Abstract
Pancreas transplantation is a complex surgical procedure performed to restore normoglycemia in patients with type 1 diabetes and includes whole/segmental organ transplant and islet cell transplantation (ICT). In the United States, simultaneous pancreas-kidney transplant (SPK) is most commonly performed due to the higher occurrence of end-stage renal disease in diabetic patients. Understanding the surgical technique and postoperative anatomy is imperative for effective and accurate surveillance following transplantation. Imaging plays an essential role in patients with pancreatic transplants and is often used to evaluate viability, vascular and parenchymal anatomy, and identify potential complications. Imaging techniques such as ultrasound, color and spectral Doppler, computed tomography (CT), magnetic resonance imaging (MRI), and angiography have a complementary role in the postoperative evaluation following a pancreas transplant. The common complications after a whole organ pancreas transplant include vascular thrombosis, graft rejection, pancreatitis, and infections. Complications can be classified into vascular (partial or complete venous thrombosis, arterial thrombosis, stenosis or pseudoaneurysm), parenchymal (pancreatitis, graft rejection), and bowel-related or miscellaneous causes (bowel obstruction, anastomotic leak, and peripancreatic fluid collections). Islet cell transplantation is an innovative therapy for patients with type 1 diabetes. It involves isolating insulin-producing islet cells from donor pancreas and transplanting into recipients, to provide long-term insulin independence or significantly reduce insulin requirements. In recent years, isolation techniques, immunosuppressive regimens, and post-transplant monitoring advancements have propelled ICT as a viable therapeutic option. This comprehensive review aims to provide insights into the current state-of-the-art imaging techniques discussing both normal and abnormal features following pancreas transplantation.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pancreatic transplantation is an important treatment option in the management of patients with diabetes, which intends to restore normoglycemia and mitigate the systemic complications linked with the disease [1]. A critical objective of pancreatic transplantation is to restore glycemic homeostasis in patients with diabetes by providing adequate functioning beta cells and avoiding or postponing the onset of diabetes-associated microangiopathies such as retinopathy, nephropathy, neuropathy, and arteriopathy [2, 3]. Pancreas-kidney transplants are associated with improved quality of life and life expectancy by reducing the need for insulin therapy and dialysis [4, 5]. The common indications for this procedure include uncontrolled type 1 diabetes mellitus, frequent insulin reactions, hypoglycemic unawareness (the absence of normal hypoglycemic warning signs, which results in alarmingly low blood sugar levels), advanced-stage kidney disease (eGFR < 20 ml/min), and type 2 diabetes associated with both low insulin resistance and poor insulin production [1, 2].
Pancreatic transplants generally belong to one of four types, depending on the presence and timing of associated renal transplants: pancreas transplant alone (PTA), simultaneous pancreas-kidney transplants (SPK), pancreas after kidney (PAK), and simultaneous pancreas and live donor kidney transplant (SPKL) [1]. Regardless of the type of pancreas transplant, the allograft is procured from a deceased donor. Among the different types, SPK is the most commonly performed procedure in the United States and is recommended in most patients with diabetes < 55 years with end-stage renal disease. According to the Organ Procurement and Transplant Network/ United Network for Organ Sharing (OPTN/ UNOS) data, SPK has seen a sustained increase in performance (77.3% in 2021 vs. 67.5% in 2010) with a notable decline in the proportions of PAK (9.6% in 2021 vs. 18.5% in 2010) and PTA (13.1% in 2021 vs. 14% in 2010) [6]. SPKs are preferred as renal transplant provides survival advantage and requires immunosuppression, justifying that for pancreas transplant. Thus, these patients perform better in terms of long-term outcomes and serum creatinine levels, a renal function assessment and rejection marker, which can also be employed as a substitute for determining pancreas graft function [2]. PAKs are often performed in patients with diabetes < 55 years, displaying hypoglycemic unawareness and other secondary complications of type 1 diabetes [2]. PAKs have significantly lower transplant wait times and a higher kidney graft survival rate than kidney transplants alone [1]. However, PAK procedures exhibit lower long-term pancreatic graft survival than SPKs. PTA is the least common transplant procedure, which is indicated in patients with diabetes in the pre-uremic phase (i.e., do not require or benefit from renal transplant) although with significant benefits justifying substitution of insulin and diabetic complications with immunosuppression and its side effects, e.g., those experiencing frequent hypoglycemic unawareness episodes [1]. Despite the differences in techniques, low 1-year patient mortality rates has been reported across all types of pancreas transplants (2.5% for PAK, 1.3% for PTA, and 3.7% for SPK), which underscores the safety of these procedures [5]. This comprehensive review discusses surgical anatomy of pancreatic allograft, various imaging techniques and their utility in evaluating normal features after pancreas transplantation and common postoperative complications. Furthermore, it delves into imaging in islet transplantation, including pre-transplantation assessment, post-transplantation monitoring, and evaluating long-term effects.
History and evolution
The first successful whole-organ cadaveric pancreas transplant was performed along with a kidney transplant by R. Lillehei and W. Kelly at the University of Minnesota in 1966 in a patient with diabetes on dialysis [7, 8]. Over the next few decades, this procedure underwent several transformations, beginning with an initial interest in the transplantation of pancreatic segments due to its reduced immunogenicity [7]. This field witnessed three major events in the 1980s, including the first use of Cyclosporine A (CsA) as a single agent to prevent rejection and the first international pancreatic transplantation meeting in 1980 which led to the inception of professional associations to discuss both improvements and failures of surgical techniques [7].
In the mid-1980s, Nghiem and Corry, at the University of Iowa, developed an innovative bladder drainage technique via a graft-to-recipient duodenocystostomy for whole pancreas grafts [7]. This technique was soon adopted by most centers in the United States and Europe for its many advantages. For SPK transplants, bladder drainage allowed for better rejection monitoring by measuring serum creatinine, thus lowering the risk of anastomotic leaks. For PTA, urine drainage permitted better rejection monitoring by analyzing urine amylase levels [7]. This technique became the most popular method until the mid-1990s when its related chronic complications, such as urinary tract infections, cystitis, and urethritis, and acidosis related to urinary bicarbonate losses, led surgeons to prefer enteric drainage [9]. Today, virtually all pancreas transplants are enterically drained [3]. Over the years, pancreatic transplants have witnessed increasing success rates resulting from improved surgical techniques, better immunosuppression regimes, and postoperative management.
Surgical technique and anatomy
Pancreatic transplant anatomy depends on the surgical technique employed, associated kidney transplantation, and type of anastomoses [9, 10]. Whole organ pancreas transplantation involves procuring the organ from the deceased donor and transplantation into the recipient after back-table preparation. The procured donor allograft pancreas is transplanted with its arterial supply, venous outflow, and short segment of the C-loop of the duodenum (Fig. 1). The native pancreas is typically retained in its original retroperitoneal location and not surgically removed. The location of the graft pancreas in the recipient is determined by the surgical technique, vascular and enteric anastomosis (Fig. 2). The most common location for the graft pancreas is in the right lower quadrant, which facilitates the extraperitoneal placement of the donor kidney in the left lower quadrant. The other common location is in the pelvis, when bladder drainage is desired. Location in the midline abdomen is chosen when portal venous and enteric drainage are preferred. The least common site of graft pancreas location is the upper abdomen, which is suited for simultaneous liver pancreas transplant and allows for portal venous and enteric drainage [2] (Fig. 2).
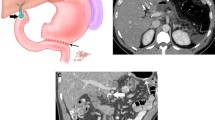
Illustrations of transplant pancreas surgical anatomy. a Explanted pancreas with donor splenic artery (dSA), donor superior mesenteric artery (dSMA), donor Y-graft (dY-graft), donor splenic vein (dSV), donor superior mesenteric vein (dSMV), donor portal vein (dPV) and donor duodenum (dD). b Transplant pancreas in the right lower quadrant with systemic vascular anastomosis to recipient iliac vessels and enteric drainage to recipient jejunum. c Transplant pancreas in the right lower quadrant with arterial anastomosis to recipient iliac artery, portal venous anastomosis to recipient superior mesenteric vein and enteric drainage to recipient jejunum. d Transplant pancreas in the pelvis with systemic vascular anastomosis to recipient iliac vessels and exocrine drainage to the recipient urinary bladder (duodenocystostomy)
Location of transplant pancreas. a Axial contrast-enhanced CT shows transplant pancreas in the right lower quadrant (arrow) and transplant kidney in the left lower quadrant (K). b Axial contrast-enhanced CT shows transplant pancreas in the right hemipelvis (arrow). c Coronal contrast-enhanced CT shows transplant pancreas in midline abdomen (arrow). d Axial contrast-enhanced CT shows transplant pancreas in the right upper quadrant (arrow)
Arterial supply
Graft pancreas arterial inflow is provided by the superior mesenteric artery (SMA), which delivers blood to the pancreatic head, and the splenic artery, which supplies blood to the body and tail of the pancreas [9]. The arterial supply to the graft pancreas and its anastomoses to the recipient is performed by the use of a donor Y graft. The Y graft used is the donor’s common iliac and its bifurcation into external, and internal iliac arteries [9]. During the back-table preparation of the graft, and prior to implantation, the donor SMA and splenic artery are anastomosed to either the external or internal iliac arteries of the Y-graft[10]. Additionally, the donor’s spleen, which is usually recovered with the pancreas, is removed on the back-table, and the donor’s splenic vessels ligated at the level of the splenic hilum. During the implantation, the common iliac artery of the donor Y-graft is anastomosed to the recipient’s common or external iliac artery, although variations in technique can occur (Figs. 1 and 3).
Postoperative anatomy of transplant pancreas. a Sagittal arterial phase MIP CT image and b volume-rendered image shows Y-graft anastomosis to recipient right external iliac artery (arrow). c Axial contrast-enhanced CT shows donor portal vein anastomosis with the recipient right common iliac vein (arrow). d Coronal non-contrast CT shows donor pancreas (arrowhead) with enteric anastomosis between donor duodenum and recipient jejunum (arrow). e, f Axial and coronal non-contrast CT shows donor duodenum anastomosis with the recipient’s urinary bladder (arrow)
Venous drainage
Venous drainage from the pancreas is essential for venous outflow and maintenance of vascular perfusion and drainage of endocrine secretions, including insulin, glucagon, somatostatin, ghrelin, and pancreatic polypeptide [11]. The donor portal vein, which constitutes the main pancreatic graft outflow vein, is formed by a confluence of donor splenic and superior mesenteric veins, which drain the pancreatic venous tributaries [9]. The venous drainage from the graft pancreas can be accomplished by anastomosis of the donor portal vein to the systemic or portal venous circulation. Systemic drainage is attained by the anastomosis of the donor’s main graft vein to the recipient’s common iliac vein or inferior vena cava, whereas, in portal venous drainage, it is anastomosed to the recipient’s superior mesenteric vein. The venous anastomosis is usually dictated by the donor and recipient anatomy and the surgeon’s preference [3]. While portal venous drainage represents a more physiologic mechanism for drainage of endocrine sections, there is no significant difference in glycemic control in both types of venous anastomoses [1] (Figs. 1 and 3).
Exocrine drainage
The C-loop of the duodenum of the graft pancreas receives exocrine secretions from the pancreatic duct, including trypsin, chymotrypsin, pancreatic amylase, and lipase [12]. It is necessary to drain all the exocrine secretions of the pancreatic gland to avoid pancreatic duct obstruction and failure of the pancreas. Drainage of exocrine secretions from the C-loop can be accomplished through either enteric or bladder anastomoses. Enteric drainage is most often accomplished through the small bowel via anastomosis of the donor duodenum to the recipient’s jejunum with or without a Roux-en-Y loop or rarely to the recipient duodenum [3]. Less commonly, drainage is facilitated by the anastomosis of the donor duodenum to the recipient’s urinary bladder (bladder drainage) [9]. However, this has fallen out of favor due to complications such as recurrent urinary tract infections, chemical cystitis, urethritis and hematuria from pancreatic secretions entering the urinary bladder and graft pancreatitis resulting from reflux of urine [2, 3, 9] (Figs. 1 and 3).
Imaging: modalities and technique
Imaging plays a pivotal role in transplant evaluation by utilizing both traditional and advanced techniques to monitor graft status and assess potential complications. Imaging provides both structural and functional information about the graft pancreas. Currently, frequently employed imaging methods comprise ultrasonography (US), computed tomography (CT), and magnetic resonance (MR) imaging. While initially employed to evaluate graft function, radionuclide studies are not routinely performed.
US and Doppler imaging
Ultrasonography is the preferred initial imaging modality to evaluate graft pancreas in the postoperative period. Its advantages include easy accessibility, portability, Doppler evaluation of vascularity, and lack of ionizing radiation exposure [2]. Accurate assessment of the pancreatic allograft requires precise techniques tailored for graft evaluation and knowledge of allograft placement and vascular/exocrine drainage anastomoses. Ultrasound offers a detailed interrogation of the pancreatic parenchyma and ductal anatomy. However, the presence of overlying bowel gas, particularly in grafts with portal drainage and intraperitoneal placement in the right or upper mid-abdomen, can obscure segments or the entirety of the organ [13]. Typically, a high-frequency linear probe, 5–12 MHz, allows optimal evaluation of the transplanted pancreas in the right iliac fossa. Applying minimal probe compression and positioning the patient in a right anterior oblique orientation can be beneficial in displacing overlying intestines [14]. Doppler imaging enables the assessment of arterial and venous anastomoses and the evaluation of parenchymal perfusion. Vascular assessment involves interrogation of donor and recipient anastomotic arteries for color flow, arterial waveforms, peak systolic flow velocity (PSV), and vascular resistive indices (RI). In addition to assessing allograft parenchyma and vasculature, ultrasound also facilitates guided biopsies.
CT imaging
CT provides a comprehensive assessment of the graft parenchyma, vascular anastomoses, and exocrine drainage and is a valuable tool for detecting and characterizing a wide range of postoperative complications [15]. It is usually recommended after an initial US or in the presence of unexplained symptoms such as fever or abdominal pain [16]. Contrast-enhanced abdomen-pelvis CT is performed with the acquisition in the arterial and portal venous phase to facilitate the evaluation of renal and pancreas allografts. While neutral oral contrast allows a superior display of vascular structures, positive oral contrast might be preferred to depict enteric drainage [17]. CT allows for sub-mm volumetric acquisition, which allows for multiplanar imaging and three-dimensional reconstructions of the graft’s vascular anatomy using two-dimensional multiplanar reformations, three-dimensional maximum intensity projection (MIP), and volume-rendered images [17]. Curved planar reformations can also accurately depict donor and recipient vascular anastomoses. In patients with renal dysfunction or compromised renal function, a non-contrast CT study is used to assess suspected intestinal obstruction and intrapancreatic or peripancreatic fluid collections, owing to nephrotoxic effects of iodinated contrast agents [18]. Similar to US, CT can serve as a valuable tool for performing guided biopsies in suspected cases of allograft rejection.
MR imaging
MRI has a problem-solving role in challenging cases though, not routinely performed due to the ease and availability of US and CT [19, 20]. MRI should be typically performed on 1.5- or 3-T systems for optimal evaluation. A typical MR protocol includes T2-weighted images, pre-contrast axial T1-weighted, coronal oblique thick-slab single-shot T2-weighted fast spin-echo sequence (MR pancreatography) and dynamic contrast-enhanced MR sequences [21]. MR pancreatography allows for superior assessment of pancreatic duct abnormalities. In patients with poor renal function, unenhanced MRI provides more acceptable information about the vasculature compared to unenhanced CT [4]. In patients with suspected vascular complications such as thrombosis, stenosis, and infarction, MR angiography permits optimal assessment of the vasculature [22].
Catheter angiography
Conventional catheter angiography remains a valuable diagnostic tool, although its role has evolved with the advent of advanced cross-sectional imaging modalities. It is particularly useful in confirming diagnoses in cases of vascular complications where other modalities yield equivocal results such as in identifying stenosis, thrombosis, pseudoaneurysm, and arteriovenous fistula. Additionally, it serves as the first step for patients requiring intricate vascular interventions such as angioplasty, endovascular stent placement, embolization, venous thrombolysis, or thrombectomy [23, 24].
Radionuclide imaging
Radionucleotide studies were initially introduced in the early 80s and were utilized to assess renal transplants. Toledo-Pereyra demonstrated that 75Se-selenomethionine imaging correlates excellently with transplant function in the initial weeks after transplantation in extraperitoneal segmental pancreas transplant. However, this technique is complementary to other imaging modalities due to cost and redundancy with information provided by plasma glucose levels [25]. Subsequently, studies have demonstrated that scintigraphic techniques, including 99mTc-sulfur colloid, 111In-labeled platelets, and 99mTc-DTPA, have utility in distinguishing normal from abnormal pancreas and kidney allografts, particularly in assessing rejection and perfusion status [26]. However these techniques are not commonly used in routine clinical practice.
Imaging: normal appearance
The imaging appearance of pancreatic allograft demonstrates features similar to normal native pancreas. On gray-scale B-mode US, the graft pancreas appears as a homogenous, low-level echogenic structure with the pancreatic duct characterized by specular echoes [27] (Fig. 4). A normal graft pancreas typically exhibits sharp systolic upstrokes, continuous antegrade diastolic flow, and an RI of 0.5–0.7, indicating reasonably lower vascular resistance within the graft (Fig. 4). The venous structures related to the allograft demonstrate a continuous monophasic waveform in an anechoic lumen. In systemic venous drainage cases, a slight cardiac phasicity may be observed in the venous flow waveform (Fig. 4). In portal drainage, it is not uncommon to experience mild, generalized constriction in the main graft vein at the anastomotic site, causing a relative flattening of the venous flow waveforms [2]. The graft pancreas might maintain normal vascular resistance even with edema resulting from inflammation or rejection due to the lack of a capsule. On CT, the pancreatic allograft, akin to a normal pancreas, exhibits uniform enhancement, with the main pancreatic duct showing minimal or no dilation with clear delineation of vascular and enteric anastomoses [27] (Fig. 5). On MRI, the graft pancreas is hyperintense on T1- and T2-weighted images relative to skeletal muscle, owing to higher protein and water-based secretions intrinsic to the gland (Fig. 6).
Ultrasound and Doppler imaging of normal transplant pancreas. a Transverse midline gray-scale US shows transplant pancreas with homogenous echotexture (arrow) and no peripancreatic inflammation. b Right lower quadrant gray-scale US shows transplant pancreas, which has homogenous echotexture (arrow) with normal graft arterial supply. c, d Transplant pancreas arterial Doppler shows a sharp systolic upstroke with continuous diastolic flow and RI of 0.7 and transplant pancreas venous Doppler shows continuous flow with mild degree of cardiac phasicity
CT imaging of normal transplant pancreas. a Coronal non-contrast CT shows a homogenous transplant pancreas in mid-abdomen (arrow) due to the previous placement of transplant kidney (K) in the right lower quadrant. b Axial contrast-enhanced CT shows a homogenously enhancing transplant pancreas in right lower quadrant (arrow) and transplant kidney in left lower quadrant (K)
MR imaging of normal transplant pancreas. a Coronal T1-weighted image shows transplant pancreas in the right lower quadrant with homogenous T1 signal intensity (arrow) (homogenously hyperintense relative to muscle). b Coronal T2-weighted image shows transplant pancreas in the right lower quadrant with homogenous parenchymal T2 signal intensity (arrow) (homogenous signal intensity between fluid and muscle) and normal caliber main pancreatic duct (arrowhead)
Imaging: post-transplantation complications
Since its first introduction in 1966, pancreas transplantation has shown improved outcomes in patients with diabetes. The 5-year survival rates for patients with type 1 and type 2 diabetes receiving pancreas allografts were 91.9 and 87.3%, respectively [5]. The 2022 OPTN/ UNOS data reports the incidence of graft failure within the first 90 days to be 6.1, 5.3, and 8.8% with PAK, PTA, and SPK, respectively [5]. The most enduring grafts have achieved a remarkable 26-year span for SPK, 24 years for PAK, and 23 years for PTA [28]. However, post-transplant complications present multifaceted challenges, and understanding these complexities is crucial for timely intervention and management. Complications can be classified into early (< 3 months) and late (≥ 3 months), depending on the duration after surgery. Early complications include acute thrombosis, hemorrhage, acute graft rejection, acute pancreatitis, anastomotic leakage, and ileus. Chronic graft rejection, pseudocyst, pseudoaneurysm, small bowel obstruction, and post-transplant lymphoproliferative disease represent the gamut of late complications. A primary reason for the allograft loss in the first 3 months is termed as technical failure, which accounts for 8% of cases [26, 27]. Technical failure can result from vascular thrombosis (50%), pancreatitis (20%), infection (18%), fistulas (6.5%), and hemorrhage (2.4%) [29]. Patients undergoing pancreas transplantation receive immunosuppressive therapy to prevent rejection, which results in intended reduced immune response and unintended heightened vulnerability to infections and gastrointestinal and metabolic complications, along with an increased propensity for the development of malignancies [30, 31]. Depending on the etiology, complications can also be categorized into vascular, parenchymal, and bowel-related complications [1].
Vascular complications
Pancreas allograft thrombosis could be due to arterial, venous, or combined involvement [32]. Venous thrombosis is the most frequent vascular complication, occurring twice as frequently as arterial thrombosis [33]. The causes of vascular thrombosis could be multifactorial, including low microcirculatory blood flow of the pancreas (especially with the lack of splenic venous drainage in the splenic vein), donor-related factors such as obesity and metabolic syndrome, back-table preparation, and cold ischemia time [33]. Venous thrombosis is more prevalent in PAK and PTA than SPK, and enteric drainage poses a greater risk than bladder drainage [34]. Severe pancreatitis, arterial wall injury, and stump thrombi formation contribute to the overall risk. Splenic vascular stumps created during the back-table preparation of the pancreas may develop clotting in the peripheral arterial and venous segments, particularly in low-flow areas. Thrombus formation, especially at the splenic vein stump in the allograft tail, can progress from a minor nidus to extensive involvement. Prophylactic anticoagulation is often initiated to prevent clot propagation in such cases despite the associated increased bleeding risks [30]. Additionally, venous obstruction also leads to pancreatic congestion and edema [35].
US demonstrates a bulky hypoechoic or heterogenous allograft with surrounding fluid. The transplant vein lumen might show echogenic foci, and the Doppler signal may be absent. Extensive venous obstruction manifests as a reverse diastolic flow on the spectral waveform and poses a risk of transplant infarction [36] (Fig. 7). Contrast-enhanced CT is required to exclude thrombosis reliably. However, unenhanced CT may suggest acute thrombus if a high-density tubular structure is observed in the expected location of the allograft vein. On MRI, thrombus may appear as a T2 hypointense, T1-hyperintense intraluminal filling defect, with absent flow void, and might need confirmation on post-contrast sequences [37]. True fast imaging with steady-state precession (FISP), gradient-echo, or time-of-flight sequences are valuable in revealing thrombosed vessels. In patients with renal failure or reduced creatinine clearance, unenhanced MRI angiography provides sufficient information for a confident assessment of vascular patency.
Pancreatic allograft venous thrombosis in a 52-year-old patient. a Color Doppler US image shows echogenic material within the transplant pancreatic vein with absent Doppler signal. b Color and spectral Doppler US image shows a reversal of arterial diastolic flow with a high RI of 1.3, suggestive of venous thrombosis
The allograft parenchyma exhibits high T2 signal intensity and lacks enhancement in patients with complete graft infarction, while hemorrhagic infarction is seen as an increased signal on T1-weighted images. Prompt pancreatectomy is often necessary to reduce infectious complications and limit mortality in cases of acute venous thrombosis. Thrombectomy and thrombolysis have limited efficacy in acute venous thrombosis and are usually suitable in short-segment thrombosis without parenchymal necrosis. Partial graft thrombosis, observed in 25% of cases, has shown promising outcomes in graft preservation when promptly managed with a combination of low molecular weight heparin and vitamin K antagonists [38]. In the rare occurrence of chronic thrombosis, collateral circulation can lead to marginal enhancement around an otherwise non-enhancing pancreas.
Diagnosing arterial thrombosis involves identifying absent arterial flow on both color and power Doppler and a missing arterial spectral waveform. CT angiography helps demonstrate the extent of the thrombus (Fig. 8). In cases where IV contrast is contraindicated, unenhanced MRI is preferred over unenhanced CT [4]. Arterial stenoses, although infrequent, can arise at any anastomotic site. Atherosclerotic plaques in many recipients can compromise vascular inflow to the allograft. High-velocity turbulent flow at the anastomosis site may indicate stenoses on Doppler ultrasound, but confirmation is typically obtained through CT or MR angiography or rarely conventional angiography (Fig. 9). During the initial postoperative period, lasting up to a week, temporary narrowing due to perianastomotic edema may occur, resolving as the anastomosis heals. However, if features of stenosis persist or newly arise after the initial postoperative week, CT angiography or MR angiography is recommended [4]. Stenoses may be amenable to endoluminal therapy, warranting the need for stent placement. In situations where diagnosing stenosis or thrombosis poses challenges, digital subtraction catheter angiography emerges as a pivotal tool for resolution, avoiding false concerns and unnecessary follow-up.
Transplant pancreas arterial thrombosis in a 42-year-old patient. a Color Doppler US image shows no color uptake in the transplant artery. b Sagittal CT Angiography shows minimal to no contrast opacification of the transplant artery immediately distal to the iliac anastomosis (arrow). c, d Right common iliac angiogram and selective angiogram of transplant pancreas artery shows very little appreciable flow within the distal aspect of transplant artery and no flow within its branches along with no demonstrable flow to the transplant pancreas (arrow). e 99m-Tc DTPA scan shows the perfusion to the location of the transplanted pancreas in the right lower quadrant is severely reduced with complete absence of activity during the flow phase of the study
Pseudoaneurysms and arteriovenous fistulas are serious, unusual complications and are often linked to surgical technique, infection, pancreatitis, or biopsy (Fig. 10). While commonly asymptomatic, they pose a high risk of hemorrhage and frequently result in graft loss [39]. Color Doppler reveals the hallmark to-and-fro yin-yang appearance within the pseudoaneurysm. At the same time, arteriovenous fistulas may display color aliasing and a distinctive high-velocity, low-resistance Doppler waveform with pulsatile flow in the draining vein [40]. On CT angiography, pseudoaneurysms manifest as contrast-filled, round, or oval structures emerging as a saccular outpouching from the artery. An arteriovenous fistula appears as a connection between a vein and an artery, often accompanied by dilation of the segmental vein associated with early contrast opacification. Treatment strategies depend on anatomy and size, with small post-biopsy fistulas often resolving independently and more significant cases requiring endovascular or surgical interventions.
Parenchymal complications
As many as 35% of patients experience mild, self-limited pancreatitis, often due to ischemia–reperfusion injury (which occurs in all organ transplants in early post-operative period), or from compromised microcirculation within 4 weeks of transplant surgery [27, 37]. Risk factors include the age of the donor, procurement preservation technique, preservation solution content, and quantity, cold ischemic time, and organ handling during surgery, with higher rates observed with bladder drainage [30]. Pancreatitis beyond the immediate postoperative phase is suspected in patients with abdominal pain or elevated serum pancreatic enzyme levels. It can manifest as graft enlargement and heterogeneity on imaging, with peripancreatic fluid and associated adjacent bowel wall thickening (Fig. 11). US can reveal other accompanying complications such as pseudocyst, vascular thrombosis, infarction, and necrosis, while CT and MRI offer greater sensitivity and specificity for detecting peritransplant collections and associated vascular complications [29].
Graft pancreatitis in a 45-year-old patient. a Gray-scale US of right lower quadrant transplant pancreas with heterogenous echotexture (arrow) and peripancreatic fluid. b Coronal contrast-enhanced CT shows an enlarged, edematous graft pancreas with mild peripancreatic fluid (arrow). Transplant kidney (K) in left lower quadrant is enlarged, edematous and shows striated appearance consistent with acute kidney injury
Acute rejection typically manifests within one week to three months after transplantation. The incidence rates following one year of pancreas transplant are 11.4, 9.0, and 10.9% for recipients aged 18–34, 35–49, and 50–64 years, respectively [5]. Diagnosing acute rejection is challenging, and early detection is crucial for timely intervention to prevent graft failure. Hyperglycemia, elevated serum amylase, and/or lipase levels should raise suspicion for allograft function loss, although the former tends to be delayed as islet cell injury related to rejection causes release of more insulin and masking of significant hyperglycemia. Imaging features of allograft rejection may not be differentiated from entities such as pancreatitis [41]. Parenchymal edema, elevated PSV, and RI (> 0.8) have been described; however, they are not highly specific [4] (Fig. 12). In practice, the major role of US and Doppler is to exclude vascular thrombosis and guide biopsy, which is the reference standard for diagnosis and confirmation of rejection [39]. Chronic rejection affects approximately 3.7–11.6% of patients and stands as the primary contributor to graft failure beyond six months [42]. Patients may present with insidious onset of loss of endocrine function and manifest as gradual parenchymal atrophy or disappearing pancreas on imaging [1] (Fig. 13).
Acute rejection in a 38-year-old patient. a, b Color and Spectral Doppler US shows elevated RI of 0.86 and 0.84 in the artery at the site of anastomosis and mid-pancreatic artery. c Axial CECT shows transplant pancreas (arrow) and kidney (K) in the right and left lower quadrants respectively- however, the transplant pancreas shows homogenous contrast enhancement. d US-guided biopsy with tip of 16 Fr needle within the hypoechoic pancreas (arrowhead). Histopathology: Grade 1 acute T cell-mediated rejection
Post-transplant lymphoproliferative disorder (PTLD), another rare long-term complication of pancreas transplantation, has a 25-year cumulative incidence rate of 1.7% and a mean time of diagnosis of 1.5 ± 0.5 years [41, 43]. While most cases are associated with a preexisting or initial Epstein-Barr virus and cytomegalovirus infections, rigorous immunosuppressive regimens also contribute to its occurrence. The common sites affected are lymph nodes, the central nervous system, the liver, the gastrointestinal tract, or the allograft [43, 44]. It is also characterized by extensive extranodal involvement, observed in 69% of patients [45, 46]. Imaging manifestation of PTLD includes the presence of solid masses in the liver and pancreas, lymphadenopathy, focal masses in the bowel, or bowel wall thickening, which is best depicted on cross-sectional imaging [46, 47]. It can often be challenging to diagnose on imaging alone and may warrant need of tissue sampling (Fig. 14).
Necrotizing and hemorrhagic pancreatitis mimicking post-transplant lymphoproliferative disorder in a 40-year-old patient. a Coronal T2 fat sat image, b contrast-enhanced CT and c whole-body FDG PET images shows areas of heterogeneity (arrow), hypoenhancement (arrow) and FDG avidity (arrow) respectively in the inferior aspect of transplant pancreas suspicious for necrotizing pancreatitis v/s post-transplant lymphoproliferative disorder. d CT-guided biopsy of transplant pancreas where four 18-gauge samples were obtained. Histopathology: acute arterial and venous thrombosis with necrotizing and hemorrhagic pancreatitis
Bowel-related and miscellaneous complications
Bowel-related complications include small bowel obstruction, anastomotic leak, extra-enteric abscess, and colitis; these occur in 19.4% of cases [48]. Small bowel obstruction, potentially exacerbated by an enteric-drained pancreas allograft placed within the peritoneal cavity, poses a higher risk for internal herniation. Internal hernia should be suspected as a cause for obstruction. CT often helps to confirm the diagnosis when dilated distal small bowel loops are seen behind the graft pancreas or donor duodenum. Obstruction caused by adhesions typically manifests in the anterior aspect of the abdomen and is generally mild. Other less common causes of bowel obstruction include narrowing at the enteric anastomosis site (Fig. 15).
Anastomotic leaks, occurring in 2–10% of patients following enteric drainage, pose a risk of intraabdominal infection and require early recognition to prevent sepsis [43, 48]. In enteric-drained transplants for duodenojejunal anastomotic leaks, prompt surgical management with revision of the anastomosis is necessary to prevent serious complications and higher rates of graft loss and patient mortality [30] (Fig. 16). In bladder-drained transplants, early leaks often occur at the site of anastomosis to the bladder, while leaks from duodenal stumps manifest much later. Such leaks can be identified using conventional cystography or CT cystography. Bladder leaks are less severe and are often managed by catheterization, but in the presence of peritonitis, conversion to enteric drainage may be necessary. Distinguishing leaks from other pancreatitis-related collections can be challenging, and positive oral contrast material administration is recommended for increased diagnostic confidence [49].
Fluid collections, common after pancreas transplantation, may be detected early or late in the postoperative period. While small collections are often clinically insignificant, larger collections may indicate intraabdominal infection [43]. Ascites, often present, is typically low in volume. The types of intraabdominal collections include seroma, hematoma, urinoma, lymphocele, and pseudocyst [2]. The presence of internal echoes on US, higher attenuation on CT, and higher intensity on T1-weighted images on MRI can identify hematomas. Percutaneous image-guided aspiration is useful for diagnosis and management. Surgical site infection can occur in up to 50% of patients, most cases being superficial and treatable with antibiotics [19]. However, deep infections are associated with more significant morbidity, graft loss, and mortality. These infections, caused by bacteria or fungi, may be diffuse in 50% of the cases [19]. Localized abscesses can be managed with percutaneous drainage alone; however, laparotomy and drainage may sometimes be required [30] (Fig. 17).
Intrapancreatic and abdominal wall abscesses in a 50-year-old patient 30th postoperative day after SPK. a Coronal and b axial contrast-enhanced CT shows peripheral rim-enhancing collection with gas within (arrow) within the transplant pancreas and a similar rim-enhancing collection with gas within in the midline subcutaneous region of anterior abdominal wall at the level of umbilicus (arrowhead)
Reporting checklist
Imaging is crucial in routine surveillance of the graft pancreas and in accurately identifying transplant-related complications. Radiologists require comprehensive knowledge of surgical anatomy and the typical postoperative imaging appearance of pancreas transplants to effectively identify and diagnose abnormal postoperative findings. Integrating a transplant evaluation checklist into imaging and reporting practices can enhance the evaluation process, facilitate standardized protocols, and improve accuracy in diagnosing and managing transplanted pancreas (Table 1).
Islet transplantation
Islet transplantation (IT) has evolved as a promising alternative for treating type 1 diabetes in patients who have experienced complications such as severe recurrent hypoglycemic episodes, labile glycemic control, and early secondary complications despite receiving insulin injections for management [46, 50]. The main aim of islet cell transplantation is to help patients achieve insulin independence to some degree [51]. Success rates range from 20 to 90% across different centers, with the center’s experience playing a significant role. However, insulin independence from islet cell infusion isn’t permanent. Patients typically need another round of infusion around every three years to sustain independence. Beta cells can still function to some extent for up to five years post-infusion. IT is currently utilized in clinical practice, but the process of harvesting, preserving, and infusing cells is evolving with ongoing discoveries from experimental research. This ongoing development aims to achieve more consistent and lasting outcomes in clinical applications [52]. Accurate imaging plays a crucial role in the islet transplantation procedure, including pre-transplantation assessment, post-transplantation monitoring, and evaluation of the procedure’s long-term effects.
Transplantation procedure
Islets are isolated from the donor by detaching the duodenum, spleen, lymph nodes, vessels, and peri-pancreatic fat from the pancreas. The head of the pancreas is then transected, and catheters are inserted into the main pancreatic duct. The pancreas is perfused with a collagenase solution, undergoes cold perfusion, and is sliced into smaller pieces before being placed into a chamber. An enzymatic solution is infused into the chamber, and temperature is manipulated until islets are cleared from acinar tissue. Islets are collected, stained with dithizone, and verified for purity, viability, potency, and stability. Islets must be infused within 72 h of harvesting for optimal survival. Donor islets, not exceeding 5 ml, are diluted with heparin and lactated Ringer before implantation. During imaging-assisted percutaneous transhepatic islet transplantation, purified islets extracted from the deceased donor’s pancreas are instilled into the recipient’s liver via the portal vein [53]. This transhepatic approach has been preferred because the liver is a significant site of insulin action, its regenerative ability, dual blood supply (hepatic artery and portal vein), and possible immunologic protection for engrafted islets [54]. The portal vein is easily accessible and offers consistent access for infusion. The infusion process takes about 15 min, with portal vein pressure monitored to ensure it stays at or below 22 mmHg. Gelatin sponges and/or coils are placed into the cannulation site once the infusion is complete (Fig. 18).
Pre-transplantation imaging
In pre-transplantation imaging, a risk–benefit analysis is necessary due to the requirement of permanent immunosuppressive therapy, which carries higher risks of malignancy and severe infections. Screening with imaging, including cross-sectional imaging techniques and Doppler US, is employed to detect any preexisting abnormalities that could complicate or preclude transplantation [55]. These abnormalities include infections (e.g., pneumonia, empyema), malignancy, focal liver lesions such as hemangioma, parenchymal liver disease such as cirrhosis or hepatic steatosis, and portal vein abnormalities such as thrombosis or portal hypertension.
Post-transplantation imaging
Post-transplantation imaging is essential for monitoring complications that may arise from the presence of grafted islet cells or adverse effects related to immunosuppressive therapy. Immediate or early complications include hemorrhage, which can occur in the subcapsular or intraparenchymal aspect of the liver or within the peritoneal or pleural cavities. Effective sealing of the intraparenchymal liver tract can prevent this complication [56]. Portal vein thrombosis represents the second most common early complication, occurring in approximately 3% of cases [57]. While complete portal venous thrombosis is rare, it requires urgent surgical intervention. Other complications may involve arteriovenous fistula, trauma to adjacent structures (gallbladder, biliary tree), or pleural trauma resulting in pneumothorax or hemothorax [58].
Late complications can include hepatic steatosis [59], which can be focal or diffuse but more commonly periportal and perivenular patterns resulting from the paracrine action of insulin secreted by transplanted islets promoting esterification of free fatty acids within hepatocytes [59]. It is not associated with elevated liver function or an inflammatory response [59] (Figs. 19 and 20). Hepatic steatosis following intraportal islet transplantation is observed in approximately 20% of C-peptide-positive individuals and 10% of those who have achieved insulin independence. The resolution of steatosis with graft function loss suggests a link between hepatic steatosis and functioning islets. However, it remains unclear whether islets in patients with steatosis are healthy or stressed [59]. Long-term immunosuppressive therapy with sirolimus can lead to the development of ovarian cysts in women, which may require surgical excision [60]. Sirolimus therapy also increases the risk of nephrotoxicity and perinephric edema, though the long-term effects on renal function are currently unknown [61]. Routine post-transplantation imaging surveillance is performed to detect early complications. It may include color Doppler abdominal US the day after the implantation, followed by an examination at regular intervals, such as annually [53]. Contrast-enhanced CT or MRI examinations could also be used for problem-solving in selected cases.
Islet transplant graft imaging
Currently, no imaging method has been established to track the viability and mass of transplanted islets following intraportal islet transplantation. However, imaging modalities hold promise for monitoring the fate of islet grafts. Due to their small size, low density, and deep abdominal location, imaging of implanted islets can be challenging [55]. Nevertheless, islets labeled with 18F-fluorodeoxyglucose (FDG) have been successfully visualized using positron emission tomography (PET) [62, 63]. Promising PET/single-photon emission computed tomography (SPECT) markers such as [11C] 5-hydroxytryptophan and radiolabeled exendin are being investigated for labeling viable pancreatic islets [57, 59]. MRI has shown the ability to image islets labeled with superparamagnetic iron oxide (SPIO). SPIO-labeled islets appear as hypointense regions dispersed within the liver parenchyma with possible detection up to 6 months post-transplantation [64, 65]. The disappearance of these hypointense spots correlates with graft loss [66]. While MRI has limited sensitivity in detecting islets compared to PET, it offers superior spatial resolution. However, commercially available SPIOs are not efficiently taken up by islet cells, requiring further development of SPIO nanoparticles.
Conclusion
Imaging plays an important role in routine surveillance following pancreatic transplantation, as well as in the evaluation of common and uncommon complications. The most commonly used imaging techniques are ultrasound, CT, and MRI. Knowledge of normal imaging appearance of pancreas allograft and manifestations of various complications is essential for early diagnosis and timely intervention. An imaging reporting checklist will be helpful for detailed assessment and standardization. Islet transplantation offers a promising solution for type 1 diabetes complications, and imaging plays a crucial role in pre-transplant evaluation, post-transplant monitoring, and long-term effects assessment. While current methods lack specificity for tracking islet mass, promising PET and MRI techniques are being developed, and the advancement of such techniques can potentially improve transplantation outcomes.
Abbreviations
- CsA:
-
Cyclosporine A
- CT:
-
Computed tomography
- dD:
-
Donor duodenum
- dPV:
-
Donor portal vein
- dSA:
-
Donor splenic artery
- dSV:
-
Donor splenic vein
- dSMA:
-
Donor superior mesenteric artery
- dSMV:
-
Donor superior mesenteric vein
- dY-graft:
-
Donor Y-graft
- 18F-FDG:
-
Fluorodeoxyglucose
- IT:
-
Islet transplantation
- MRI:
-
Magnetic resonance imaging
- OPTN/ UNOS:
-
Organ Procurement and Transplant Network/ United Network for Organ Sharing
- PAK:
-
Pancreas after kidney transplant
- PET:
-
Positron emission tomography
- PSV:
-
Peak systolic velocity
- PTA:
-
Pancreas transplant alone
- PTLD:
-
Post-transplant lymphoproliferative disorder
- RI:
-
Resistive index
- SMA:
-
Superior mesenteric artery
- SPECT:
-
Single-photon emission computed tomography
- SPIO:
-
Superparamagnetic iron oxide
- SPK:
-
Simultaneous pancreas-kidney transplant
- SPKL:
-
Simultaneous cadaveric-donor pancreas and live-donor kidney transplant
- US:
-
Ultrasound
References
M. T. Heller and P. Bhargava, “Imaging in pancreatic transplants,” Indian J Radiol Imaging, vol. 24, no. 4, pp. 339–349, Nov. 2014, doi: https://doi.org/10.4103/0971-3026.143896.
P. P. Tolat, W. D. Foley, C. Johnson, M. D. Hohenwalter, and F. A. Quiroz, “Pancreas transplant imaging: how I do it,” Radiology, vol. 275, no. 1, pp. 14–27, Apr. 2015, doi: https://doi.org/10.1148/RADIOL.15131585.
N. Antunes, R. Santos, F. G. Almeida, and N. Carrilho, “Pancreatic transplantation: what the radiologist needs to know. Transplante Pancreático: O que o Radiologista Deve Saber,” vol. 29, pp. 1–13, 2017.
R. B. O’Malley, M. Moshiri, S. Osman, C. O. Menias, and D. S. Katz, “Imaging of Pancreas Transplantation and Its Complications,” Radiol Clin North Am, vol. 54, no. 2, pp. 251–266, Mar. 2016, doi: https://doi.org/10.1016/J.RCL.2015.09.012.
R. Kandaswamy et al. OPTN/SRTR 2022 annual data report: pancreas. Am J Transplant, vol. 24, 2S1, pp. S119–S175, 2024, doi: https://doi.org/10.1016/J.AJT.2024.01.013.
R. Kandaswamy et al., “OPTN/SRTR 2021 annual data report: pancreas,” Am J Transplant, vol. 23, no. 2 Suppl 1, pp. S121–S177, Feb. 2023, doi: https://doi.org/10.1016/J.AJT.2023.02.005.
J. P. Squifflet, R. W. G. Gruessner, and D. E. R. Sutherland, “The history of pancreas transplantation: past, present and future,” Acta Chir Belg, vol. 108, no. 3, pp. 367–378, 2008, doi: https://doi.org/10.1080/00015458.2008.11680243.
D. Casanova, “Pancreas transplantation: 50 years of experience,” Cir Esp, vol. 95, no. 5, pp. 254–260, May 2017, doi: https://doi.org/10.1016/J.CIRESP.2017.02.005.
J. L. Chen et al., “Imaging spectrum after pancreas transplantation with enteric drainage,” Korean J Radiol, vol. 15, no. 1, pp. 45–53, Feb. 2014, doi: https://doi.org/10.3348/KJR.2014.15.1.45.
M. Hameed, S. Hameed, C. Harvey, S. Moser, and A. Muthusamy, “Imaging in whole organ pancreatic transplants and a multimodality review of its complications,” Br J Radiol, vol. 94, no. 1122, Jun. 2021, doi: https://doi.org/10.1259/BJR.20200106.
S. A. El Sayed and S. Mukherjee, Physiology, pancreas. StatPearls, May 2023, Accessed: 17 Mar 2024. Available: https://www.ncbi.nlm.nih.gov/books/NBK459261/
H. Sarles, “The Exocrine Pancreas,” Int Rev Physiol, vol. 12, pp. 173–221, 2010, doi: https://doi.org/10.4199/C00026ED1V01Y201102ISP014.
P. Nikolaidis et al., “Role of sonography in pancreatic transplantation,” Radiographics, vol. 23, no. 4, pp. 939–949, 2003, doi: https://doi.org/10.1148/RG.234025160.
K. Sandrasegaran, C. Lall, W. A. Berry, T. Hameed, and D. D. T. Maglinte, “Enteric drainage pancreatic transplantation,” Abdom Imaging, vol. 31, no. 5, pp. 588–595, Oct. 2006, doi: https://doi.org/10.1007/S00261-005-0076-3.
A. H. Dachman, G. M. Newmark, J. R. Thistlethwaite, A. Oto, D. S. Bruce, and K. A. Newell, “Imaging of pancreatic transplantation using portal venous and enteric exocrine drainage,” AJR Am J Roentgenol, vol. 171, no. 1, pp. 157–163, 1998, doi: https://doi.org/10.2214/AJR.171.1.9648780.
M. C. Freund et al., “Spectrum of imaging findings after pancreas transplantation with enteric exocrine drainage: Part 2, posttransplantation complications,” AJR Am J Roentgenol, vol. 182, no. 4, pp. 919–925, 2004, doi: https://doi.org/10.2214/AJR.182.4.1820919.
R. Perez-Johnston, D. K. Lenhart, and D. V. Sahani, “CT angiography of the hepatic and pancreatic circulation,” Radiol Clin North Am, vol. 48, no. 2, 2010, doi: https://doi.org/10.1016/J.RCL.2010.02.021.
B. L. Holbert and N. Lalwani, “Imaging in pancreas transplantation,” Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas: Volume 1, pp. 195–207, Jan. 2020, doi: https://doi.org/10.1016/B978-0-12-814833-4.00016-2.
F. Q. Vandermeer, M. A. Manning, A. A. Frazier, and J. J. Wong-You-Cheong, “Imaging of whole-organ pancreas transplants,” Radiographics, vol. 32, no. 2, pp. 411–435, Mar. 2012, doi: https://doi.org/10.1148/RG.322115144/-/DC1.
G. Low et al., “Imaging of vascular complications and their consequences following transplantation in the abdomen,” Radiographics, vol. 33, no. 3, pp. 633–652, May 2013, doi: https://doi.org/10.1148/RG.333125728.
P. Boraschi et al., “Pancreatic transplants: Secretin-stimulated MR pancreatography,” Abdom Imaging, vol. 32, no. 2, pp. 207–214, Apr. 2007, doi: https://doi.org/10.1007/S00261-007-9178-4/FIGURES/6.
K. D. Hagspiel et al., “Contrast-enhanced MR angiography after pancreas transplantation: normal appearance and vascular complications,” AJR Am J Roentgenol, vol. 184, no. 2, pp. 465–473, 2005, doi: https://doi.org/10.2214/AJR.184.2.01840465.
A. P. Wasnik, A. A. Aslam, J. D. Millet, A. Pandya, and R. O. Bude, “Multimodality imaging of pancreas-kidney transplants,” Clin Imaging, vol. 69, pp. 185–195, Jan. 2021, doi: https://doi.org/10.1016/J.CLINIMAG.2020.07.028.
Angiographic evaluation of pancreas transplants correlation... : transplantation. Accessed: 19 Apr. 2024. Available: https://journals.lww.com/transplantjournal/fulltext/1999/04150/angiographic_evaluation_of_pancreas_transplants.694.aspx
L. H. Toledo-Pereyra1, Scintigraphy of pancreatic transplants.
R. A. Low, C. C. Kuni, and J. G. Letourneau, Pancreas transplant imaging: an overview, 1990.
M. A. Pozniak, P. A. Propeck, F. Kelcz, and H. Sollinger, “Imaging of pancreas transplants.,” Radiol Clin North Am, vol. 33, no. 3, pp. 581–94, May 1995.
D. E. R. Sutherland et al., “Lessons learned from more than 1,000 pancreas transplants at a single institution,” Ann Surg, vol. 233, no. 4, pp. 463–501, 2001, doi: https://doi.org/10.1097/00000658-200104000-00003.
T. Grochowiecki et al., “Multivariate analysis of complications after simultaneous pancreas and kidney transplantation,” Transplant Proc, vol. 46, no. 8, pp. 2806–2809, Oct. 2014, doi: https://doi.org/10.1016/J.TRANSPROCEED.2014.08.010.
C. Troppmann, “Complications after pancreas transplantation,” Curr Opin Organ Transplant, vol. 15, no. 1, pp. 112–118, Feb. 2010, doi: https://doi.org/10.1097/MOT.0B013E3283355349.
J. Maupoey Ibáñez, A. Boscà Robledo, and R. López-Andujar, “Late complications of pancreas transplant,” World J Transplant, vol. 10, no. 12, p. 404, Dec. 2020, doi: https://doi.org/10.5500/WJT.V10.I12.404.
A. Hakeem et al., “Pancreatic allograft thrombosis: Suggestion for a CT grading system and management algorithm,” Am J Transplant, vol. 18, no. 1, pp. 163–179, Jan. 2018, doi: https://doi.org/10.1111/AJT.14433.
A. S. R. Muthusamy, P. L. F. Giangrande, and P. J. Friend, “Pancreas allograft thrombosis,” Transplantation, vol. 90, no. 7, pp. 705–707, Oct. 2010, doi: https://doi.org/10.1097/TP.0B013E3181EB2EA0.
S. A. White, J. A. Shaw, and D. E. Sutherland, “Pancreas transplantation,” Lancet, vol. 373, no. 9677, pp. 1808–1817, 2009, doi: https://doi.org/10.1016/S0140-6736(09)60609-7.
G. W. Burke et al., “Hypercoagulable state associated with kidney–pancreas transplantation. Thromboelastogram-directed anti-coagulation and implications for future therapy,” Clin Transplant, vol. 18, no. 4, pp. 423–428, Aug. 2004, doi: https://doi.org/10.1111/J.1399-0012.2004.00183.X.
J. W. Harbell et al., “Splenic vein thrombosis following pancreas transplantation: identification of factors that support conservative management,” Am J Transplant, vol. 17, no. 11, pp. 2955–2962, Nov. 2017, doi: https://doi.org/10.1111/AJT.14428.
K. D. Hagspiel et al., “Evaluation of vascular complications of pancreas transplantation with high-spatial-resolution contrast-enhanced MR angiography,” Radiology, vol. 242, no. 2, pp. 590–599, Feb. 2007, doi: https://doi.org/10.1148/RADIOL.2422041261.
W. H. Kopp et al., “Retrospective study on detection, treatment, and clinical outcome of graft thrombosis following pancreas transplantation,” Transplant International, vol. 32, no. 4, p. 410, Apr. 2019, doi: https://doi.org/10.1111/TRI.13384.
M. M. Barth, K. Khwaja, S. Faintuch, and D. Rabkin, “Transarterial and transvenous embolotherapy of arteriovenous fistulas in the transplanted pancreas,” J Vasc Interv Radiol, vol. 19, no. 8, pp. 1231–1235, Aug. 2008, doi: https://doi.org/10.1016/J.JVIR.2008.04.028.
E. Orsenigo et al., “Successful endovascular treatment for gastroduodenal artery pseudoaneurysm with an arteriovenous fistula after pancreas transplantation,” Transpl Int, vol. 16, no. 9, pp. 694–696, Sep. 2003, doi: https://doi.org/10.1007/S00147-003-0611-5.
N. L. Nelson et al., “Pancreas allograft rejection: correlation of transduodenal core biopsy with Doppler resistive index,” Radiology, vol. 200, no. 1, pp. 91–94, 1996, doi: https://doi.org/10.1148/RADIOLOGY.200.1.8657950.
A. Humar et al., “Chronic rejection: The next major challenge for pancreas transplant recipients,” Transplantation, vol. 76, no. 6, pp. 918–923, Sep. 2003, doi: https://doi.org/10.1097/01.TP.0000079457.43199.76.
J. Goodman and Y. T. Becker, “Pancreas surgical complications,” Curr Opin Organ Transplant, vol. 14, no. 1, pp. 85–89, Feb. 2009, doi: https://doi.org/10.1097/MOT.0B013E328320A8EC.
J. R. Dillman, K. M. Elsayes, R. O. Bude, J. F. Platt, and I. R. Francis, “Imaging of pancreas transplants: postoperative findings with clinical correlation,” J Comput Assist Tomogr, vol. 33, no. 4, pp. 609–617, 2009, doi: https://doi.org/10.1097/RCT.0B013E3181966988.
N. Issa et al., “Posttransplant lymphoproliferative disorder following pancreas transplantation,” Am J Transplant, vol. 9, no. 8, pp. 1894–1902, Aug. 2009, doi: https://doi.org/10.1111/J.1600-6143.2009.02691.X.
A. A. Borhani, K. Hosseinzadeh, O. Almusa, A. Furlan, and M. Nalesnik, “Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation,” Radiographics, vol. 29, no. 4, pp. 981–1000, Jul. 2009, doi: https://doi.org/10.1148/RG.294095020.
F. J. Krendl et al., “Post-transplant malignancies following pancreas transplantation: Incidence and implications on long-term outcome from a single-center perspective,” J Clin Med, vol. 10, no. 21, p. 4810, Nov. 2021, doi: https://doi.org/10.3390/JCM10214810/S1.
C. G. Lall, K. Sandrasegaran, D. T. Maglinte, and J. A. Fridell, “Bowel complications seen on CT after pancreas transplantation with enteric drainage,” AJR Am J Roentgenol, vol. 187, no. 5, pp. 1288–1295, Nov. 2006, doi: https://doi.org/10.2214/AJR.05.1087.
D. S. Nath, A. Gruessner, R. Kandaswamy, R. W. Gruessner, D. E. R. Sutherland, and A. Humar, “Late anastomotic leaks in pancreas transplant recipients - clinical characteristics and predisposing factors,” Clin Transplant, vol. 19, no. 2, pp. 220–224, Apr. 2005, doi: https://doi.org/10.1111/J.1399-0012.2005.00322.X.
S. Paraskevas et al., “Posttransplant lymphoproliferative disorder in pancreas transplantation: a single-center experience,” Transplantation, vol. 80, no. 5, pp. 613–622, Sep. 2005, doi: https://doi.org/10.1097/01.TP.0000168366.07896.D7.
J. R. T. Lakey, M. Mirbolooki, and A. M. J. Shapiro, “Current status of clinical islet cell transplantation,” Methods Mol Biol, vol. 333, pp. 47–104, 2006, doi: https://doi.org/10.1385/1-59745-049-9:47.
A. Gamble, A. R. Pepper, A. Bruni, and A. M. J. Shapiro, “The journey of islet cell transplantation and future development,” Islets, vol. 10, no. 2, pp. 80–94, Mar. 2018, doi: https://doi.org/10.1080/19382014.2018.1428511.
G. Low et al., “Role of imaging in clinical islet transplantation,” Radiographics, vol. 30, no. 2, pp. 353–366, Mar. 2010, doi: https://doi.org/10.1148/RG.302095741.
N. Onaca, G. B. Klintmalm, and M. F. Levy, “Pancreatic islet cell transplantation: a treatment strategy for type I diabetes mellitus,” Nutr Clin Pract, vol. 19, no. 2, pp. 154–164, 2004, doi: https://doi.org/10.1177/0115426504019002154.
D. Cahill, F. Zamboni, and M. N. Collins, “Radiological Advances in Pancreatic Islet Transplantation,” Acad Radiol, vol. 26, no. 11, pp. 1536–1543, Nov. 2019, doi: https://doi.org/10.1016/J.ACRA.2019.01.006.
P. Villiger et al., “Prevention of bleeding after islet transplantation: lessons learned from a multivariate analysis of 132 cases at a single institution,” Am J Transplant, vol. 5, no. 12, pp. 2992–2998, Dec. 2005, doi: https://doi.org/10.1111/J.1600-6143.2005.01108.X.
E. A. Ryan et al., “Five-year follow-up after clinical islet transplantation,” Diabetes, vol. 54, no. 7, pp. 2060–2069, Jul. 2005, doi: https://doi.org/10.2337/DIABETES.54.7.2060.
E. A. Ryan et al., “Assessment of the severity of hypoglycemia and glycemic lability in type 1 diabetic subjects undergoing islet transplantation,” Diabetes, vol. 53, no. 4, pp. 955–962, Apr. 2004, doi: https://doi.org/10.2337/DIABETES.53.4.955.
R. Bhargava et al., “Prevalence of hepatic steatosis after islet transplantation and its relation to graft function,” Diabetes, vol. 53, no. 5, pp. 1311–1317, May 2004, doi: https://doi.org/10.2337/DIABETES.53.5.1311.
E. Alfadhli et al., “High prevalence of ovarian cysts in premenopausal women receiving sirolimus and tacrolimus after clinical islet transplantation,” Transpl Int, vol. 22, no. 6, pp. 622–625, Jun. 2009, doi: https://doi.org/10.1111/J.1432-2277.2009.00839.X.
P. A. Senior, A. M. J. Shapiro, T. E. Ackerman, E. A. Ryan, B. W. Paty, and R. Bhargava, “Magnetic resonance-defined perinephric edema after clinical islet transplantation: a benign finding associated with mild renal impairment,” Transplantation, vol. 78, no. 6, pp. 945–948, Sep. 2004, doi: https://doi.org/10.1097/01.TP.0000134971.15093.07.
M. McCall and A. M. James Shapiro, “Update on islet transplantation,” Cold Spring Harb Perspect Med, vol. 2, no. 7, 2012, doi: https://doi.org/10.1101/CSHPERSPECT.A007823.
O. Eriksson et al., “Positron Emission Tomography to Assess the Outcome of Intraportal Islet Transplantation,” Diabetes, vol. 65, no. 9, pp. 2482–2489, Sep. 2016, doi: https://doi.org/10.2337/DB16-0222.
W. A. Eter, D. Bos, C. Frielink, O. C. Boerman, M. Brom, and M. Gotthardt, “Graft revascularization is essential for non-invasive monitoring of transplanted islets with radiolabeled exendin,” Scientific Reports 2015 5:1, vol. 5, no. 1, pp. 1–10, Oct. 2015, doi: https://doi.org/10.1038/srep15521.
C. Toso et al., “Clinical magnetic resonance imaging of pancreatic islet grafts after iron nanoparticle labeling,” Am J Transplant, vol. 8, no. 3, pp. 701–706, 2008, doi: https://doi.org/10.1111/J.1600-6143.2007.02120.X.
M. L. Malosio et al., “MR imaging monitoring of iron-labeled pancreatic islets in a small series of patients: islet fate in successful, unsuccessful, and autotransplantation,” Cell Transplant, vol. 24, no. 11, pp. 2285–2296, 2015, doi: https://doi.org/10.3727/096368914X684060.
Funding
This manuscript received no specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Avinash Kambadakone: Research grants (GE, Philips Healthcare, and PanCAN; not relevant to this manuscript). None of the other authors have any relevant financial relationships to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Srinivas Rao, S., Pandey, A., Mroueh, N. et al. Comprehensive review of imaging in pancreas transplantation: a primer for radiologists. Abdom Radiol 49, 2428–2448 (2024). https://doi.org/10.1007/s00261-024-04383-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-024-04383-9