Abstract
Ovarian cancer patients with persistent (platinum-resistant) or progressive (platinum-refractory) disease respond poorly to second line chemotherapy and have low survival expectancy. New and improved therapeutic approaches are needed and immune biologics are one possibility. Interleukin-2 (IL-2) is a T-cell growth factor believed to be important in anti-tumor immunity. We performed a phase II clinical trial with intraperitoneal (IP) recombinant IL-2 administered in weekly infusions of 6 × 105 IU/m2. Thirty-one subjects were sequentially entered into the study and clinical responses were surgically confirmed in 24 patients. The primary end point of this study was clinical response with immunologic measurements as secondary end points. The IP regimen was generally well tolerated. Of the 24 patients assessed for response, there were 6 (4 complete, 2 partial) responses for an overall response rate of 25.0% [95% confidence interval (CI) of 11–45]. The median survival of the 31 patient cohort was 2.1 years (95% CI of 1.3–4.4), but for the 6 patients with responses the median survival has not been reached (range 24–120+ months). Eosinophil and lymphocyte numbers were continuously monitored during treatment. Peripheral blood eosinophils were markedly increased at the completion of treatment (p < 0.0001) and associated with increased circulating eotaxin (p = 0.03). We also found significant associations between changes in CD3 counts and survival (p = 0.05) and between IFNγ-secreting CD8 T cells at early time points and survival (p = 0.04). This study provides important evidence for IP IL-2 in platinum-resistant ovarian cancer and identifies several immune correlates of survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is the most lethal gynecologic cancer responsible for more than 15,000 deaths in the US and 125,000 deaths worldwide each year [1, 2]. Despite a 75% objective response rate after first line therapy, 80% or more of patients eventually die from disease recurrence. The worst prognosis is for patients with advanced stages of disease who have persistent (platinum-resistant) or progressive (platinum-refractory) disease. Most second-line therapies in this setting produce responses in the range of 3–12% with an overall survival of only 12–24 months [3, 4], emphasizing the need for new and improved therapeutic approaches.
The confinement of persistent disease to the peritoneal cavity has led to the use of intraperitoneal (IP) infusion of cytotoxic and biologic agents. While front-line IP chemotherapy has improved survival as compared to intravenous (IV) infusions [5–7], IP approaches for persistent disease remain controversial. Interleukin-2 (IL-2) is a T-cell growth factor that plays a critical role in T cell-dependent immunity and is believed to be important in anti-tumor immunity [8], although the in vivo IL-2-mediated mechanisms are not yet fully defined. Systemic high dose IL-2 is US Food and Drug Administration (FDA)—approved for the treatment of metastatic melanoma and renal cell cancer where a small percentage of patients consistently show durable anti-tumor responses [9]. Given this modest yet consistent success of IL-2 in melanoma and renal cancer, its indication for other solid tumors, including ovarian cancer, continues to be explored [10]. Notwithstanding the clinical benefits for a subset of patients, high dose IV IL-2 triggers extreme toxicities like hypotension, oliguria and pulmonary edema. We have previously reported a phase I/II dose-escalation variable schedule study of IL-2 infused directly into the peritoneal cavity of ovarian cancer patients [11] and identified that weekly intermittent infusions were better tolerated than the daily infusions and produced a significant number of durable complete responses with a 26% response rate and a median duration of response exceeding 7 years. Based on these findings, we designed a phase II IP IL-2 trial in persistent ovarian cancer after platinum-based chemotherapy (PCT). The primary end point of this study was clinical response, with immunologic measurements as secondary end points. We postulated that moderate doses of IL-2 are better tolerated when administered IP and may induce durable tumor regression and increase survival in ovarian cancer patients with persistent disease.
Patients and methods
Clinical trial design
This clinical trial was conducted from 1995 to 1999 at Magee Womens Hospital in Pittsburgh, according to University of Pittsburgh Medical Center Institutional Review Board-approved protocol. Subjects were enrolled after informed consent. Patients’ characteristics are described in Table 1 and the study CONSORT diagram is displayed in Fig. 1.
Patients with biopsy-proven persistent ovarian or “extra-ovarian Müllerian” cancer after first or second-line platinum-based chemotherapy (PCT) were eligible for this trial. Patients must have completed greater than five courses of platinum or taxane-containing regimen with a disease-free interval of less than 6 months. Eligible subjects had an ECOG performance score of 0 or 1, normal renal and liver function and no history of autoimmune or cardiac disease. Disease status was evaluated surgically no more than 6 weeks prior to being enrolled. The disease burden was required to be intra-abdominal without brain, liver, lung parenchyma, or bone metastases.
Treatment plan, toxicity assessment and stopping rules
The protocol was designed for 16 weekly infusions of 6 × 105 IU/m2 IL-2 (Proleukin, Aldesleukin, Chiron), administered through an indwelling (Bard™) peritoneal catheter with subcutaneous reservoir in 2 l of sterile 5% dextrose. Pre-warmed IL-2 was infused into the peritoneum with an infusion pump.
Toxicities were graded using the ECOG toxicity scale, with additional scales for grading peritoneal catheter associated toxicities for two etiologies: infection and mechanical complications, graded as described in Table 2. Altogether, catheter obstruction or infusion pain resulted in five (16%) subjects not completing the study. Infusion of IL-2 was stopped for any reported grade III or IV toxicity other than fever and re-initiated with a 20% dose reduction once the presenting toxicity abated. Patients were removed from the study if a grade III or IV toxicity recurred after an initial dose reduction. Loss of catheter access (in four patients) was managed by replacement (in two patients), usually by laparoscopy, with a 2-week treatment delay. Two patients chose to not have their catheter replaced and were withdrawn from the study.
Criteria for evaluation of response and survival
Platinum-resistant disease was defined as persistent visible disease after primary PCT therapy, or disease that recurred within 6 months of completing platinum therapy [12].
Clinical responses were assessed 4–8 weeks after protocol completion. Complete clinical responses (CR) were defined by the disappearance of all clinically detectable disease and no detection of new malignant lesions. All CR were confirmed by biopsy of prior tumor sites via open laparoscopy or laparotomy. Partial response (PR) was defined as a decrease of more than 25% in tumor size. Progressive disease (PD) was defined by a 25% or greater increase in the product of perpendicular cross diameters at laparotomy or two-dimensional area by exam, computed tomography (CT) or magnetic resonance imaging (MRI). Stable disease (SD) represents disease for which dimensions failed to meet the above criteria for progression or regression.
Survival data were obtained from multiple sources, including the tumor registries of Magee-Womens Hospital and office records. As of 31 March 2009, one patient was still alive.
Sample collection
Peripheral blood, tumor, and peritoneal aspirates were collected at each surgery. Peripheral blood and peritoneal aspirates were collected just prior to and 15 min after each weekly IP IL-2 infusion. Heparinized sterile vacutainer tubes (BD) were used for cell isolation and non-heparinized tubes for serum collection. Processing included Ficoll separation via centrifugation and cryopreservation in liquid nitrogen of the mononuclear cell fraction, until ready to use.
Flow cytometry
Peripheral blood mononuclear cells (PBMC) were stained with the following T lymphocyte surface markers: PerCP anti-CD3, PE-Cy7 anti-CD4, APC-Cy7 anti-CD8 (BD). Intracellular staining for FOXP3 was performed according to manufacturer’s instructions (eBioscience). For cytokine detection, the cells were rested overnight in culture medium (cRPMI, 10% FBS) and later stimulated with PMA (50 ng/ml) and Ionomycin (500 ng/ml) (both from Sigma) for 4 h at 37°C, in the presence of Brefeldin A (BD Biosciences). After stimulation the cells were stained for surface markers, washed twice with FACS buffer (5% FBS in phosphate buffered saline, PBS), resuspended in fixation/permeabilization solution (BD Biosciences) and then finally stained with APC anti-IFNγ (BD Biosciences). The data were analyzed using a LSR II flow cytometer and FACDiva software (BD Biosciences).
Cytometric bead array
Cytometric bead array (CBA) technology was used to simultaneously measure serum eotaxin and vascular endothelial growth factor (VEGF) in a 50 μl total sample volume (1:10 dilution, duplicates), according to the recommended manufacturer’s protocol (BD Biosciences), using a BD LSR II flow cytometer. Data acquired with BD FACSDiva software was analyzed with FCAP Array v1.0.1 software (BD Biosciences). The VEGF measurements were confirmed by ELISA.
ELISA
Vascular endothelial growth factor (VEGF) presence in the peritoneal wash of women treated with IL-2 was measured using a commercial ELISA kit (R&D System). The samples were run in duplicates and the assay results were calculated using Ascent Software for Multiskan version 2.6 (Thermo Scientific).
Statistical analysis
The overall survival (OS) was calculated as the time interval between start date of treatment and date of death. It was censored by the last follow-up date if a patient was still alive and was estimated by the Kaplan–Meier method. Greenwood formula was used to estimate the 95% CI for OS. A log rank test was used to assess the association between OS and clinical response. Kruskal–Wallis tests were used to test the association between early, late, or changes in cell counts and response. Cox Regression was used to study the effect of early, late, or change in cell counts on survival. Wilcoxon Signed Rank Tests were used to test the difference between early and late marker expression. Analyses were performed using SAS v 9.1 (Cary, NC) and significance level of 0.05 was assumed.
Results
Adverse events
A total of 31 ovarian cancer patients were treated with weekly IP injections of 6 × 105 IL-2 IU/m2. Characteristics of the patients are summarized in Table 1. The median time from the diagnosis of cancer to the initiation of IP IL-2 was 12 months. All 31 patients were previously treated with greater than 5 courses of PCT before receiving IL-2.
IL-2 is a highly inflammatory molecule which triggers numerous adverse events when administered IV [13, 14]. Weekly IP administrations of 6 × 105 IU/m2 employed here were generally well tolerated, although constitutional grade 1 and grade 2 toxicities were seen uniformly in patients (Table 3), but ameliorated with medical therapy without dose restriction. Most of the toxicities were possibly therapy-associated although it was difficult to differentiate between therapy-associated toxicity and progression of disease symptoms in some cases. Non-catheter related grade III adverse events were limited to nausea, abdominal distension, and deep venous thrombosis.
Seventeen (55%) subjects completed all 16 weeks of therapy and the median number of weekly infusions for those who did not finish was 8. Most patients experienced some mechanical catheter malfunctions which were preceded by infusion pain and were limited to grade I/II.
Clinical response and correlation with survival
All 31 patients enrolled received at least one infusion of IL-2. The clinical responses were evaluated by laparoscopy in 24 patients (Table 4): a complete response (CR) was seen in 4 patients, a partial response (PR) in 2 patients and stable disease (SD) in 7 patients. Eleven patients showed progressive disease (PD). By the intention-to-treat analysis, the response rate (CR + PR) would be 6/31 or 19.4% with a 95% confidence interval of (0.09–0.36). The seven patients who did not undergo response assessment received a median of six infusions and the protocol was terminated due to toxicities (catheter infection, obstruction or infusion pain) in five patients or subject withdrawal in the remaining two patients.
Of the 20 patients enrolled with papillary serous and poorly differentiated adenocarcinoma, five demonstrated a response to treatment. One patient with persistent grade III immature teratoma that progressed on PCT was enrolled and had a pathologically negative second look. There were no mucinous tumors enrolled and none of the ten patients enrolled with endometrioid or clear cell histology demonstrated a response to IP IL-2. The clinical response was independent of initial disease volume (p = 0.8929).
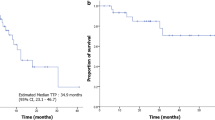
The median survival of the cohort was 2.1 years with a 95% confidence interval of 1.3–4.1 years. Figure 2a demonstrates the Kaplan–Meier curve with 95% confidence bands for the entire 31 patient cohort. The survival analysis of patients evaluable for response (n = 24, Fig. 2b) shows that survival was significantly affected by the type of clinical response (p = 0.0063). The median survival for patients who did not respond to IP IL-2 (PD, n = 11) was 1.5 years (0.68–2.3 95% CI). For the 13 responding patients (CR + SD + PR), the median survival has not been reached (as of the time of this publication) and the lower confidence interval is above 2 years.
Kaplan–Meier curve of overall survival with 95% pointwise confidence limits (grey areas). b Association between OS and clinical response calculated by log rank test (p = 0.0063). Responders are complete (CR) + partial responders (PR) + stable disease (SD). Non-responders are patients with progressive disease (PD)
IP IL-2 induces lymphocytosis, eosinophilia and increased eotaxin
Complete blood counts (CBC) and white blood cell (WBC) differential counts were evaluated early during treatment and close to treatment completion and correlated to objective response and survival. Of the 24 patients who were evaluable for clinical response, serial analysis of immune parameters was only possible in 18. Although at early time points, the eosinophil (Eos) counts were within normal limits, they were drastically increased (p < 0.0001) by the end of the treatment, suggestive of treatment-induced eosinophilia (Table 5, Supplemental Fig. 1). The gradual increase in peripheral blood Eos started soon after treatment was instituted (not shown) and often reached more than 50% of the circulating leukocytes. Furthermore, Eos also accumulated in the peritoneal fluid (not shown), where white blood cell counts reveal them as the vastly predominant population, often reaching 80–100% of resident cells. However, neither the early or late (end-of-treatment) Eos counts nor the count changes from early to late time points significantly correlated with clinical response or survival (not shown).
Eosinophils migrate in response to eotaxin and release inflammatory granules in response to ligand-binding of surface receptors. We identified a significant (p = 0.031) increase in eotaxin (Table 5, Supplemental Fig. 1) in patients’ sera at late time points. In addition to eosinophilia, the patients also showed increased lymphocyte counts with treatments (Table 5, Supplemental Fig. 1), in accordance to findings from renal cell carcinoma [15–17].
Correlation between number and activity of peripheral blood lymphocytes and survival
In order to identify the effects of IL-2 on T lymphocytes and their cytokine secretion profile, 36 PBMC samples collected at two time points during treatment were used for multicolor flow cytometry analysis. The cells collected at either early or late times during treatment were stained for CD3, C4, CD8, FOXP3, and for IFN-γ following PMA/ionomycin polyclonal stimulation. Variations in percentages of CD3 T lymphocytes FOXP3 + CD4 T cells and IFNγ-secreting CD8 T lymphocytes from four patients (1 CR, 1PR, 1SD and 1PD) are shown in Supplemental Fig. 2. No significant changes from early to late times were seen for the cohort and no significant associations between any of the above markers (either the counts at early, late or early-to-late changes) and clinical response were demonstrated. However, we identified a borderline significant association between the early values of IFNγ- secreting CD8 T cells and survival (p = 0.05) and the statistical analysis suggests that patients with more circulating IFNγ-secreting CD8 T cells at early time points had better chances of survival (HR = 0.971). We also identified a significant association between the change (p = 0.04) in the total CD3 T cell number and survival. Notably, the patients who had a decrease in T cell counts had better chances of survival than those patients that had an increase. In addition, we did not detect significant changes in the number of circulating regulatory FOXP3 + CD4 T cells (Tregs), which largely remained within normal limits.
Serum and IP fluid VEGF measurements in treated patients
We also explored VEGF as a marker of response and survival and found no correlations with values at early, late or with changes from early to late time points. However, all patients showed a significant increase in circulating VEGF at treatment completion (p = 0.025) (Table 5, Supplemental Fig. 3). In contrast, measurements in the IP fluid suggested a decrease in local VEGF, although the difference did not reach significance (p = 0.058).
Discussion
First line therapy in ovarian cancer combines cytoreductive surgery followed by chemotherapy. Because ovarian tumors remain largely confined within the peritoneal cavity, IP administration of drugs could maximize efficacy and reduce toxicity. Several large randomized studies [5–7] have each shown improved responses with either IP or combination IV/IP chemotherapy, demonstrating clear benefit for this approach. Nevertheless, the exploration of peritoneal therapy for ovarian cancer still requires improvements in delivery systems and administration techniques to make this approach feasible in routine clinical settings. To help standardize this, we have proposed a toxicity grading system (Table 2) which may assist with the future developing of improved peritoneal infusion systems.
The use of the IP route for administration of immune biologics, including IL-2, in ovarian cancer, has not been extensively studied. However, intravenous administration of IL-2 as monotherapy or in association with immunotherapy has been tested in several clinical trials, demonstrating a 15–20% response rate in melanoma and metastatic renal cancer, as well as a significant activity in other neoplasms such as lymphoma and lung, colorectal, gastric and pancreatic cancers [10]. Furthermore, collective results in ovarian cancer from several studies, including our previous phase I/II trial, show that from a total of 96 patients with platinum resistant disease [10], clinical activity was achieved in 21 patients (22%), an acceptable level of efficacy given the unfavorable nature of the patient population tested. Increasing this success rate may ultimately depend on our ability to identify those patients most likely to benefit from therapy and to define the optimal administration regimen with the lowest treatment-associated toxicity.
Using a moderate weekly dose and a newly developed toxicity grading system, we show that IP IL-2 is relatively well tolerated and with apparent clinical efficacy. Despite the inherent inflammatory nature of IL-2 and the significant number of previous therapies administered to our patients, 55% of the cohort was able to complete 16 weekly infusions. Notably absent from this trial were the episodes of pronounced hypotension, gastrointestinal fistulas, peritoneal fibrosis, and pelvic abscesses seen in our phase I study [11], likely due to the weekly intermittent regimen and the reduced IL-2 dose selected. The use of IP therapy in persistent and recurrent cancer has been reported in a number of trials but the toxicities due to the IP infusion have not been well delineated. Our study of second and third line IP therapy attempts to do this and shows a surprisingly high completion rate, higher than seen with front line therapy by Armstrong et al. (42%, [7]) and comparable to other Gynecologic Oncology Group (GOG) front-line trials [5, 18]. For the 45% who did not complete the trial, the reason was largely catheter-associated toxicity, most commonly pain or obstruction. The role of progressive cancer in these subjects is difficult to define, but two of the seven subjects withdrawn for toxicity succumbed to their cancer within 3 months of starting the study.
Ovarian cancer patients with persistent (platinum-resistant) or progressive (platinum-refractory) disease respond poorly to second line chemotherapy and have low survival expectancy. Although a control arm was not included in this study, the survival for progressive and persistent ovarian cancer has been reported to be between 12 and 24 months [3, 4]. The overall survival for our patient cohort was 2.1 years, but for the 6 patients with responses the median survival has not been reached (range 24–120+ months and the lower confidence interval longer than 2 years), with one patient surviving for 10 years and one still alive, suggesting that the durability of responses seen with IL-2 in melanoma and renal cell carcinoma can also be elicited in certain platinum-resistant ovarian cancer patients.
The complete in vivo IL-2 mechanisms of action are not fully defined. IL-2 is a cytokine mainly released by activated T cells and acts as a T cell growth factor, enhancing the cytotoxic activity of previously activated T cells [19–23]. It also stimulates growth and differentiation of B-lymphocytes and natural killer (NK) cells and may act as a chemoattractant for Eos, which express the IL-2 receptor [24, 25].
Our results demonstrate that following IP IL-2, the number of circulating Eos increases significantly from early to late time points during treatment and is paralleled by increases in serum eotaxin, a potent in vitro and in vivo chemoattractant for Eos. A variety of cell types may release eotaxin in vivo, including macrophages, T lymphocytes and Eos themselves [26–28]. Furthermore, Eos were the predominant cell population in the peritoneal fluid and were also detected infiltrating the tumor (not shown). Although we did not demonstrate correlation of Eos with either clinical response or survival, studies on colorectal and lung cancer have demonstrated that blood or tissue eosinophilia correlates with significantly better prognosis [29–31] while others suggest the opposite [32–34]. The factors that influence the balance between tumor-killing by IgE-binding Eos [35] and tumor-promoting due to Eos-derived pro-inflammatory and pro-angiogenic factors [36] may ultimately shed light into their roles in the tumor microenvironment and define their prognostic value in anti-tumor immunity.
Unlike Eos, whose roles in anti-tumor immunity are only now being deciphered, lymphocytes and their modulations by immune biologics have been extensively studied in cancer. Nevertheless, only very few lymphocytic correlates of in vivo efficacy (objective response) and survival have been described to date [15, 17, 37, 38] and none are currently standardized. Although correlations of tumor-infiltrating CD8 T cell numbers and survival have been described [39–41], no associations of cytokine secreting CD8 T cell subsets and survival have been so far reported in ovarian cancer. We found a significant correlation with survival of early counts of systemic CD8 T cells secreting IFNγ, a hallmark of cytotoxicity. CD8 cytotoxic T cells are modulated by CD4 Th1 cells and increased Th1 immunity in the host is generally regarded as the most efficient phenotype in fighting cancer [42–44]. We did not however, identify any association between IFNγ-secreting CD4 Th1 T cells with either clinical response or survival in our patients, suggesting alternative modulation, potentially via IL-12. We also acknowledge that since only early IFNγ-secreting CD8 T cells were found to correlate with survival, this measurement may not serve as an immune biomarker for IL-2 therapy, but potentially as a biomarker for response to treatment in general; we postulate that improving overall baseline immune status in patients with immune modulators that boost Th1 immunity and/or foster IFNγ CD8 T cells [45, 46] may provide a better environment for the subsequent generation of anti-tumor responses with cancer vaccines or immune biologics.
Last, we also report here the effect of treatment on circulating VEGF, currently tested as therapeutic target in many types of cancer [47, 48]. Interestingly, the VEGF values were increased in serum while the IP fluid measurements seemed to decline with treatment. Given the inherent variability associated with IP fluid sampling, further evaluation of this marker in serum versus the tumor microenvironment is needed. The increased serum VEGF may have triggered increased vascular permeability [9] and migration of CD3 T cells into periphery and potentially at the tumor site, explaining our observed association with change in circulating T lymphocytes and survival.
In summary, ovarian cancer patients with persistent or progressive disease have limited treatment options and reduced survival. Systemic high dose IL-2, although associated with considerable toxicity, is FDA-approved for metastatic renal cell carcinoma and melanoma, where few patients show robust responses. Our studies with IP IL-2 in ovarian cancer, demonstrate that loco-regional IL-2 in a moderate weekly dose is better tolerated and with apparent clinical efficacy. The durability of the responses is striking as it is for both renal cancer and melanoma. Our results show sufficient benefit with reasonable toxicity and call for further studies in platinum-resistant ovarian cancer patients and for additional validation of immune findings in bigger control arm trials or combination therapy with other biologics.
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ (2005) Cancer statistics, 2005. CA Cancer J Clin 55:10–30
Parkin DM, Bray F, Ferlay J, Pisani P (2005) Global cancer statistics, 2002. CA Cancer J Clin 55:74–108
Cannistra SA (2004) Cancer of the ovary. N Engl J Med 351:2519–2529
Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ (2001) Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. J Clin Oncol 19:3312–3322
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335:1950–1955
Markman M, Blessing JA, Moore D, Ball H, Lentz SS (1998) Altretamine (hexamethylmelamine) in platinum-resistant and platinum-refractory ovarian cancer: a Gynecologic Oncology Group phase II trial. Gynecol Oncol 69:226–229
Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, Copeland LJ, Walker JL, Burger RA (2006) Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 354:34–43
Rosenberg SA, Lotze MT, Muul LM, Leitman S, Chang AE, Ettinghausen SE, Matory YL, Skibber JM, Shiloni E, Vetto JT et al (1985) Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med 313:1485–1492
Elias L, Hunt WC (2001) A literature analysis of prognostic factors for response and quality of response of patients with renal cell carcinoma to interleukin-2-based therapy. Oncology 61:91–101
Grande C, Firvida JL, Navas V, Casal J (2006) Interleukin-2 for the treatment of solid tumors other than melanoma and renal cell carcinoma. Anticancer Drugs 17:1–12
Edwards RP, Gooding W, Lembersky BC, Colonello K, Hammond R, Paradise C, Kowal CD, Kunschner AJ, Baldisseri M, Kirkwood JM, Herberman RB (1997) Comparison of toxicity and survival following intraperitoneal recombinant interleukin-2 for persistent ovarian cancer after platinum: twenty-four-hour versus 7-day infusion. J Clin Oncol 15:3399–3407
Markman M, Reichman B, Hakes T, Lewis JL Jr, Jones W, Rubin S, Barakat R, Curtin J, Almadrones L, Hoskins W (1992) Impact on survival of surgically defined favorable responses to salvage intraperitoneal chemotherapy in small-volume residual ovarian cancer. J Clin Oncol 10:1479–1484
Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, Paradise C, Kunkel L, Rosenberg SA (1999) High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17:2105–2116
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC (1995) Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13:688–696
Donskov F, Bennedsgaard KM, Von Der Maase H, Marcussen N, Fisker R, Jensen JJ, Naredi P, Hokland M (2002) Intratumoural and peripheral blood lymphocyte subsets in patients with metastatic renal cell carcinoma undergoing interleukin-2 based immunotherapy: association to objective response and survival. Br J Cancer 87:194–201
Fumagalli L, Lissoni P, Di Felice G, Meregalli S, Valsuani G, Mengo S, Rovelli F (1999) Pretreatment serum markers and lymphocyte response to interleukin-2 therapy. Br J Cancer 80:407–411
Jeong IG, Han KS, Joung JY, Choi WS, Hwang SS, Yang SO, Seo HK, Chung J, Lee KH (2007) Analysis of changes in the total lymphocyte and eosinophil count during immunotherapy for metastatic renal cell carcinoma: correlation with response and survival. J Korean Med Sci 22:S122–S128
Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, Wadler S, Sickel J (2001) Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 19:1001–1007
Bordin V, Giani L, Meregalli S, Bukovec R, Vaghi MM, Mandala M, Paolorossi F, Ardizzoia A, Tancini G, Barni S, Frigerio F, Fumagalli L, Bordoni A, Valsuani G, Di Felice G, Lissoni P (2000) Five-year survival results of subcutaneous low-dose immunotherapy with interleukin-2 alone in metastatic renal cell cancer patients. Urol Int 64:3–8
Lissoni P, Barni S, Ardizzoia A, Crispino S, Paolorossi F, Andres M, Scardino E, Tancini G (1994) Prognostic factors of the clinical response to subcutaneous immunotherapy with interleukin-2 alone in patients with metastatic renal cell carcinoma. Oncology 51:59–62
Palmer PA, Atzpodien J, Philip T, Negrier S, Kirchner H, Von der Maase H, Geertsen P, Evers P, Loriaux E, Oskam R et al (1993) A comparison of 2 modes of administration of recombinant interleukin-2: continuous intravenous infusion alone versus subcutaneous administration plus interferon alpha in patients with advanced renal cell carcinoma. Cancer Biother 8:123–136
von Rohr A, Ghosh AK, Thatcher N, Stern PL (1993) Immunomodulation during prolonged treatment with combined interleukin-2 and interferon-alpha in patients with advanced malignancy. Br J Cancer 67:163–171
Wersall P, Mellstedt H (1995) Increased LAK and T cell activation in responding renal cell carcinoma patients after low dose cyclophosphamide, IL-2 and alpha-IFN. Med Oncol 12:69–77
Degrate L, Nobili C, Franciosi C, Caprotti R, Brivio F, Romano F, Leone BE, Trezzi R, Uggeri F (2009) Interleukin-2 immunotherapy action on innate immunity cells in peripheral blood and tumoral tissue of pancreatic adenocarcinoma patients. Langenbecks Arch Surg 394:115–121
Rand TH, Silberstein DS, Kornfeld H, Weller PF (1991) Human eosinophils express functional interleukin 2 receptors. J Clin Invest 88:825–832
Lamkhioued B, Renzi PM, Abi-Younes S, Garcia-Zepada EA, Allakhverdi Z, Ghaffar O, Rothenberg MD, Luster AD, Hamid Q (1997) Increased expression of eotaxin in bronchoalveolar lavage and airways of asthmatics contributes to the chemotaxis of eosinophils to the site of inflammation. J Immunol 159:4593–4601
Mattoli S, Stacey MA, Sun G, Bellini A, Marini M (1997) Eotaxin expression and eosinophilic inflammation in asthma. Biochem Biophys Res Commun 236:299–301
Ying S, Robinson DS, Meng Q, Rottman J, Kennedy R, Ringler DJ, Mackay CR, Daugherty BL, Springer MS, Durham SR, Williams TJ, Kay AB (1997) Enhanced expression of eotaxin and CCR3 mRNA and protein in atopic asthma Association with airway hyperresponsiveness and predominant co-localization of eotaxin mRNA to bronchial epithelial and endothelial cells. Eur J Immunol 27:3507–3516
Fernandez-Acenero MJ, Galindo-Gallego M, Sanz J, Aljama A (2000) Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer 88:1544–1548
Rivoltini L, Viggiano V, Spinazze S, Santoro A, Colombo MP, Takatsu K, Parmiani G (1993) In vitro anti-tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int J Cancer 54:8–15
Samoszuk M (1997) Eosinophils and human cancer. Histol Histopathol 12:807–812
Leighton SE, Teo JG, Leung SF, Cheung AY, Lee JC, van Hasselt CA (1996) Prevalence and prognostic significance of tumor-associated tissue eosinophilia in nasopharyngeal carcinoma. Cancer 77:436–440
Moroni M, Porta C, De Amici M, Quaglini S, Cattabiani MA, Buzio C (2000) Eosinophils and C4 predict clinical failure of combination immunotherapy with very low dose subcutaneous interleukin-2 and interferon in renal cell carcinoma patients. Haematologica 85:298–303
van Driel WJ, Hogendoorn PC, Jansen FW, Zwinderman AH, Trimbos JB, Fleuren GJ (1996) Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum Pathol 27:904–911
Karagiannis SN, Bracher MG, Hunt J, McCloskey N, Beavil RL, Beavil AJ, Fear DJ, Thompson RG, East N, Burke F, Moore RJ, Dombrowicz DD, Balkwill FR, Gould HJ (2007) IgE-antibody-dependent immunotherapy of solid tumors: cytotoxic and phagocytic mechanisms of eradication of ovarian cancer cells. J Immunol 179:2832–2843
Coussens LM, Werb Z (2001) Inflammatory cells and cancer: think different. J Exp Med 193:F23–F26
Cesana GC, Romano F, Piacentini G, Scotti M, Brenna A, Bovo G, Vaghi M, Aletti G, Caprotti R, Kaufman H, Uggeri F (2007) Low-dose interleukin-2 administered pre-operatively to patients with gastric cancer activates peripheral and peritumoral lymphocytes but does not affect prognosis. Ann Surg Oncol 14:1295–1304
Donskov F, Bennedsgaard KM, Hokland M, Marcussen N, Fisker R, Madsen HH, Fode K, von der Maase H (2004) Leukocyte orchestration in blood and tumour tissue following interleukin-2 based immunotherapy in metastatic renal cell carcinoma. Cancer Immunol Immunother 53:729–739
Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Han G, Ceballos K, Cadungog MG, Huntsman DG, Coukos G, Gilks CB (2008) Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol 22:393–402
Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K (2005) Intraepithelial CD8 + tumor-infiltrating lymphocytes and a high CD8 +/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA 102:18538–18543
Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G (2003) Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 348:203–213
Egeter O, Mocikat R, Ghoreschi K, Dieckmann A, Rocken M (2000) Eradication of disseminated lymphomas with CpG-DNA activated T helper type 1 cells from nontransgenic mice. Cancer Res 60:1515–1520
Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C (2008) Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med 358:2698–2703
Muller-Hermelink N, Braumuller H, Pichler B, Wieder T, Mailhammer R, Schaak K, Ghoreschi K, Yazdi A, Haubner R, Sander CA, Mocikat R, Schwaiger M, Forster I, Huss R, Weber WA, Kneilling M, Rocken M (2008) TNFR1 signaling and IFN-gamma signaling determine whether T cells induce tumor dormancy or promote multistage carcinogenesis. Cancer Cell 13:507–518
Grange JM, Bottasso O, Stanford CA, Stanford JL (2008) The use of mycobacterial adjuvant-based agents for immunotherapy of cancer. Vaccine 26:4984–4990
Schon MP, Schon M (2008) TLR7 and TLR8 as targets in cancer therapy. Oncogene 27:190–199
Ellis LM, Hicklin DJ (2008) VEGF-targeted therapy: mechanisms of anti-tumour activity. Nat Rev Cancer 8:579–591
Longo R, Gasparini G (2008) Anti-VEGF therapy: the search for clinical biomarkers. Expert Rev Mol Diagn 8:301–314
Acknowledgments
This work was supported by NIH R21 CA74105-02S1, American Cancer Society, Chiron Therapeutics, PA Department of Health and the Magee-Womens Health Foundation.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Vlad, A.M., Budiu, R.A., Lenzner, D.E. et al. A phase II trial of intraperitoneal interleukin-2 in patients with platinum-resistant or platinum-refractory ovarian cancer. Cancer Immunol Immunother 59, 293–301 (2010). https://doi.org/10.1007/s00262-009-0750-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-009-0750-3