Abstract
Purpose
To evaluate angle-corrected peak systolic cystic artery velocity (CAv) as a predictor of acute cholecystitis among patients presenting to the emergency department (ED) with right upper quadrant (RUQ) pain.
Methods
In this IRB-approved and retrospective study, CAv was evaluated in 73 patients, 43 who underwent definitive treatment with cholecystectomy or percutaneous cholecystostomy and 30 control patients without clinical suspicion for cholecystitis. In addition to CAv, the following were reviewed by 3 radiologists: CBD diameter, cholelithiasis, impacted stone in the neck, sludge, gallbladder wall thickness > 3 mm, gallbladder transverse dimension ≥ 4 cm, longitudinal dimension ≥ 8 cm, tensile gallbladder fundus sign, pericholecystic fluid, pericholecystic echogenic fat, and sonographic Murphy sign.
Results
Of the 43 patients who underwent definitive treatment, 25 had acute cholecystitis (34%) and 18 (25%) had chronic cholecystitis. Average CAv measurements were 50 ± 16 cm/s (acute), 28 ± 8 cm/s (chronic), and 22 ± 8 cm/s (control; p < 0.0001). In univariate analysis, among patients who underwent definitive therapy, CAv ≥ 40 cm/s, gallbladder wall thickness, stone impaction, GB long dimension ≥ 8 cm, and elevated WBC were associated with acute cholecystitis (p < 0.05). In multivariate analysis, CAv ≥ 40 cm/s was the only statistically significant variable (p = 0.016). CAv ≥ 40 cm/s alone had a PPV of 94.7% and overall accuracy of 81.4% in diagnosing acute cholecystitis.
Conclusion
CAv ≥ 40 cm/s is highly associated with acute cholecystitis in patients presenting to the ED with RUQ pain.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute right upper quadrant abdominal pain is a common chief complaint among patients presenting to the emergency department [1]. Numerous societal guidelines including the American College of Radiology (ACR) and the Tokyo Guidelines recommend abdominal ultrasound as the first-line imaging modality for patients with right upper quadrant pain and suspected biliary disease [1, 2]. Although ultrasound is over 96% accurate in the diagnosis of cholelithiasis, the sensitivity and specificity of ultrasound for acute cholecystitis is variable in the literature and may be related to the limited number of sonographic features studied [3, 4] or the relative familiarity of reviewers with ultrasound [4].
Gallbladder hyperemia has been observed in acute cholecystitis due to increased cystic artery flow [2, 5, 6]. We hypothesize that the peak systolic cystic artery velocity (CAv) may serve as an objective, quantitative imaging biomarker of acute inflammation and a helpful sonographic finding that may improve the accuracy of ultrasound in the diagnosis of acute cholecystitis. The purposes of our study were to compare CAv in patients with acute cholecystitis to those without, using surgical pathology or percutaneous cholecystostomy tube as the reference standard, and to determine if a threshold velocity may help optimally diagnose acute cholecystitis. Our secondary objective was to compare the diagnostic accuracy of CAv to other common sonographic findings of acute cholecystitis.
Materials and methods
Patients
Our Institutional Review Board approved this retrospective study and waived the need for informed consent. All ultrasound examinations between August 2019 through April 2020 were reviewed on an independent workstation Sectra PACS ID57 (Sectra AB, Linköping, Sweden) to identify adults (18 years or older) who presented to the emergency department with right upper quadrant pain and underwent cystic artery measurement (127 patients). The electronic medical record was reviewed to identify patients who underwent definitive treatment within 6 days of ultrasound examination (43 patients). Patients with suspected biliary pathology who did not undergo definitive treatment within 6 days and patients treated for alternative diagnoses were excluded (84 patients) given that we could not definitively exclude the possibility of acute cholecystitis in these patients without pathologic proof. Additionally, patients were excluded if they had a history of ongoing pregnancy, cirrhosis, hepatocellular carcinoma, transjugular intrahepatic portosystemic shunt (TIPS), or hepatic metastases (10 patients).
To establish a control group, prospective measurement of cystic artery velocity was attempted in 108 consecutive outpatients undergoing abdominal ultrasound. Patients with clinical suspicion of acute cholecystitis or other biliary disease (specifically, patients with fever, tachycardia, abnormal liver function tests, or right upper quadrant pain) were excluded. Cystic artery velocity was measurable in 30 out of 51 asymptomatic patients.
In total, 73 patients comprised the final sample: 22 had acute cholecystitis on surgical pathology, 3 had acute cholecystitis based on cholecystostomy tube placement, 18 had chronic cholecystitis, and 30 comprised the control group.
Ultrasonography technique
All ultrasounds were performed by sonographers certified by the American Registry for Diagnostic Medical Sonography and checked at the time of the exam by a radiologist. Sonographers were asked to measure the cystic artery velocity in patients presenting to the ED with right upper quadrant pain but were unaware of the significance or threshold of the cystic artery velocity at the time of imaging. Curved array or vector transducers 2.5–5.5 MHz were used to obtain Grayscale and color Doppler images of the gallbladder using GE Logiq E9 or E10 (GE Healthcare, Wausheka WI), ACUSON S2000 (Siemens Medical Solutions, Mountain View CA), or Canon Aplio i900 (Canon Medical Systems USA, Inc, Tustin, CA) ultrasound machines. A right lateral intercostal or subcostal approach with spectral Doppler was used to measure the peak systolic velocity of the cystic artery (or branch of the cystic artery) along the long axis of the gallbladder wall where it was best visualized with color Doppler. Angle correction less than or equal to 60° was made with the artery or gallbladder wall in longitudinal dimension. The peak systolic hepatic artery velocity (HAv) was measured with angle-corrected spectral Doppler where the artery courses parallel to the portal vein in the hepatoduodenal ligament. For both the cystic and hepatic arteries, the peak systolic velocities were reported in centimeters per second. If velocities were sampled more than once, the average velocity was reported. Each ultrasound was reviewed by a radiology resident (MP) to determine if CAv and HAv were measured and whether the technique was adequate and appropriate for inclusion.
Image review
Images of each patient were independently reviewed by 3 board-certified radiologists with subspecialty expertise in abdominal imaging (2, 15, and > 25 years of experience) who were blinded to the original report and pathologic diagnosis. Images were evaluated for cholelithiasis, CBD diameter (measured from inner wall to inner wall), stone impacted in the neck of the gallbladder, sludge, wall thickness > 3 mm, gallbladder distention (transverse dimension ≥ 4 cm, gallbladder longitudinal dimension ≥ 8 cm), tensile gallbladder fundus sign (defined as identification of a bulging gallbladder fundus against the anterior abdominal wall due to resistance from being flattened by the abdominal wall as previously described by An et al.) [7], pericholecystic fluid, and pericholecystic echogenic fat. Sonographic Murphy sign (as documented at time of examination by radiologist or sonographer), gallbladder transverse and longitudinal dimension, CBD diameter, angle-corrected peak systolic hepatic artery velocity, and angle-corrected peak systolic cystic artery velocity were documented at the time of examination and were recorded but not re-evaluated on retrospective review by the three radiologists.
Clinical review
The electronic medical record was reviewed to document age, sex, white blood cell count, heart rate at presentation, and days between imaging to surgery or interventional procedure for each patient.
Reference standard
Final pathology reports were used as a reference standard among those who underwent cholecystectomy. Pathology reports were not available for patients who underwent cholecystostomy tube placement, but patients were considered positive for acute cholecystitis if there was documentation of turbid bile, bacterial growth from aspirated fluid, or identification of cystic duct obstruction during cholangiography.
Statistical analysis
With the three reviewers, a majority rules approach was used to determine the presence or absence of a sonographic finding (i.e., if two out of three radiologists agreed there was pericholecystic fluid, for instance, this was considered to be positive). Cohen’s Kappa statistic was calculated to determine inter-rater agreement, defined as poor (< 0.20), fair (0.20–0.39), moderate (0.40–0.59), substantial (0.60–0.79), almost perfect (0.80–0.99), or perfect (1.0). Continuous variables were compared using one-way ANOVA and post hoc Tukey’s for multiple comparisons when comparing all 3 groups (acute cholecystitis, chronic cholecystitis, and control) and two-tailed Student’s t-test when comparing between acute cholecystitis and chronic cholecystitis. Categorical variables were compared with two-tailed Fisher’s exact test in univariate analysis. Multivariate analysis was performed to determine independent predictors of acute cholecystitis among statistically significant univariate variables. A threshold of p < 0.05 was used to determine statistical significance.
Logistic regression models were used to assess the association between cystic artery velocity and acute cholecystitis. Receiver operator characteristic (ROC) curves from logistic regression were used to determine performance and a threshold was determined to maximize accuracy.
Statistical analyses to describe the diagnostic performance and inter-rater reliability were performed between the acute cholecystitis and chronic cholecystitis groups, as these were the patients clinically suspected to have acute cholecystitis.
Statistical analysis was performed using GraphPad Prism Windows Version 8.4.2 (GraphPad Software, La Jolla, CA) and Stata Version 16.1 (Stata Corporation, College Station, TX).
Results
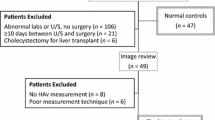
Of 73 patients, 43 underwent definitive treatment: 40 patients underwent cholecystectomy while 3 underwent percutaneous cholecystostomy tube placement. All 3 patients who underwent percutaneous cholecystostomy had additional findings to support the diagnosis of acute cholecystitis. Of those with definitive treatment, 25 patients had acute cholecystitis and 18 had chronic cholecystitis. Of the 25 patients with acute cholecystitis, 5 had evidence of gangrenous change and 2 had necrotizing cholecystitis at pathology. The control group with no clinical suspicion of acute cholecystitis was comprised of 30 patients. The process to derive the final sample is summarized in Fig. 1.
Baseline demographics between the two groups with definitive treatment are summarized in Table 1. Patients with chronic cholecystitis were more likely to be women (p = 0.006). There were no statistically significant differences in other baseline characteristics, including patient age, tachycardia, fever, and mean white blood cell count. Additionally, there was no statistically significant difference between time interval and definitive treatment, averaging 1.4 ± 1.4 days for those with acute cholecystitis and 1.3 ± 1.2 for those with chronic cholecystitis (p = 0.831).
Inter-rater agreements for each sonographic feature are summarized in Table 2. The presence of cholelithiasis was the only feature with substantial or higher agreement. The remaining features were moderate (impacted stone, thickened wall, pericholecystic fluid) or fair (sludge, tensile gallbladder, echogenic fat). Distribution of each sonographic feature is summarized in Table 3.
Mean CAv was elevated in patients with acute cholecystitis, measuring 50 ± 16 cm/s, versus 28 ± 8 cm/s among those with chronic cholecystitis (p < 0.0001) and 22 ± 8 cm/s among the control group (p < 0.0001; Fig. 2a). There were no statistically significant differences between mean CAv for patients with chronic cholecystitis and the control group (p = 0.173). Examples of CAv waveforms are provided in Figs. 3 and 4.
a Scatter plot depicting distribution of Cystic artery velocity (Cav) among the control group, chronic cholecystitis, and acute cholecystitis, with lines drawn at the mean and one standard deviation. b Scatter plot depicting distribution of hepatic artery velocity (HAv) among the control group, chronic cholecystitis, and acute cholecystitis, with lines drawn at the mean and one standard deviation
41-year-old man with pathology-proven acute cholecystitis. Spectral Doppler evaluation showed the angle-corrected peak systolic CAv was measured to be 61 cm/s. Angle correction is parallel to the gallbladder wall in longitudinal dimension. Cholelithiasis and wall thickening were also seen in this patient
Mean HAv was also elevated in patients with acute cholecystitis, measuring 121 ± 62 cm/s, versus 86 ± 45 cm/s (chronic cholecystitis; p = 0.038) and 71 ± 24 cm/s (control; p = 0.0004; Fig. 2b). Similarly, there were no statistically significant differences between mean HAv for patients with chronic cholecystitis and the control group (p = 0.521).
In comparison of patients with acute cholecystitis versus chronic cholecystitis, univariate analysis showed that cystic artery velocity (p = 0.002), gallbladder wall thickness > 3 mm (p = 0.023), stone impaction (p = 0.013), and GB long dimension ≥ 8 cm (0.006) were statistically significant predictors of acute cholecystitis. Other sonographic features (common bile duct size, cholelithiasis, sludge, sonographic Murphy sign, transverse gallbladder dimension, pericholecystic fluid, tensile gallbladder fundus sign, and pericholecystic echogenic fat) were not statistically significantly. Of clinical variables, univariate analysis showed that only elevated WBC count, defined as greater than 12 × 106/liter, was statistically significant (p = 0.007). Multivariate analysis of above variables demonstrated that cystic artery velocity outperformed all other statistically significant variables in the diagnosis of acute cholecystitis (p = 0.016; Table 4).
ROC curve (Fig. 5a) and accuracy curve (Fig. 5b) analysis determined cystic artery velocity greater than or equal to 40 cm/s as the threshold with highest overall accuracy for differentiating acute cholecystitis from chronic cholecystitis. Using this threshold, cystic artery velocity had a diagnostic performance of 72% sensitivity, 94% specificity, 95% PPV, 71% NPV, and 81% accuracy (Table 3).
a Receiver operating characteristic curve demonstrating the ability of CAv to predict acute cholecystitis. The area under the curve is 0.82. Maximum accuracy of 0.81 is obtained using a threshold of CAv ≥ 40 cm/s. b Accuracy curve in predicting acute cholecystitis based on CAv threshold determined by ROC curve analysis. Maximum accuracy of 0.81 is determined using a threshold of CAv ≥ 40 cm/s
Discussion
We found that the cystic artery velocity was an excellent sonographic biomarker for acute cholecystitis in patients who presented with right upper quadrant pain. A threshold of 40 cm/s was able to identify patients with acute cholecystitis with 95% positive predictive value, and in multivariate analysis, was the only statistically significant variable associated with acute cholecystitis.
Several prior studies have evaluated gallbladder wall hyperemia as a potentially useful finding in the diagnosis of acute cholecystitis, but systematic evaluation of the angle-corrected peak systolic velocity of the cystic artery itself has been limited [5, 8]. Measurement of the cystic artery velocity is ideal because it provides an objective, quantitative measure that is not reliant on gestalt or potentially influenced by variable machine parameters. Jeffrey et al., first evaluated the cystic artery qualitatively [5] and found that 26% of patients with acute cholecystitis had cystic artery length greater than half of the anterior gallbladder wall compared to 2% of normal controls. They did not, however, measure the angle-corrected velocity of the artery itself. In 2004, Tochio et al. studied the utility of cystic artery velocity in diagnosis of acute cholecystitis in cirrhotic patients. Similar to our study, they found that a velocity of 40 cm/s was a useful threshold; however, their study included only cirrhotic patients, of whom only 6 had acute cholecystitis [9]. Thus, it was not clear if their findings could be extrapolated to the non-noncirrhotic population. Our study not only confirms the findings by Tochio et al., but also shows that this threshold has a higher diagnostic accuracy for acute cholecystitis when applied to patients presenting to the ED with right upper quadrant pain than in cirrhotic patients.
Acute cholecystitis is postulated to be secondary to chemical irritation and inflammation of the gallbladder due to cystic duct obstruction most commonly by gallstones. The gallbladder mucosa is directly irritated by the detergent action of bile upon the mucosal epithelium [10] and develops reactive hyperemia. Since the cystic artery is the only blood supply to the gallbladder with no collateral circulation, measurement of the cystic artery velocity may serve as a proxy for inflammation. Interestingly, an older study by Warren et al. in 1992 confirmed that acute gallstone-related cholecystitis gallbladder arteriograms showed significant arterial dilatation compared to controls, whereas acalculous cholecystitis gallbladders showed small vessel occlusion [11]. More recently, the 2018 Tokyo Guideline diagnostic criteria for acute cholecystitis comments on gallbladder wall hyperemia as an imaging finding seen with acute cholecystitis yet because of difficulty in quantification, they do not recommend routine use of this common finding in diagnosis. Our findings of the cystic artery velocity may help incorporate this well-known phenomenon of gallbladder wall hyperemia in a more quantifiable metric to aid diagnosis of acute calculous cholecystitis.
The cystic artery typically arises from the proper hepatic artery, which has previously been shown to have an increased likelihood of elevated peak systolic velocity in acute cholecystitis, possibly due to increased perfusion demands [12]. An inflammatory process in the gallbladder would thus increase both the CAv and HAv. Indeed, we observed that both elevated mean HAv and CAv were statistically significantly associated with acute cholecystitis when compared against chronic cholecystitis and normal controls. However, we found that CAv performed significantly better than HAv. Many other conditions are known to cause an increase in HAv, such as acute alcoholic hepatitis [13], end-stage cirrhosis [14], ascending cholangitis [15], as well as other structural hepatobiliary pathologies [16]. As a result, the positive predictive value of an elevated HAv in acute cholecystitis may be diminished by these other potential alternative etiologies for right upper quadrant pain. CAv measurement is a more direct biomarker of gallbladder wall hyperemia and may offer increased specificity for gallbladder pathology compared to the HAv.
Despite the relatively high incidence of acute cholecystitis in the general population, this diagnosis has long been challenging to establish radiographically because of the myriad imaging manifestations that may (or may not) be present. A common misconception is that the diagnosis of acute cholecystitis is simple to establish with ultrasound. For example, one of the most highly cited papers in the literature regarding sonographic diagnosis of acute cholecystitis by Ralls et al. in 1985 reported that the combination of gallstones and a sonographic Murphy sign are the only two findings necessary to establish the diagnosis, with a “92% PPV and 95% NPV” [17]. No study since this paper has been able to replicate such extraordinary numbers and in practice, we have found that reliance on only these two criteria results in a very low accuracy in diagnosis of acute cholecystitis. Indeed, the combination of these two findings in our study was only 20% sensitive and had a NPV of 44%. This low performance may in part be related to the increasing prevalence of gallstones in the general population and because many patients are given analgesic medications prior to ultrasound imaging. A sonographic Murphy sign is defined as maximal pain when the gallbladder is localized sonographically [17]. However, the pain needed to elicit the sonographic Murphy sign may be diminished or even eliminated by analgesic medications, thereby making this sign less reliable. In fact, it is now standard of care at our institution and many others to administer analgesic medications as needed to control pain without consideration of how this may affect a diagnostic ultrasound of the right upper quadrant/gallbladder.
Other commonly accepted imaging findings of acute cholecystitis that significantly improve accuracy include gallbladder wall thickening, gallbladder enlargement, pericholecystic fluid, and an impacted stone in the neck of the gallbladder [18]. However, no single finding or even select combinations of findings are definitive, leading to continued challenges in the diagnosis of acute cholecystitis [19]. More recently described ancillary findings of acute cholecystitis (i.e., tensile gallbladder fundus sign, echogenic pericholecystic fat) had high PPV in our study but low NPV and only fair inter-rater agreement. CAv overcomes many of the above obstacles by serving as an objective and quantifiable biomarker that is both sensitive and specific for acute cholecystitis. We emphasize, however, that this finding should not be used in isolation but rather applied within the context of right upper quadrant pain and other sonographic and clinical findings suggestive of acute cholecystitis.
We purposely used a control group of patients without clinical suspicion of acute cholecystitis. Although we had a relatively large number of patients with RUQ pain and cystic artery velocity measurements who did not go on to surgical resection of the gallbladder within 6 days, these patients could not be used as a control group for the following reasons: (1) it is entirely possible that these patients had acute cholecystitis but defervesced with or without antibiotics, (2) patients may have been discharged from our hospital but treated definitively at another institution, and (3) definitive treatment may have occurred greater than 6 days between ultrasound and surgery. Because of these and many other potential reasons, the integrity of the non-surgical cohort of patients with RUQ pain could not be guaranteed. Thus, we elected to use as controls, the CAv measured in outpatients who presented to us for abdominal ultrasound with indications that did not overlap with acute cholecystitis. Interestingly, in these control patients, only ~ 60% had a measurable cystic artery velocity, suggesting that a non-visualized cystic artery is also likely to be a normal finding. We did not, however, document the number of times that a patient with acute cholecystitis did not have a measurable cystic artery velocity, nor did we investigate whether higher body mass index (BMI) contributed to non-visualization of the CAv. Both of these points are worthy of future investigation. Nonetheless, in patients with right upper quadrant pain in whom the CAv is non-measurable or non-visible, it is reasonable to still evaluate and utilize findings of the HAv which tends to be an easier vessel to interrogate given its relatively good performance in our current study as well as prior studies [12].
We did not study the resistive index in either the cystic artery or the hepatic artery in our patients. Loehfelm et al. previously found that the hepatic artery resistive index is not predictive of acute cholecystitis even when the peak systolic velocity was predictive [12] Further, in a study by Lafortune et al., the hepatic artery resistive index was shown to increase after a recent meal, which they hypothesized was likely due to an increase in portal venous inflow causing a reciprocal constriction of the hepatic artery via the hepatic arterial buffer response [20] They only studied the resistive index and did not evaluate the effects of a recent meal on the hepatic artery velocity. Although we did not record the fasting state of our ED patients, our outpatients are instructed to fast for at least 6 h prior to their ultrasound. Thus, the control group’s hepatic artery would be expected to be higher if indeed the fasting state had an effect on our results. Since those with acute cholecystitis had higher hepatic artery and cystic artery velocities, we do not think the potential variability in fasting state of ED patients affected our results.
Our study had several limitations. First, our study only included patients who presented to our emergency department with right upper quadrant pain who went on to have definitive treatment with surgery or cholecystostomy tube. Our patient population did not include inpatients suspected to have acalculous cholecystitis who typically are critically ill or in the intensive care unit. The pathophysiology of acalculous cholecystitis is thought to be related to an ischemic insult to the gallbladder from prolonged hypotension or other causes of arterial compromise to the gallbladder rather than an obstructing stone in the cystic duct. Thus, it is unclear if our results can be applied to patients with suspected acalculous cholecystitis. Nonetheless, because our acute cholecystitis group included patients with gangrenous and necrotizing cholecystitis, we feel that our findings of CAv can be applied in those suspected to have gangrenous progression of acute cholecystitis.
Second, this was a single-institution, retrospective study with a relatively small sample size. This was in part due to the fact that we only recently began to ask our sonographers to measure the cystic artery velocity in patients with right upper quadrant pain. Because the utility of CAv was not known and because of the large number of sonographers at our institution, measurement of the cystic artery velocity was not performed consistently in all patients who presented with right upper quadrant pain. From those patients who had CAv measured, only those who went on to surgical resection were included in our final study sample in order to ensure the validity of our standard of reference. A short time interval between ultrasound and treatment (average of 1.3 days) helped to preserve the ground truth in pathologic correlation but further decreased our study sample size. All of these factors contributed to our limited study population. However, because our sonographers are now aware of the powerful predictive value of the CAv, future multi-institutional studies with larger samples may be warranted to validate our findings.
Third, because we limited our sample to those with right upper quadrant pain presenting to the ED, we do not know if other conditions may also contribute to elevation of the CAv velocity and if so, how they may affect the diagnostic accuracy of the CAv for acute cholecystitis. For instance, an older study by Hayakawa et al. showed that the cystic artery velocity increases to > 30 cm/s in gallbladder cancer [21]. Further, we hypothesize that other conditions such as end-stage liver disease, large or multiple intrahepatic metastases, and portal vein occlusion may also increase the CAv due to alterations in liver hemodynamics; future investigations focusing on these alternative diagnoses would be helpful in fully understanding the specificity of CAv in acute cholecystitis.
Finally, we did not have any patients with histologically proven normal gallbladders; all surgical patients had either chronic or acute cholecystitis. This was an unavoidable limitation given the relative unlikeliness of performing a cholecystectomy in a completely normal gallbladder. We overcame this limitation by obtaining CAv measurements in asymptomatic patients to establish a control group.
Conclusion
Elevated peak systolic cystic artery velocity is an objective imaging biomarker that may help improve accuracy of abdominal ultrasound in diagnosis of acute cholecystitis in patients presenting to the ED with right upper quadrant abdominal pain.
References
1.Expert Panel on Gastrointestinal I, Peterson CM, McNamara MM, et al. ACR Appropriateness Criteria((R)) Right Upper Quadrant Pain. J Am Coll Radiol. 2019;16(5S):S235-S243.
2.Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):41-54.
3.Hwang H, Marsh I, Doyle J. Does ultrasonography accurately diagnose acute cholecystitis? Improving diagnostic accuracy based on a review at a regional hospital. Can J Surg. 2014;57(3):162-168.
4.Wertz JR, Lopez JM, Olson D, Thompson WM. Comparing the Diagnostic Accuracy of Ultrasound and CT in Evaluating Acute Cholecystitis. AJR Am J Roentgenol. 2018;211(2):W92-W97.
5.Jeffrey RB, Jr., Nino-Murcia M, Ralls PW, Jain KA, Davidson HC. Color Doppler sonography of the cystic artery: comparison of normal controls and patients with acute cholecystitis. J Ultrasound Med. 1995;14(1):33-36.
6.Olcott EW, Jeffrey RB, Jr., Jain KA. Power versus color Doppler sonography of the normal cystic artery: implications for patients with acute cholecystitis. AJR Am J Roentgenol. 1997;168(3):703-705.
7.An C, Park S, Ko S, Park MS, Kim MJ, Kim KW. Usefulness of the tensile gallbladder fundus sign in the diagnosis of early acute cholecystitis. AJR Am J Roentgenol. 2013;201(2):340-346.
8.Soyer P, Brouland JP, Boudiaf M, et al. Color velocity imaging and power Doppler sonography of the gallbladder wall: a new look at sonographic diagnosis of acute cholecystitis. AJR Am J Roentgenol. 1998;171(1):183-188.
9.Tochio H, Nishiuma S, Okabe Y, Orino A, Kudo M. Diagnosis of acute cholecystitis in patients with liver cirrhosis: waveform analysis of the cystic artery by color Doppler imaging. J Med Ultrason (2001). 2004;31(1):21-28.
10.Crawford J. Chapter 18 Liver and biliary tract In: Robbins and Cotran Pathologic Basis of Disease Seventh ed.: Elsevier Saunders 2005:877.
11.Warren BL. Small vessel occlusion in acute acalculous cholecystitis. Surgery. 1992;111(2):163-168.
12.Loehfelm TW, Tse JR, Jeffrey RB, Kamaya A. The utility of hepatic artery velocity in diagnosing patients with acute cholecystitis. Abdom Radiol (NY). 2018;43(5):1159-1167.
13.Han SH, Rice S, Cohen SM, Reynolds TB, Fong TL. Duplex Doppler ultrasound of the hepatic artery in patients with acute alcoholic hepatitis. J Clin Gastroenterol. 2002;34(5):573-577.
14.Park HS, Desser TS, Jeffrey RB, Kamaya A. Doppler Ultrasound in Liver Cirrhosis: Correlation of Hepatic Artery and Portal Vein Measurements With Model for End-Stage Liver Disease Score. J Ultrasound Med. 2017;36(4):725-730.
15.Tse JR, Liang T, Jeffrey RB, Kamaya A. Does measurement of the hepatic artery velocity improve the sonographic diagnosis of cholangitis? Abdom Radiol (NY). 2019;44(12):4004-4010.
16.Tse JR, Jeffrey RB, Kamaya A. Performance of Hepatic Artery Velocity in Evaluation of Causes of Markedly Elevated Liver Tests. Ultrasound Med Biol. 2018;44(11):2233-2240.
17.Ralls PW, Colletti PM, Lapin SA, et al. Real-time sonography in suspected acute cholecystitis. Prospective evaluation of primary and secondary signs. Radiology. 1985;155(3):767-771.
18.Hertzberg B, Middleton W. The Gallbladder. In: Ultrasound Requisites. Third ed.: Elsevier 2016:32-50.
19.Trowbridge RL, Rutkowski NK, Shojania KG. Does this patient have acute cholecystitis? JAMA. 2003;289(1):80-86.
20.Lafortune M, Dauzat M, Pomier-Layrargues G, et al. Hepatic artery: effect of a meal in healthy persons and transplant recipients. Radiology. 1993;187(2):391-394.
21.Hayakawa S, Goto H, Hirooka Y, et al. Colour Doppler-guided spectral analysis of gall-bladder wall flow. J Gastroenterol Hepatol. 1998;13(2):181-185.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Aya Kamaya: Book royalties from Elsevier. Marcelina G. Perez, Justin R. Tse, Kristen Bird, Tie Liang, and R. Brooke Jeffrey disclose that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Perez, M.G., Tse, J.R., Bird, K.N. et al. Cystic artery velocity as a predictor of acute cholecystitis. Abdom Radiol 46, 4720–4728 (2021). https://doi.org/10.1007/s00261-021-03020-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03020-z