Abstract
CD46 or membrane cofactor protein (MCP) is a complement regulatory protein that has been identified on all nucleated cells and which protects them from attack by autologous complement. Breast carcinomas are reported to consistently express CD46.
Aim and methods: Our previous immunohistochemical study showed that in breast carcinomas, loss of CD59 and CD55 correlated with poor survival. This study aimed to investigate the prognostic significance of CD46 on breast tumours using a rabbit polyclonal anti-CD46 antibody with a standard immunohistochemistry method. A total of 510 breast tissues from patients with primary operable breast cancer diagnosed between 1987 and 1992 had previously been included in tissue microarrays. They included patients 70 years of age or less (mean = 54 years) with a long-term follow-up (median = 82 months).
Results: Immunohistochemical study revealed that 507/510 (99.4%) of breast tumours expressed CD46. Strong immunoreactivity was exhibited by 136/510 (27%) tumours, while moderate and weak staining was observed in 43% and 29% of tumours, respectively. Intensity of CD46 expression was significantly associated with tumour grade (p<0.05), histological type of tumour (p<0.001) and tumour recurrence (p<0.05). There was no correlation with lymph node stage or the presence of vascular invasion, nor with patient age or menopausal status. Interestingly, as most tumours expressed CD46, it would appear that poor-prognosis tumours that lose CD55 and CD59 still express CD46.
Conclusion: Breast tumours express high levels of CD46 that correlates with tumour grade and recurrence. It is therefore likely that loss of CD55 and CD59 could be compensated by expression of CD46. However, loss of CD55 and CD59, even for tumours that still express CD46, is still associated with a poor prognosis. This may suggest that CD46 alone can protect from complement lysis but that loss of CD55 and CD59 are associated with other roles in immune regulation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To prevent complement-mediated autologous tissue damage, host cells express a number of membrane-bound complement regulatory proteins (CRPs) including CD59, CD55 (DAF) and CD46. Membrane cofactor protein (MCP, CD46), a widely distributed regulator of complement activation, is a cofactor for the factor I–mediated degradation of C3b and C4b deposited on host cells [17, 30]. CD46 therefore has a complementary activity to CD55 with which it acts jointly to inhibit C3b and C4b deposition on self-tissue [16]. Except for erythrocytes, MCP has been identified on all human peripheral blood cells [2, 31], and cells of epithelial, endothelial and fibroblast lineages [20, 27].
The molecule consists of four contiguous short consensus repeat (SCR) domains and an alternatively spliced region rich in serines, threonines and prolines that is heavily O-glycosylated. However, CD46 has three sites for N-glycosylation in SCR1, SCR2 and SCR4 and inserts into the cell membrane through a transmembrane domain, unlike the other two regulatory proteins that are GPI-anchored. SCR2, SCR3 and SCR4 are all involved in C3b, C4b and cofactor binding [17, 26, 29]. CD46 is a potent inhibitor of alternative pathway activation [12] and counteracts the amplification phase of C3b deposition [4], but has less effect controlling classical pathway and C3b/C4b deposition [12].
It has been shown that most tumour cell lines, except those of B-cell lineage, possess twofold to eightfold more MCP in comparison with their normal counterparts [32]. Most normal surface epithelia express CRPs; however, tumours show variable expression of these proteins. It has been presumed that overexpression of one of the CRPs may counteract for the loss of another [15]. Ductal carcinomas of the breast demonstrate the most variation in phenotype; some tumours express only one inhibitor, while others express various combinations of two or three inhibitors. Previous immunohistochemical studies on a limited number of freshly frozen tumours have shown that CD46 was consistently expressed in breast carcinomas, predominantly on the cell membrane with no stromal deposition [22, 35]. In 1994, Hofman et al. [11] reported that CD46 reactivity was significantly greater in malignant compared with benign breast disorders.
Our previous immunohistochemical study showed that in breast carcinoma loss of CD59 and CD55 correlated with poor survival [18, 19]. To our knowledge, however, there has been no study of CD46 expression on a large number of paraffin-embedded breast tumours. As none of the commercially available anti-CD46 monoclonal antibodies can be used on formalin-fixed sections, this study aimed to investigate the prognostic significance of CD46 on breast tumours with a new rabbit polyclonal anti-CD46 antibody employing a standard immunohistochemistry method.
Materials and methods
Patients
The present study comprises 510 cases of primary operable invasive breast carcinoma from patients aged 70 years or less diagnosed between 1987 and 1992 obtained from the Nottingham Tenovus Primary Breast Carcinoma Series. Patient characteristics including age and menopausal status were also collected, and information on local, regional and distant recurrence and survival was also retrieved. At the time of diagnosis, patient age ranged from 27 to 70 years (mean 53). From a total of 487 with recorded menopausal status, 184 (38%) of patients were premenopausal and 303 (62%) were postmenopausal.
Patients were followed up at 3-month intervals initially, then 6 monthly then annually for a median period of 82 months, presenting a mean survival of 76 months (1–151 months). Distant recurrence was observed in 123 (24%) of a total of 510 patients.
Patient management was based on tumour characteristics by calculating the Nottingham Prognostic Index (NPI). Those women with an NPI score ≤3.4 received no adjuvant therapy, and those with a NPI score >3.4 received tamoxifen if oestrogen receptor (ER) positive (± Zoledex, if premenopausal), or CMF (classical chemotherapy) if ER negative and fit enough for chemotherapy to be appropriate.
Tissue preparation
The excised tumours were sliced, fixed immediately and placed in neutral buffered formalin and then processed through to embedding in paraffin wax to minimise any diffusion problems. Tumour samples were arrayed as described by Kononen et al. [13]. From each sample, one representative tumour region was included. Microarray samples with a diameter of 0.6 mm were punched from selected regions of each “donor” block using a Beecher instrument (Manual Tissue Arrayer) and precisely arrayed into a new recipient paraffin block. The tissue microarray (TMA) blocks were constructed in three copies, each containing one sample from a different region of the tumour. It has been shown that, contrary to expectations, tissue heterogeneity did not negatively influence the predictive power of the TMA results [1, 37].
This is a well-characterised series of primary operable breast cancer treated in a uniform manner and has been used to study a wide range of potential prognostic factors and markers including CD59 [18] and CD55 expression [19]. Tumour characteristics, including histological grade [6], tumour type [5], vascular invasion [25], menopausal status [36], tumour size, lymph node stage and NPI [7] are routinely assessed and recorded in a database. The NPI score was calculated for each patient based on the following equation [10]: NPI=0.2 × tumour size (cm) + histological grade (1–3) + lymph node stage (1–3). This index predicts survival of patients with invasive breast cancer and is utilised clinically in three groups: good prognosis (NPI≤3.4), moderate prognosis (3.41<NPI≤5.4) or poor prognosis (NPI>5.4) according to the score obtained.
In addition, assays to determine the ER status of each tumour section were conducted (at the Tenovus Institute, Cardiff) by a dextran-coated charcoal technique. A cutoff of 10 fmol/mg protein was used to determine positivity. ER status was known in 286 patients; 87 (31%) of patients were ER negative and 199 (69%) were ER positive.
Finally, tumour sections were classified in four prognostic type groups [7, 24]:
-
1.
Excellent prognosis type (>80% 10-year survival), includes tubulo-lobular, tubular, mucinous and invasive cribriform carcinoma.
-
2.
Good types (60–80% 10-year survival), includes tubular mixed, mixed ductal with special type and alveolar lobular carcinoma.
-
3.
Moderate prognosis types (50–60% 10-year survival), includes classical lobular, medullary, atypical medullary and lobular mixed carcinoma.
-
4.
Poor prognosis types (≤50% 10-year survival), includes ductal NST, solid lobular, mixed ductal and lobular carcinoma.
Among 510 tumours, 28 (5%) were group 1, 111 (22%) were group 2, 58 (11%) group 3 and 313 (62%) were classified as group 4 or poor prognostic type.
Of 510 tumours, 106 (21%) were grade 1, 173 (34%) were grade 2 and 231 (45%) were histological grade 3. Among 506 patients whose axillary lymph nodes had been examined; 321 (63%) were node negative and 185 (37%) had axillary node metastatic disease.
Immunohistochemistry
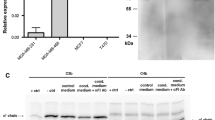
The rabbit polyconal anti-CD46 antibody was raised against affinity-purified CD46 [33]. Following production, the serum was affinity-purified on CD46 and validated using competition ICC with CD46 antigen (data not shown). The polyclonal antibody has also compared to a monoclonal anti-CD46 antibody (E4.3) by indirect immunofluorescence on a CD46-expressing cell line (FACS profile is shown in Fig. 1).
Sections 4-μm thick were cut and stained using the standard avidin-biotin complex method. Briefly, deparaffinized sections were immersed in methanol containing 0.3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Microwave pretreatment in citrate buffer (pH 6.0) was performed 10 min at high power followed by 10 min at low power to retrieve antigenicity. The sections were then incubated with normal swine serum (NSS) for 10 min to block nonspecific antibody binding sites. After that, primary antibody was incubated on the slides for 1 h with the optimal dilution found to be 1:200. Primary antibody was omitted from the negative control, which was left incubating in NSS. Normal breast tissue adjacent to tumour was used as a positive control. The sections were then incubated in biotinylated goat antimouse/rabbit IgG (Dako, Denmark) for 30 min followed by streptavidin-biotinylated horseradish peroxidase complex (Dako, Denmark) for 1 h at room temperature with the addition of 3,3′-diaminobenzidine with 0.03% hydrogen peroxidase (Dako, Denmark) to achieve visualisation of the antigen. The sections were lightly counterstained with haematoxylin (Dako, Denmark), dehydrated in alcohol, cleared in xylene xylene (Genta Medica, York, UK) and mounted with distyrene, plasticiser and xylene (DPX) (BDH, Poole, England).
Scoring system
Immunostaining of CD46 was evaluated using a semiquantitative system by one author (Z. Madjd), blinded to patient outcome and other clinical findings. Additional cases were assessed by two observers (Z. Madjd, S.E. Pinder) to confirm agreement. The immunostaining results were classified into four categories: 0 = no immunostaining present, 1 = weak immunostaining, 2 = moderate immunostaining and 3 = strong immunostaining. The percentage of tumour cells showing positivity was also assessed semiquantitatively as 1 (<25%), 2 (25–50%), 3 (51–75%) or 4 (>75%).
Statistical analysis
SPSS version 10.1 for Windows was used for statistical analysis. Univariate associations of CD46 intensity with clinicopathological parameters of breast cancer were analysed by cross-tabulation, and Pearson χ2 tests. To give sizes of effect and to look at the independence of effects, CD46 intensity was reclassified as a binary outcome (low [0 or 1] and high [2 or 3] expression), and effects of clinicopathological parameters were assessed using multiple logistic regression to give adjusted odds ratios and 95% confidence intervals. The effect of CD46 expression upon survival was analysed by Kaplan–Meier, and statistical significance by the log-rank test. p values lower than 0.05 were identified as statistically significant.
Results
Previous studies on frozen breast sections had suggested that expression of CD46 was variable and may correlate with tumour prognosis. The Nottingham series is formed from a large number of well-defined breast cancer patients with long follow-up. To complete our analysis of complement control proteins in these breast arrays, a CD46 polyclonal serum was derived to overcome the lack of commercially available antisera that work on formalin-fixed paraffin-embedded material. The antisera was used on a panel of 510 breast arrays, and expression levels compared against prognostic factors and patient outcomes.
Immunohistochemical expression of CD46
Immunostaining was predominantly observed in the tumour cell membrane and heterogeneously within the cytoplasm (Fig. 2). Normal epithelium of ducts and lobules adjacent to tumour lesions consistently and strongly expressed CD46. In this series of breast carcinomas, CD46 expression was identified in 507 of the 510 tumours investigated (99.4%). One hundred and thirty-six of the 510 (27%) tumours exhibited strong immunoreactivity, while moderate and weak staining was observed in 43% and 29% of tumours, respectively (Table 1, Fig. 2). A variable percentage of CD46-positive cells was observed; 58% (n=298) of cases showed extensive expression of CD46 (>75% positive cells), whereas 6% (n=30) showed CD46 immunoreactivity in less than 25% of tumour cells (Table 2).
Comparison with existing prognostic factors
The level of expression of CD46 in the tumour samples was compared with existing prognostic parameters (histological grade, tumour type, vascular invasion, lymph node stage, tumour size, ER status and NPI), patient characteristics (age and menopausal status) and outcome (overall survival, development of distant metastases, local and regional recurrence) (Table 3).
The intensity of CD46 expression (categorised in four groups: no staining, weak, moderate and strong staining) was negatively associated with histological grade of invasive tumour (p=0.003) (Table 4, Fig. 3), such that the odds ratios for high intensity of CD46 in those with a poor histological grade compared with those with a well-differentiated tumour was 0.59 (95% CI, 0.35–1.0) (Table 5). Moreover, an inverse association was demonstrated between CD46 intensity and histological tumour type group (p<0.001); strong staining of CD46 was less often seen in poor prognosis types (ductal/NST, solid lobular, lobular mixed, mixed NST and lobular types) compared with excellent prognosis type tumours (tubulo-lobular, tubular, mucinous and invasive cribriform types) (Table 4), with an odds ratio of 0.56 (0.22–1.45) (Table 5).
We also observed a positive association between CD46 intensity and patient’s age (p=0.018); higher expression of CD46 (moderate vs strong intensity) was more often seen in breast tumours of older patients (>50 years, Table 4), with an odds ratio of 3.57 (1.70–7.49) (Table 5).
No correlations were found between intensity of CD46 expression and other prognostic factors including the presence or absence of vascular invasion, lymph node stage, NPI, ER status and follow-up events including overall survival (p=0.602), or the development of distant metastasis (Table 3). Similarly no correlation was found between the percentage of cells showing immunoreactivity and prognostic factors (Table 3).
In multiple logistic regression, there were independent effects of age with adjusted odds ratios of 1.31 (1.06–1.62) (data not shown).
Discussion
Small early tumours may escape immune recognition; however, there is increasing evidence that as they grow and develop, tumours can stimulate innate, humoral and cellular immunity. Thus tumours that survive and progress evoke a wide variety of measures to protect themselves from immune attack. One method is to overexpress complement inhibitory proteins (CIPs) that prevent C3b deposition and complement lysis [21]. We have previously shown that breast tumours that lose either CD55 or CD59 have a significantly poor prognosis when compared with tumours expressing these CIPs [18, 19]. This is counterintuitive as tumour cells lacking CIPs should be more susceptible to complement attack. However, several studies have shown that either CD55 or CD46 alone can inhibit both C3b deposition and complement-mediated lysis. The present study therefore looked for expression of CD46 on the same series of 510 breast tumours that had previously been examined for CD55 and CD59 expression.
Most of the tumours expressed CD46 (507/510) with 70% showing strong to moderate expression. This is in accordance with early studies performed on small samples of frozen breast sections [11, 22, 35] but is in contrast to renal and lung cancers which frequently lose expression of CD46 but retain expression of CD55 [22]. The results presented here suggest that in breast cancer, expression of CD46 alone is sufficient to protect from complement-mediated attack but that loss of CD55 and CD59 may be associated with other roles in immune regulation.
Overexpression of CD55 on breast tumours may be due to selective pressure from complement activation within the tumour environment. Surviving cells may express higher levels of CD55. It may be predicted that these tumours would be more aggressive as they should be resistant to complement attack. However, our previous study showed that these breast tumours were less aggressive [19]. CD55 also interacts with CD97, an early activation antigen on T cells [9], and may have a role in T-cell regulation. During tumour immunosurveillance, tumours may initially overexpress CD55 to protect from complement lysis, but this may make them more susceptible to cellular attack. Loss of CD55 would then become a selective advantage. Our previous studies have also shown that loss of CD59 is also associated with aggressive tumours. CD59 has also been shown to bind to CD2, a costimulatory molecule that reduces the threshold for T-cell activation and release of effector function [3, 8]. Although later studies failed to show a strong binding association between CD59 and CD2, this does not disprove a lower affinity interaction [38]. Furthermore, monoclonal antibodies to CD59 have been shown to initiate signal transduction [14, 23, 34]. Thus tumours that lose expression of CD55 and CD59 but retain expression of CD46 may be less susceptible to cellular attack but still be resistant to attack by complement.
The intensity of CD46 staining negatively correlated with tumour grade, histological type, tumour size and tumour recurrence. However there was no overall correlation with patient survival. These results suggest that tumours with aggressive pathology lose expression of CD46 and may allow escape from complement control, leading to enhanced recurrence, although this was not reflected in reduced survival suggesting that other factors may have a more profound influence. Similar results were obtained when breast tumours were analysed for CD46 mRNA expression [28]. In contrast, a previous study suggested that increased CD46 expression was associated with increased tumour grade; however, this was a small study (38 samples) predominantly on ductal carcinomas [11]. In the present study we found that ductal carcinomas stained less intensely than other histological types and were therefore not representative of the larger breast tumour group.
In summary, invasive breast carcinomas appear to preferentially retain CD46 expression to protect them from both C3b deposition and complement-mediated lysis. Whereas loss of CD55 and CD59 appears to confer poor prognosis. This may be related to other emerging roles that CIPs appear to have in immune regulation via their interaction with ligands other than complement.
References
Bubendorf L, Nocito A, Moch H, Sauter G (2001) Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol 195:72–79
Cole JL, Housley GA Jr, Dykman TR, MacDermott RP, Atkinson JP (1985) Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A 82:859–863
Deckert M, Kubar J, Bernard A (1992) CD58 and CD59 molecules exhibit potentializing effects in T cell adhesion and activation. J Immunol 148:672–677
Devaux P, Christiansen D, Fontaine M, Gerlier D (1999) Control of C3b and C5b deposition by CD46 (membrane cofactor protein) after alternative but not classical complement activation. Eur J Immunol 29:815–822
Ellis IO, Galea M, Broughton N, Locker A, Blamey RW, Elston CW (1992) Pathological prognostic factors in breast cancer, II: histological type—relationship with survival in a large study with long-term follow-up. Histopathology 20:479–489
Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer, I: the value of histological grade in breast cancer—experience from a large study with long-term follow-up. Histopathology 19:403–410
Galea MH, Blamey RW, Elston CE, Ellis IO (1992) The Nottingham Prognostic Index in primary breast cancer. Breast Cancer Res Treat 22:207–219
Hahn WC, Menu E, Bothwell AL, Sims PJ, Bierer BE (1992) Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science 256:1805–1807
Hamann J, Vogel B, van Schijndel GM, van Lier RA (1996) The seven-span transmembrane receptor CD97 has a cellular ligand (CD55, DAF). J Exp Med 184:1185–1189
Haybittle JL, Blamey RW, Elston CW, Johnson J, Doyle PJ, Campbell FC, Nicholson RI, Griffiths K (1982) A prognostic index in primary breast cancer. Br J Cancer 45:361–366
Hofman P, Hsi BL, Manie S, Fenichel P, Thyss A, Rossi B (1994) High expression of the antigen recognized by the monoclonal antibody GB24 on human breast carcinomas: a preventive mechanism of malignant tumor cells against complement attack? Breast Cancer Res Treat 32:213–219
Kojima A, Iwata K, Seya T, Matsumoto M, Ariga H, Atkinson JP, Nagasawa S (1993) Membrane cofactor protein (CD46) protects cells predominantly from alternative complement pathway-mediated C3-fragment deposition and cytolysis. J Immunol 151:1519–1527
Kononen J, Bubendorf L, Kallioniemi A, Barlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP (1998) Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 4:844–847
Korty PE, Brando C, Shevach EM (1991) CD59 functions as a signal-transducing molecule for human T cell activation. J Immunol 146:4092–4098
Li L, Spendlove I, Morgan J, Durrant LG (2001) CD55 is over-expressed in the tumour environment. Br J Cancer 84:80–86
Liszewski MK, Post TW, Atkinson JP (1991) Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol 9:431–455
Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP (1996) Control of the complement system. Adv Immunol 61:201–283
Madjd Z, Pinder SE, Paish C, Ellis IO, Carmichael J, Durrant LG (2003) Loss of CD59 expression in breast tumours correlates with poor survival. J Pathol 200:633–639
Madjd Z, Durrant LG, Bradley R, Spendlove I, Ellis IO, Pinder SE (2004) Loss of CD55 is associated with aggressive breast tumors. Clin Cancer Res 10:2797–2803
McNearney T, Ballard L, Seya T, Atkinson JP (1989) Membrane cofactor protein of complement is present on human fibroblast, epithelial, and endothelial cells. J Clin Invest 84:538–545
Morgan BP, Harris CL (1999) Complement regulatory proteins. Academic Press, San Diego
Niehans GA, Cherwitz DL, Staley NA, Knapp DJ, Dalmasso AP (1996) Human carcinomas variably express the complement inhibitory proteins CD46 (membrane cofactor protein), CD55 (decay-accelerating factor), and CD59 (protectin). Am J Pathol 149:129–142
Okada H, Nagami Y, Takahashi K, Okada N, Hideshima T, Takizawa H, Kondo J (1989) 20 KDa homologous restriction factor of complement resembles T cell activating protein. Biochem Biophys Res Commun 162:1553–1559
Pereira H, Pinder SE, Sibbering DM, Galea MH, Elston CW, Blamey RW, Robertson JF, Ellis IO (1995) Pathological prognostic factors in breast cancer, IV: should you be a typer or a grader? A comparative study of two histological prognostic features in operable breast carcinoma. Histopathology 27:219–226
Pinder SE, Ellis IO, Galea M, O’Rouke S, Blamey RW, Elston CW (1994) Pathological prognostic factors in breast cancer, III: vascular invasion—relationship with recurrence and survival in a large study with long-term follow-up. Histopathology 24:41–47
Post TW, Liszewski MK, Adams EM, Tedja I, Miller EA, Atkinson JP (1991) Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J Exp Med 174:93–102
Purcell DF, McKenzie IF, Lublin DM, Johnson PM, Atkinson JP, Oglesby TJ, Deacon NJ (1990) The human cell-surface glycoproteins HuLy-m5, membrane co-factor protein (MCP) of the complement system, and trophoblast leucocyte-common (TLX) antigen, are CD46. Immunology 70:155–161
Rushmere NK, Knowlden JM, Gee JM, Harper ME, Robertson JF, Morgan BP, Nicholson RI (2004) Analysis of the level of mRNA expression of the membrane regulators of complement, CD59, CD55 and CD46, in breast cancer. Int J Cancer 108:930–936
Russell SM, Sparrow RL, McKenzie IF, Purcell DF (1992) Tissue-specific and allelic expression of the complement regulator CD46 is controlled by alternative splicing. Eur J Immunol 22:1513–1518
Seya T, Turner JR, Atkinson JP (1986) Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med 163:837–855
Seya T, Ballard LL, Bora NS, Kumar V, Cui W, Atkinson JP (1988) Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol 18:1289–1294
Seya T, Hara T, Matsumoto M, Akedo H (1990) Quantitative analysis of membrane cofactor protein (MCP) of complement: high expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J Immunol 145:238–245
Singhrao SK, Neal JW, Rushmere NK, Morgan BP, Gasque P (1999) Differential expression of individual complement regulators in the brain and choroid plexus. Lab Invest 79:1247–1259
Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H (1991) GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254:1016–1019
Thorsteinsson L, O’Dowd GM, Harrington PM, Johnson PM (1998) The complement regulatory proteins CD46 and CD59, but not CD55, are highly expressed by glandular epithelium of human breast and colorectal tumour tissues. Apmis 106:869–878
Todd JH, Dowle C, Williams MR, Elston CW, Ellis IO, Hinton CP, Blamey RW, Haybittle JL (1987) Confirmation of a prognostic index in primary breast cancer. Br J Cancer 56:489–492
Torhorst J, Bucher C, Kononen J, Haas P, Zuber M, Kochli OR, Mross F, Dieterich H, Moch H, Mihatsch M, Kallioniemi OP, Sauter G (2001) Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 159:2249–2256
van der Merwe PA, Barclay AN, Mason DW, Davies EA, Morgan BP, Tone M, Krishnam AK, Ianelli C, Davis SJ (1994) Human cell-adhesion molecule CD2 binds CD58 (LFA-3) with a very low affinity and an extremely fast dissociation rate but does not bind CD48 or CD59. Biochemistry 33:10149–10160
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madjd, Z., Durrant, L.G., Pinder, S.E. et al. Do poor-prognosis breast tumours express membrane cofactor proteins (CD46)?. Cancer Immunol Immunother 54, 149–156 (2005). https://doi.org/10.1007/s00262-004-0590-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-004-0590-0