoint proteins would indicate a more robust immune response and not necessarily correlate with immune downregulation. There could also be significant post-translational modification, including protein degradation so that these immune checkpoint proteins would not ultimately be a part of the TME milieu. Further research is needed to better understand the differential prognostic impact of tumor and TIIC expression of VISTA between primary and metastatic melanoma.
Our study has several limitations including those inherent as part of a retrospective study. The sample size was relatively modest which limited our statistical power. This may have decreased our ability to detect significant effects on survival of PD-1/PD-L1 or TIL density. Despite having information on stage and histologic data including depth and ulceration, we were limited in our ability to perform subgroup analyses because of limited case numbers of some subtypes. It is difficult to separately assess the impact of VISTA in the T-cell component of the TME by IHC, due to the very low density of VISTA expression on this population. Future studies may benefit from advances in multiplex fluorescence microscopy methods, which will aid in exploring complex interactions within the TME.
In conclusion, we identified associations between TIIC VISTA expression and TIIC PD-1 expression in the setting of primary melanoma, with negative impacts on disease-specific survival, likely due to immune evasion by the tumor. Our results suggest a novel mechanism in the pathogenesis of melanoma as well as future avenues for translational research into drug therapies that may be able to disrupt this interaction in early stage disease and improve outcomes.
Abbreviations
- AJCC:
-
American Joint Committee on Cancer
- CI:
-
Confidence interval
- HR:
-
Hazard ratio
- TCGA:
-
The cancer genome atlas
- TIIC:
-
Tumor-infiltrating inflammatory cells
References
Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM (2011) Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist 16(1):5–24
Geller AC, Zhang Z, Sober AJ, Halpern AC, Weinstock MA, Miller DR et al (2003) The first 15 years of the American Academy of Dermatology skin cancer screening programs: 1985–1999. J Am Acad Dermatol 48(1):34–41
Jemal A, Saraiya M, Patel P, Cherala SS, Barnholtz-Sloan J, Kim J et al (2011) Recent trends in the cutaneous melanoma incidence and death rates in the United States, 1992–2006. J Am Acad Dermatol 65(5 Suppl 1):S17–S25 (e1–3)
Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N et al (2001) Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19(16):3622–3634
Clemente CG, Mihm MC Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N (1996) Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 77(7):1303–1310
Clark WH Jr, From L, Bernardino EA et al (1969) The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res 29(3):705–727
Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW et al (2012) Tumor-infiltrating lymphocyte grade is an independent predictor of sential lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol 30(21):2678–2683
Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL et al (2012) Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 4(127):127ra37
Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H et al (2000) Engagement of PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 192(7):1027–1034
Wang L, Rubinstein R, Lines JL, Wasiuk A, Ahonen C, Guo Y et al (2011) VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 208(3):577–592
Lines JL, Pantazi E, Mak J, Sempere LF, Wang L, O’Connell S et al (2014) VISTA is an immune checkpoint molecule for human T cells. Cancer Res 74(7):1924–1932
Flies DB, Wang S, Xu H, Chen L (2011) Cutting edge: a monoclonal antibody specific for programmed death-1 homolog prevents graft-versus-host disease in mouse models. J Immunol 187(4):1537–1541
Lines JL, Sempere LF, Broughton T, Wang L, Noelle R (2014) VISTA is a novel broad-spectrum negative checkpoint regulator for cancer immunotherapy. Cancer Immunol Res 2(6):510–517
Le Mercier I, Chen W, Lines JL, Day M, Li J, Sergent P et al (2014) VISTA regulates the development of protective antitumor immunity. Cancer Res 74(7):1933–1944
Piras F, Colombari R, Minerba L, Murtas D, Floris C, Maxia C et al (2005) The predictive value of CD8, CD4, CD68, and human leukocyte antigen-D-related cells in the prognosis of cutaneous malignant melanoma with vertical growth phase. Cancer 104(6):1246–1254
The Cancer Genome Atlas Network (2015) Genomic classification of cutaneous melanoma. Cell 161(7):1681–1696
Wu L, Deng WW, Huang CF, Bu LL, Yu GT, Lao L et al (2017) Expression of VISTA correlated with immunosuppression and synergized with CD8 to predict survival in human oral squamous cell carcinoma. Cancer Immunol Immunother 66(5):627–636
Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III and IV melanoma. N Engl J Med 377(19):1824–1835
Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H et al (2016) Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med 375(19):1845–1855
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Topalian SL, Taube JM, Anders RA, Pardoll DM (2016) Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 16(5):275–287
Hino R, Kabashima K, Kato Y, Yagi H, Nakamura M, Honjo T et al (2010) Tumor expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer 116(7):1757–1766
Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M et al (2014) Programmed death ligand-1 expression in non-small cell lung cancer. Lab Investig 94(1):107–116
Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE et al (2009) Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114(8):1537–1544
Oba J, Nakahara T, Abe T, Hagihara A, Moroi Y, Furue M (2014) Expression of programmed death receptor ligand 1 in melanoma may indicate tumor progression and poor patient survival. J Am Acad Dermatol 70(5):954–956
Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L et al (2014) PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515(7528):568–571
Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J et al (2015) PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res 28(3):245–253
Wang Q, He J, Flies DB, Luo L, Chen L (2017) Programmed death one homolog maintains the pool size of regulatory T cells by promoting their differentiation and stability. Sci Rep 7(1):6086
Liu J, Yuan Y, Chen W, Putra J, Suriawinata AA, Schenk AD et al (2015) Immune-checkpoint proteins VISTA and PD-1 nonredundantly regulate murine T-cell responses. Proc Natl Acad Sci USA 112(21):6682–6687
Gadiot J, Hoojikaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C (2011) Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 117(10):2192–2201
Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS et al (2006) Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 66(7):3381–3385
Tsang JY, Au WL, Lo KY, Ni YB, Hlaing T, Hu J et al (2017) PD-L1 expression and tumor infiltrating PD-1+ lymphocytes associated with outcome in HER2+ breast cancer patients. Breast Cancer Res Treat 162(1):19–30
Danilova L, Wang H, Sunshine J, Kaunitz GJ, Cottrell TR, Xu H et al (2016) Association of PD-1/PD-L axis expression with cytolytic activity, mutational load, and prognosis in melanoma and other solid tumors. Proc Natl Acad Sci USA 113(48):E7769–E7777
Kakavand H, Jacket LA, Menzies AM, Gide TN, Carlino MS, Saw RPM et al (2017) Negative immune checkpoint regulation by VISTA: a mechanism of acquired resistance to anti-PD-1 therapy in metastatic melanoma patients. Mod Pathol 30(12):1666–1676
Funding
This work was supported by National Cancer Institute Grant NCI P30CA023108, Dartmouth Hitchcock Melanoma Funds, and funding provided by Immunext. In addition, Dr. Randolph Noelle has support from NCI RO1 AI098007 and NCI RO1 CA214062, Dr. Mary Jo Turk from NCI RO1 CA214062, and Dr. Marc Ernstoff from NCI PO1 CA206980 and NCI P30 CA016056.
Author information
Authors and Affiliations
Contributions
LFK: interpreted data and wrote the manuscript. SY: conceived the study, interpreted data, and wrote the manuscript. ZL: analyzed data. JLF: interpreted data and wrote the manuscript. CC: analyzed data. RJN: edited the manuscript. CVA: edited the manuscript. MJT: edited the manuscript. MSE: conceived and supervised the study, interpreted data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Randolph J. Noelle is the co-founder of ImmuNext. All other authors declare no conflicts of interest.
Ethical approval
This study was approved by the Dartmouth College Committee for the Protection of Human Subjects/Institutional Review Board, approval number 23388, and was in compliance with ethical guidelines according to the Declaration of Helsinki.
Informed consent
Informed consent was waived by the Institutional Review Board on the grounds of being a retrospective study using tumor tissue already archived by the Dartmouth-Hitchcock Medical Center Department of Pathology tumor bank. Many patients were deceased. All data was abstracted from the medical record and had previously been obtained for the purposes of medical care.
Rights and permissions
About this article
Cite this article
Kuklinski, L.F., Yan, S., Li, Z. et al. VISTA expression on tumor-infiltrating inflammatory cells in primary cutaneous melanoma correlates with poor disease-specific survival. Cancer Immunol Immunother 67, 1113–1121 (2018). https://doi.org/10.1007/s00262-018-2169-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-018-2169-1