Abstract
Purpose
Cytotoxic T lymphocytes (CTL)- and T-helper cell-specific, and major histocompatibility complex (MHC) class-I and class-II peptides, respectively, of the HER-2/neu protein, induce immune responses in patients. A major challenge in developing cancer peptide vaccines is breaking tolerance to tumor-associated antigens which are functionally self-proteins. An adequate CD4+ T-helper response is required for effective and lasting responses.
Methods
Stimulating anti-cancer CD4+ T cell responses by MHC class-II epitope peptides has been limited by their weak potency, at least compared with tight-binding MHC class-I epitope peptides. Previously, a potent T-cell response to a MHC class-II epitope was engineered by coupling the N-terminus of the pigeon cytochrome C [PGCC(95–104)] MHC class-II epitope to the C-terminus of an immunoregulatory segment of the Ii protein (hIi77–81, the Ii-Key peptide) through a polymethylene spacer.
Results
In vitro presentation of the MHC class-II epitope to a T hybridoma was enhanced greatly (>250 times). Now, an Ii-Key/HER-2/neu (777–789) MHC class-II epitope hybrid peptide stimulated lymphocytes from both a healthy donor and a patient with metastatic breast carcinoma. The in vitro primary stimulation with the hybrid peptide strongly activated IFN-γ release, whereas the epitope-only peptide was weakly active. In fact, the hybrid stimulated IFN-γ release as well as the wild-type peptide when augmented with IL-12; however, the hybrid was comparable to free peptide in stimulating IL-4 release. This pattern is consistent with preferential activation along a non-tolerogenic Th1 pathway.
Conclusion
Such Ii-Key/MHC class-II epitope hybrid peptides have both diagnostic and therapeutic applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
HER-2/neu tumor-associated antigen
HER-2/neu is a good immunotherapeutic target in patients with early breast cancer. Over-expression of HER-2/neu in tumors of approximately 32% of patients with invasive breast cancer predicts poor clinical outcome in early stage node-negative and node-positive disease and in women with metastatic disease [6, 9, 37]. The immunohistochemical pattern and level of HER-2/neu over-expression are comparable in primary and metastatic lesions [30]. Because micrometastases are a primary source of relapse following initial therapies, and HER-2/neu is over-expressed in metastases, HER-2/neu immunotherapies target consolidation of response locally and eradication of micrometastases. Anti-HER-2/neu immunotherapies can augment chemotherapy regimens for relapsing patients [37].
HER-2/neu: a passive immunotherapeutic target
Herceptin (trastuzumab) is a monoclonal antibody that is in patients with metastatic breast cancer receiving chemotherapy: significantly increases time to disease progression (7.4 vs 4.6 mo, p<0.0001), a higher rate of objective response (50 vs 32%, p<0.001), a longer duration of response (median 9.1 vs 6.1, p<0.001), a higher 1-year survival rate (78 vs 67%, p=0.008), longer survival (median survival 25.1 vs 20.3 mo, p=0.046), and a 20% reduction in the risk of death [37]. Trastuzumab disables the HER-2/neu EGF receptor and mediates antibody-dependent cellular cytotoxicity (ADCC) [34]. The ADCC is not linked to induction of antigen-specific memory in T or B lymphocytes, or proliferation of antigen-specific cytotoxic T cells. Reilly et al. demonstrated in neu transgenic mice expressing the nontransforming rat proto-oncogene, that both humoral and cellular responses against HER-2/neu are required to completely eradicate HER-2/neu-positive tumors [35]. Cure is not likely to be achieved with trastuzumab alone since this therapy does not exploit the full range of immune responses against HER-2/neu as an immunogenic target in the treatment of breast cancer.
HER-2/neu: an active immunotherapeutic target
Tumors over-expressing HER-2/neu are immunogenic, since 11% of patients with advanced stage breast and ovarian cancers, 16% of patients with androgen-independent prostate cancer, and 14% of patients with metastatic colorectal cancer exhibited antigen-specific in vitro immune responses to HER-2/neu protein and peptides [13, 14, 31, 40]. This fact has led to various active immunotherapies against HER-2/neu. Vaccines against HER-2/neu require methods to overcome tolerance to self-proteins [37]. Autologous T cells appear to recognize and become tolerant to dominant epitopes of self-proteins, but they “ignore” subdominant epitopes [8, 10, 16, 26, 32]. Vaccination with a collection of putative T-cell epitope peptides from the intracellular domain of rat neu (15–18 amino acids in length) elicited peptide- and protein-specific immune responses in rats; however, vaccination with whole protein did not [12]. On the basis of these findings, clinical trials of a variety of major histocompatibility complex (MHC) class-I and MHC class-II epitopes were conducted [24]. The epitopes used in such clinical trials are typically selected for eliciting strong immune responses in in vitro stimulation assays with lymphocytes from the peripheral blood of healthy donors and patients with HER-2/neu over-expressing malignancies, as well as lymphocytes from metastatic lymph nodes of patients with HER-2/neu over-expressing malignancies.
One well-characterized vaccine epitope is HER-2/neu(776–790), an HLA-DR4-restricted epitope of the intracellular domain of HER-2/neu. The epitope is promiscuous, presented at least by DRB1*0405, DRB5*0101, and DRB1*0701, thereby offering a broad population coverage [38]. T cells of 10 of 18 patients with metastatic breast cancer recognized HER-2(777–789) in cultures with a Th1 pattern of cytokine release (relatively higher levels of interferon-γ than IL-4 or IL-10) [27]. Furthermore, in vitro stimulation of peripheral blood mononuclear cells (PBMC) with HER-2(776–789) expanded the immune response to another epitope HER-2/neu (884–899) in both an ovarian cancer patient with progressive disease and a healthy donor, both of whom shared HLA-DR11 [3]. In a study of putative MHC class-II epitope HER-2 peptides, four epitopes, including HER-2(777–789), stimulated PBMC from healthy donors as well as 25 patients with ovarian cancer [18]. The PBMC primed with HER-2(777–789) survived longer and had augmented antigen-specific cytotoxic T lymphocytes (CTL) lysis following stimulation with the HLA-A2-restricted peptide HER-2(369–377) than PBMC not first primed with HER-2(777–789) prior to stimulation with HER-2(369–377) [28]. In peptide vaccination trials conducted with MHC class-II epitopes, including HER-2(776–790), the majority of patients receiving all six immunizations developed HER-2/neu peptide and protein-specific T-cell responses with determinant spreading [15].
A clinically acceptable and potent adjuvant should enable Th1 responses that can overcome tolerance. Intradermal GM-CSF compared favorably with either Freund’s complete adjuvant or alum in eliciting strong humoral and cellular immunity. Rat neu peptides inoculated with GM-CSF elicit in rats a strong delayed-type hypersensitivity reaction (DTH), whereas peptides alone were non-immunogenic [11], and GM-CSF potentiates intracutaneous injections clinically [5].
Clearly, strategies to augment antigen presentation are important in the design of clinically effective peptide vaccine approaches. In order to assess the feasibility of clinical trials with Ii-Key/HER-2(MHC II epitopes), we evaluated T-cell proliferation and cytokine release to HER-2(777–789) peptide and Ii-Key/HER-2(777–789) hybrid peptide in PBMC and lymph nodes of patients with metastatic breast cancer.
Creating a potent MHC class-II vaccine peptide
Coupling the Ii-Key segment of the immunoregulatory Ii protein to an MHC class-II epitope substantially increases the potency of presentation of the epitope. The Ii-Key peptide from the Ii protein acts at an allosteric site on MHC class-II molecules to catalyze binding or release of antigenic peptides from the antigenic peptide binding site in vitro [1]. This Ii-Key peptide-enhanced presentation of MHC class-II epitope peptides from pigeon cytochrome C and hen egg lysozyme by I-E molecules on living or fixed APC lines to respective T hybridomas [2]. Similarly Ii-Key peptides catalyze release, exchange, and binding of a human myelin basic protein peptide to purified exomembranal HLA-DR1 molecules [41]. Nested deletions from the C-terminus revealed maximal activity in a 7-amino-acid-core LRMKLPK, with half-maximal activity retained in LRMK [2].
Creation of an Ii-Key/MHC class-II antigenic epitope hybrid by coupling LRMK to the pigeon cytochrome C (PGCC) epitope potentiated presentation of the tethered antigenic epitope more than 250 times, relative to the antigenic epitope [19]. Both the length of the Ii-Key derivative and linker composition was varied within the series. Homologs were tested, each coupled to the PGCC epitope, with LRMK, LRMKLPK, LRMKLPKPVS and LRMK-ava, and LRMK-ava-ava (where ava is δ-amino-valeric acid, or 5-aminopentanoic acid). Activity was measured by their ability to induce cellular proliferation and interleukin release in the murine T-cell hybridoma cell line Tpc9.1, which is specific for the PGCC peptide antigenic epitope. While all of the Ii-Key/PGCC antigenic epitope hybrids were considerably more potent than the antigenic epitope peptide, a peptide of simplest chemical structure LRMK-ava- PGCC(95–104) antigenic epitope was the most potent. Since synthetic hybrids containing linkers of either the natural sequence of Ii-protein extending from the LRMK motif or 5-aminopentanoic acid were active, no specific side-chain interactions were required between the linker and the alpha helices of the antigen-binding site; thus, the specific amino acids forming a spacer region appeared not to be relevant. Spacing by one ava residue might be more potent than two ava residues, because binding of the MHC class-II epitope in the antigenic peptide binding trough pulls the LRMK motif out of the allosteric site. We now apply this technology to evaluate Ii-Key/antigenic epitope hybrids to enhance MHC class-II epitope peptide vaccines against HER-2/neu.
Materials and methods
Patients
The University of Texas M.D. Anderson Cancer Center Institutional Review Board approved the study, and participants in the study gave informed consent to participate. The PBMC donor was a healthy male with no known HER-2/neu over-expressing tumors. The patient was a woman who presented with stage-IIb (T3N2M0) breast cancer who received pre-operative chemotherapy. At surgery, lymph nodes were positive and HER-2/neu over-expression was documented.
Immunohistochemistry for HER-2/neu status
The HER-2/neu status of the primary tumor was determined by immunohistochemical staining of 4-µm-thick sections cut from a representative paraffin-embedded block of invasive carcinoma. The slides were incubated with the anti-HER-2/neu mAb e24001 (1:100 dilution) on a Dako autostainer (Dako Corp., Carpinteria, Calif.) with the LSAB-2 peroxidase kit (Dako) using 3–3 diaminobenzedine. The percentage of cells with complete membrane staining and the intensity of the staining were evaluated on a semiquantitative scale of 0–3+, with scores defined as follows: 3+, strong complete membrane staining in more than 10% of tumor cells; 2+, weak to moderate complete membrane staining in more than 10% of tumor cells; and 1+, faint or barely perceptible membrane staining in more than 10% of tumor cells. In all cases exhibiting any positivity (1+ to 3+), immunoreactivity was confirmed by performing in situ hybridization analysis to determine the HER-2/neu gene copy number. The Path Vysion HER-2/neu kit (Vysis, Downers Grove, Ill.) employed two directly labeled fluorescent DNA probes; one was specific for the HER-2/neu gene locus, and the second is specific for the alpha satellite DNA sequence at the centrimeric region of chromosome 17. Signals were counted for 40 tumor cells by using an epiflorescence microscope, and the ratio of HER-2/neu to chromosome 17 was calculated. A ratio >2.0 was considered to represent HER-2/neu gene amplification and was considered to be a positive test result.
HER-2/neu peptides
The HER-2/neu peptides were prepared by Commonwealth Biotechnology, Inc. (Richmond, Va.). Peptides were assayed for amino acid composition, HPLC homogeneity and mass spectrometry weight, and dissolved in phosphate-buffered saline (PBS), aliquoted at 2 mg/ml, and stored frozen at −20°C until used.
Isolation, stimulation, and propagation of lymphocytes from PBMC
Peripheral blood mononuclear cells (PBMC) cultures were assayed for the production of cytokines and for cellular proliferation, either after primary stimulation or after re-stimulation in the presence or absence of IL-12. The PBMC were suspended in supplemented RPMI-1640 medium at 6×106cells/ml. Then, 50-μl aliquots of that suspension were added to each assay well of a 96-well, flat-bottomed microtiter plate, yielding 3×105 cells/well. Peptides were dissolved at a concentration of 100 μg/ml in supplemented RPMI-1640 medium. Then, 50 μl of this solution was added to respective wells yielding a final peptide concentration of 25 μg/ml. Next, 50 μl of a 1:5 dilution of the stock solution of peptides was added to the other wells yielding a final peptide concentration of 5 μg/ml. Then, 100 μl of a 4-IU/ml solution of IL-12 was added to the wells to be brought to a final concentration of 2 IU IL-12/ml. For the wells without IL-12, 100-μl aliquots were aspirated and replaced with RPMI-1640 medium with 10% FCS and 150 IU/ml IL-2 to bring the wells to a final concentration of 75 IU/ml. After the cultures were incubated for 3 days at 37°C in a 5% CO2 atmosphere, [3H]thymidine (2 μCi/well) was added. The cells were incubated an additional 16 h and harvested. For the assay of released cytokines, additional cultures were set up receiving 100 μl/RMPI medium with 10% FCS instead of 100 μl of the IL-2 stock solution. After 3 days, supernatants from those cultures were collected and assayed for cytokines. Cytokines were detected with ELISA kits for INF-γ and IL-4, according to the manufacturer’s instructions (Biosource, Camarillo, Calif.). For the cell proliferation assay, each culture was run in duplicate. For the cytokine assay each culture was run in duplicate and each culture supernatant was assayed in duplicate as well.
Isolation, stimulation, and propagation of lymphocytes from lymph nodes
Standard axillary dissection was performed, and the axillary contents were immediately examined grossly by a pathologist under a sterile hood located just outside the operating rooms. Lymph nodes were bivalved and microscopically examined for the presence of breast cancer axillary metastases using frozen section or touch preparation techniques, or both. Half of each lymph node was submitted for permanent section examination to confirm the presence or absence of metastases.
The other half of each lymph node was immediately mechanically dispersed and then plated at a concentration of 20×105 cells per well with RPMI 1640 medium (Life Technologies, Grand Island, N.Y.) with 5% human serum and 40 µg/ml gentamycin. When no proliferation was evident on peptide stimulation, interleukin-12 (IL-12; PharMingen, San Diego, Calif.) was added as a co-stimulator at a concentration of 200 pg/ml. IL-12 has demonstrated significant antitumor activity in several preclinical tumor models [7, 36]; therefore, it was of interest to investigate the effects if IL-12 in the current breast cancer model. For re-stimulation experiments, after 4 days of stimulation with HER-2/neu peptides, the cultures were expanded with IL-2 (Cetus Corporation, Emeryville, Calif.) at 20 U/ml for 1 week. The cultures were maintained for 3 days in the absence of IL-2 to induce antigen-specific T cells. The non-adherent cells were then stimulated with irradiated (10 Gy) adherent cells that were pulsed with HER-2/neu peptides at 50 µg/ml for 90 min or with PBS alone [18]. Cultures were incubated for 72 h in a humidified incubator at 37°C in 5% CO2, and cytokine production was measured.
Proliferation assay
For cell proliferation assays, 20×105 lymphocytes isolated from diseased lymph nodes and PBMC were cultured in triplicate in 96-well plates in 200 µl as follows: (a) without peptide (NP: no peptide); (b) with each peptide at 5 and 25 μg/ml; and (c) with phytohemagglutinin (PHA) at 10 μg/ml (GIBCO Life Technologies, Grand Island, N.Y.) for 96 h [39]. During the final 16 h of culture, 1 µCi tritiated thymidine (3H-TdR) was added to measure proliferation. The cells were then harvested, and the radioactivity was measured in a Beckman LS3501 liquid scintillation counter (Beckman Coulter, Fullerton, Calif.) [21].
Cytokine production
For cytokine production assays, lymphocytes isolated from axillary lymph nodes and PBMC were cultured either without or with peptides. Supernatants were collected after 72 h and stored frozen at −20°C until assayed for cytokine levels. The cytokines interferon-γ (IFN-γ) and IL-4 were measured by double-sandwich, enzyme-linked immunosorbent assay (ELISA) using the corresponding kits (Biosource International, Camarillo, Calif.).
Statistical analysis
Values obtained for 3H-TdR incorporation by the lymph node lymphocytes incubated with PBS, PHA, or HER-2 peptides or without peptide were compared using the Student’s t test, and differences between groups were considered significant when p<0.05.
Results
Analysis of healthy donor PBMC
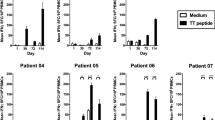
In vitro IFN-γ release studies following primary stimulation of PBMC from the healthy donor indicated greater IFN-γ release in the absence of IL-12 co-stimulation for Ii-Key/HER-2(777–789) as compared with HER-2(777–789): 968 and 308 pg/ml, respectively, at 72 h (Fig. 1). The level of IFN-γ release with Ii-Key/HER-2(777–789) was similar to the PHA-positive control: 968 and 974 pg/ml, respectively. In the setting of IL-12 co-stimulation, the peptides induced similar levels of IFN-γ release, which were virtually identical to PHA-positive control. On secondary stimulation (where IL-12 was used in the primary stimulation of all PBMC), there was virtually no difference in IFN-γ release at 72 h between Ii-Key/HER-2(777–789) and HER-2(777–789): 1401 and 1350 pg/ml (data not shown).
Interferon-γ release from peripheral blood mononuclear cells (PBMC). Lymphocytes derived from healthy-donor PBMC were cultured either without peptide (NP no peptide) or with indicated peptides (25 μg/ml). Experiments were performed without IL-12 co-stimulation (black bars) and with IL-12 co-stimulation (hatched bars) using 200 pg/ml. Supernatants were collected after 72 h and stored frozen at −20°C until assayed for cytokine levels. Interferon-γ was measured by double-sandwich, enzyme-linked immunosorbent assay (Biosource International, Camarillo, Calif.). PHA: phytohemagglutinin
Although cellular proliferation studies on primary stimulation revealed generally greater responses to Ii-Key/HER-2(777–789) than HER-2(777–789) (Table 1), the differences were not statistically significant. The PHA-positive control provided statistically greater proliferative responses than either Ii-Key/HER-2(777–789) or HER-2(777–789) peptides. On secondary stimulation, there were no statistically significant differences between the Ii-Key/HER-2(777–789; 3984 cpm) and HER-2(777–789) peptides (3428 cpm) at 25 μg/ml (p=0.33; data not shown).
Analysis of lymph node specimens
Following primary stimulation, in vitro cytokine release studies on lymph node specimens from the patient with metastatic disease indicated greater IFN-γ release in the absence of IL-12 co-stimulation for Ii-Key/HER-2(777–789) as compared with HER-2(777–789): 24.9 vs 2.3 pg/ml, respectively, at 72 h (Table 2). IFN-γ release was increased significantly without a concomitant induction of IL-4. In the presence of IL-12 co-stimulation, similar levels of IFN-γ were induced by Ii-Key/HER-2(777–789) and HER-2(777–789) (Table 2). Cellular proliferation studies revealed 4462 cpm and 3750 cpm for Ii-Key/HER-2(777–789) and HER-2(777–789) peptides, respectively (p=0.1558, two-sided t test), following secondary stimulation.
Discussion
Previously, we vaccinated patients with the immunodominant HLA-A2-restricted epitope HER-2(369–377) peptide, which induced cytolysis of the ovarian cancer cell line SKOV.3 following in vitro stimulation of PBMC from 5 of 10 healthy donors [4, 17, 36]. In a phase-I trial, 5 of 9 patients with advanced breast and ovarian cancers vaccinated with HER-2(369–377) peptide plus GM-CSF had significantly increased proliferation of peripheral blood lymphocytes with ex vivo exposure to peptide as compared with NP. In 4 of 8 patients tested IFN-γ production and in vitro CTL activity against peptide-pulsed targets and HER2+, HLA-A2+ tumor cell lines were significantly augmented by adding IL-12 [33]. In another study HER-2(369–377) vaccination in Freund’s incomplete adjuvant induced IFN-γ production when peripheral blood CD8+ cells were incubated with peptide; however HER-2+, HLA-A2+ tumor cells were not lysed [42]. In a clinical study of HER-2(369-377) vaccination with GM-CSF in patients with HER-2/neu over-expressing tumors, 2 of 4 patients developed increased HER-2/neu peptide-specific responses, which were short-lived and not detected 5 months after the final inoculation [25].
Knutson et al. also conducted a clinical trial of a mixture of three HER-2/neu helper epitopes HER-2(369–384), HER-2(688–703), and HER-2(971–984), within each of which is an HLA-A2 binding motif [24]. After vaccination, the mean HLA-A2 peptide-specific T-cell frequency increased in the majority of patients, the peptide-specific T cells lysed tumors, and the responses were long-lived (detectable for more than 1 year after final vaccination). Of 21 HER-2(369–377)-responding clones from 1 patient vaccinated with the peptides HER-2(369–384), HER-2(688–703), and HER-2(971–984), 19/21, 16/21, and 5/21 clones were, respectively, CD3+, CD8+/CD4−, and CD4+/CD8−, despite being generated with an HLA-A2 motif peptide [23].
Responses to MHC class-I epitopes are enhanced by stimulating CD4+ T-helper cells. The CD4+ T-helper cell response also extends the duration of lytic CD8+ cell responses and promotes accumulation of antigen-presenting cells at the tumor site [20]. The MHC class-II peptides with included MHC-I epitopes provide enhanced and diverse immune responses compared with MHC class-I epitope-only peptides. We evaluated HER-2 (777–789) MHC class-II epitope plus CTL epitope HER-2(369–377) in vitro and found that HER-2(777–789) enhanced expansion and antigen-specific cytolysis upon priming with HER-2(369–377), but inhibited survival of HER-2(369–377) specific CTLs upon re-stimulation [28]. Augmented helper T-cell responses derived from more potent antigen presentation of the MHC class-II epitope may modulate the HER-2(369–377) response observed on re-stimulation. The magnitude of the IFN-γ responses seen with the fresh PBMCs are significantly greater than those seen with lymphocytes isolated from the lymph nodes of the patient with breast cancer. This difference might reflect a relative immune inhibitory tendency in breast patient than in healthy people. More samples are needed to confirm or rule out this possibility.
In this preliminary study, we set out to determine whether the Ii-Key moiety modulates the response of a well-characterized, promiscuous MHC class-II epitope, HER-2/neu(777–789), in human systems. We examined whether the augmented antigen presentation properties of Ii-Key, previously demonstrated in pre-clinical models, provides enhancement of immune function of human lymphocytes from PBMC. Since HER-2/neu is expressed in a variety of normal tissues and other investigators have used PBMC from normal donors to evaluate the immunogenicity of peptide vaccines in overcoming tolerance for the treatment of melanoma, our approach in this preliminary study is justified [29].
There was observed a markedly enhanced level of IFN-γ release from PBMC in response to Ii-Key/HER-2(777–789) as compared with HER-2(777–789)—968 and 308 pg/ml, respectively—in the absence of IL-12 co-stimulation. The level seen with Ii-Key/HER-2(777–789) was equivalent to the PHA-positive control. In as much as the epitope-peptide only HER-2(777–789) elicited IFN-γ responses equivalent to the PHA-positive control in the setting of IL-12 co-stimulation, the Ii-Key moiety provided a level of adjuvanticity similar to IL-12 in this system. Considering the toxicities associated with IL-12 used in the clinic, covalent modification of MHC class-II epitopes with the Ii-Key moiety offers an attractive alternative to provide potent adjuvant effects in vivo. Secondary stimulation studies of IFN-γ release (where IL-12 was used in the primary stimulation of all PBMC) revealed no difference between Ii-Key/HER-2(777–789) as compared with HER-2(777–789). This observation, taken with the markedly enhanced effect observed at primary stimulation in the absence of IL-12, further suggests that the Ii-key moiety provides a level of adjuvanticity comparable to IL-12. Furthermore, the enhancement of IFN-γ release is specific to the HER-2(777–789) peptide since the Ii-key moeity itself does not bind to the MHC class-II groove as proven by competitive inhibition assays [41]. Even though there was no significant increase in proliferative responses subsequent to primary or secondary stimulation, the Ii-key/HER-2(777–789) hybrid enhanced IFN-γ release of lymphocytes from both a healthy donor and a patient with metastatic breast carcinoma following primary stimulation. This observation is similar to our finding that an Ii-Key/HIV gag(279–290) hybrid stimulated a potent interferon-γ response in injected mice [22].
This was the first study of Ii-Key modified MHC class-II epitopes with human cells, carried out to determine whether clinical trials of Ii-Key-modified HER-2/neu MHC class-II epitopes are warranted. Taken together, the results demonstrate that covalent coupling of a segment of the Ii protein, the LRMK tetrapeptide Ii-Key core site, to the MHC class-II epitope HER-2(777–789) greatly augments IFN-γ release in healthy PBMC and lymphocytes from metastatic lymph nodes. The responses are very strong, comparable to those of phytohemagglutinin controls. In addition, the responses are comparable to those achieved with epitope-only peptide plus exogenous IL-12. These studies point the way to clinical studies with Ii-Key/HER-2(MHC-II epitope) hybrids, either alone or in combination with immunodominant and subdominant MHC class-I epitopes of HER-2/neu and HER-2/neu DNA vaccination. In addition, these studies indicate the use of Ii-Key hybrids in diagnostic applications, including the evaluation and quantification of baseline immunity to HER-2/neu.
References
Adams S, Humphreys RE (1995) Invariant-chain peptides enhancing or inhibiting the presentation of antigenic peptides by major histocompatibility complex class-II molecules. Eur J Immunol 25:1693
Adams S, Albericio F, Alsina J, Smith ER, Humphreys RE (1997) Biological activity and therapeutic potential of homologs of an Ii peptide which regulates antigenic peptide binding to cell surface MHC class-II molecules. Arzneimittelforschung 47:1069
Anderson BW, Kudelka AP, Honda T, Pollack MS, Gershenson DM, Gillogly MA, Murray JL, Ioannides CG (2000) Induction of determinant spreading and of Th1 responses by in vitro stimulation with HER-2 peptides. Cancer Immunol Immunother 49:459
Anderson BW, Peoples GE, Murray JL, Gillogly MA, Gershenson DM, Ioannides CG (2000) Peptide priming of cytolytic activity to HER-2 epitope 369–377 in healthy individuals. Clin Cancer Res 6:4192
Bernhard H, Salazar L, Schiffman K, Smorlesi A, Schmidt B, Knutson KL, Disis ML (2002) Vaccination against the HER-2/neu oncogenic protein. Endocr Relat Cancer 9:33
Carr JA, Havstad S, Zarbo RJ, Divine G, Mackowiak P, Velanovich V (2000) The association of HER-2/neu amplification with breast cancer recurrence. Arch Surg 135:1469
Chen L, Chen D, Block E, O’Donnell M, Kufe DW, Clinton SK (1997) Eradication of murine-bladder carcinoma by intratumor injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol 159:351
Cibotti R, Kanellopoulos JM, Cabaniols JP, Halle-Panenko O, Kosmatopoulos K, Sercarz E, Kourilsky P (1992) Tolerance to a self-protein involves its immunodominant but does not involve its subdominant determinants. Proc Natl Acad Sci USA 89:416
De Placido S, Gallo C, Perrone F, Marinelli A, Pagliarulo C, Carlomagno C, Petrella G, D’Istria M, Delrio G, Bianco AR (1990) Prolactin receptor does not correlate with oestrogen and progesterone receptors in primary breast cancer and lacks prognostic significance. Ten year results of the Naples adjuvant (GUN) study. Br J Cancer 62:643
Disis ML, Calenoff E, McLaughlin G, Murphy AE, Chen W, Groner B, Jeschke M, Lydon N, McGlynn E, Livingston RB (1994) Existent T-cell and antibody immunity to HER-2/neu protein in patients with breast cancer. Cancer Res 54:16
Disis ML, Bernhard H, Shiota FM, Hand SL, Gralow JR, Huseby ES, Gillis S, Cheever MA (1996) Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood 88:202
Disis ML, Gralow JR, Bernhard H, Hand SL, Rubin WD, Cheever MA (1996) Peptide-based, but not whole-protein, vaccines elicit immunity to HER-2/neu, oncogenic self-protein. J Immunol 156:3151
Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA (1997) High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 15:3363
Disis ML, Knutson KL, Schiffman K, McNeel DG (2000) Pre-existent immunity to the HER-2/neu oncogenic protein in patients with HER-2/neu overexpressing breast and ovarian cancer. Breast Cancer Res Treat 62:245
Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, Knutson KL, Schiffman K (2002) Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol 20:2624
Elson CJ, Barker RN, Thompson SJ, Williams NA (1995) Immunologically ignorant autoreactive T cells, epitope spreading, and repertoire limitation. Immunol Today 16:71
Fisk B, Blevins TL, Wharton JT, Ioannides CG (1995) Identification of an immunodominant peptide of HER-2/neu protooncogene recognized by ovarian tumor-specific cytotoxic T lymphocyte lines. J Exp Med 181:2109
Fisk B, Hudson JM, Kavanagh J, Wharton JT, Murray JL, Ioannides CG, Kudelka AP (1997) Existent proliferative responses of peripheral blood mononuclear cells from healthy donors and ovarian cancer patients to HER-2 peptides. Anticancer Res 17:45
Humphreys RE, Adams S, Koldzic G, Nedelescu B, Hofe E von, Xu M (2000) Increasing the potency of MHC class II-presented epitopes by linkage to Ii-Key peptide. Vaccine 18:2693
Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H (1998) The central role of CD4(+) T cells in the antitumor immune response. J Exp Med 188:2357
Ioannides CG, Platsoucas CD, Freedman RS (1990) Immunological effects of tumor vaccines: II. T cell responses directed against cellular antigens in the viral oncolysates. In Vivo 4:17
Kallinteris NL, Hu H, Li Y, Lu X, Wu S, Gulfo GV, Humphreys RE, Xu M (in press) Ii-key/ MHC class II epitope hybrid peptide vaccines for HIV. Vaccine 21:4128
Knutson KL, Disis ML (2002) Clonal diversity of the T-cell population responding to a dominant HLA-A2 epitope of HER-2/neu after active immunization in an ovarian cancer patient. Hum Immunol 63:547
Knutson KL, Schiffman K, Disis ML (2001) Immunization with a HER-2/neu helper peptide vaccine generates HER-2/neu CD8 T-cell immunity in cancer patients. J Clin Invest 107:477
Knutson KL, Schiffman K, Cheever MA, Disis ML (2002) Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide: results in short-lived peptide-specific immunity. Clin Cancer Res 8:1014
Kobayashi H, Wood M, Song Y, Appella E, Celis E (2000) Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res 60:5228
Kuerer HM, Peoples GE, Sahin AA, Murray JL, Singletary SE, Castilleja A, Hunt KK, Gershenson DM, Ioannides CG (2002) Axillary lymph node cellular immune response to HER-2/neu peptides in patients with carcinoma of the breast. J Interferon Cytokine Res 22:583
Lee TV, Johnston DA, Thomakos N, Honda T, Efferson CL, Ioannides CG (2002) Helper peptide G89 (HER-2:777–789) and G89-activated cells regulate the survival of effectors induced by the CTL epitope E75 (HER-2, 369–377). Correlation with the IFN-gamma: IL-10 balance. Anticancer Res 22:1481
Lewis JJ, Janetzki S, Schaed S, Panageas KS, Wang S, Williams L, Meyers M, Butterworth L, Livingston PO, Chapman PB, Houghton AN (2000) Evaluation of CD8(+) T-cell frequencies by the Elispot assay in healthy individuals and in patients with metastatic melanoma immunized with tyrosinase peptide. Int J Cancer 87:391
Masood S, Bui MM (2000) Assessment of HER-2/neu over expression in primary breast cancers and their metastatic lesions: an immunohistochemical study. Ann Clin Lab Sci 30:259
McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML (2000) Antibody immunity to prostate-cancer-associated antigens can be detected in the serum of patients with prostate cancer. J Urol 164:1825
Moudgil KD, Sercarz EE (2000) The self-directed T cell repertoire: its creation and activation. Rev Immunogenet 2:26
Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, Hortobagyi GN, Kudelka AP, Grabstein KH, Cheever MA, Ioannides CG (2002) Toxicity, immunogenicity, and induction of E75-specific tumor-lytic CTLs by HER-2 peptide E75 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2+ patients with metastatic breast and ovarian cancer. Clin Cancer Res 8:3407
Pegram MD, Lipton A, Hayes DF, Weber BL, Baselga JM, Tripathy D, Baly D, Baughman SA, Twaddell T, Glaspy JA, Slamon DJ (1998) Phase-II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J Clin Oncol 16:2659
Reilly RT, Machiels JP, Emens LA, Ercolini AM, Okoye FI, Lei RY, Weintraub D, Jaffee EM (2001) The collaboration of both humoral and cellular HER-2/neu-targeted immune responses is required for the complete eradication of HER-2/neu-expressing tumors. Cancer Res 61:880
Rongcun Y, Salazar-Onfray F, Charo J, Malmberg KJ, Evrin K, Maes H, Kono K, Hising C, Petersson M, Larsson O, Lan L, Appella E, Sette A, Celis E, Kiessling R (1999) Identification of new HER2/neu-derived peptide epitopes that can elicit specific CTL against autologous and allogeneic carcinomas and melanomas. J Immunol 163:1037
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783
Sotiriadou R, Perez SA, Gritzapis AD, Sotiropoulou PA, Echner H, Heinzel S, Mamalaki A, Pawelec G, Voelter W, Baxevanis CN, Papamichail M (2001) Peptide HER2 (776–788) represents a naturally processed broad MHC class II-restricted T cell epitope. Br J Cancer 85:1527
Tuttle TM, Anderson BW, Thompson WE, Lee JE, Sahin A, Smith TL, Grabstein KH, Wharton JT, Ioannides CG, Murray JL (1998) Proliferative and cytokine responses to class II HER-2/neu-associated peptides in breast cancer patients. Clin Cancer Res 4:2015
Ward RL, Hawkins NJ, Coomber D, Disis ML (1999) Antibody immunity to the HER-2/neu oncogenic protein in patients with colorectal cancer. Hum Immunol 60:510
Xu M, Jackson R, Adams S, Humphreys RE (1999) Studies on activities of invariant chain peptides on releasing or exchanging of antigenic peptides at human leukocyte antigen-DR1. Arzneimittelforschung 49:791
Zaks TZ, Rosenberg SA (1998) Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res 58:4902
Acknowledgement
The authors gratefully acknowledge the advice of C. Ioannides in performing the analyses and interpreting the results.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gillogly, M.E., Kallinteris, N.L., Xu, M. et al. Ii-Key/HER-2/neu MHC class-II antigenic epitope vaccine peptide for breast cancer. Cancer Immunol Immunother 53, 490–496 (2004). https://doi.org/10.1007/s00262-003-0463-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-003-0463-y